Abstract
Sirtuin 1 (SIRT1) is an NAD+-dependent deacetylase that connects cellular energy levels to homeostatic responses by deacetylating and modulating the activities of many transcriptional regulators. Discovered as a longevity protein in yeast, the mammalian SIRT1 has been intensively studied because of its great potential as a therapeutic target to benefit human health by preventing and improving many age-related diseases. There has been, therefore, substantial interest in developing agents that upregulate SIRT1 expression and activity. SIRT1 is regulated at multiple levels, including post-transcriptionally by microRNAs (miRs), powerful regulators of diverse biological pathways. Here we discuss how expression and activity of SIRT1 and other sirtuins are inhibited by miRs and further discuss the therapeutic potential of targeting miRs for age-related diseases that involve SIRT1 dysfunction, focusing on obesity-related diseases.
Keywords: aging, deacetylase, NAD, NAMPT, obesity, therapeutics
INTRODUCTION
Sirtuin 1 (SIRT1) is a highly conserved NAD+-dependent protein deacetylase that increases life-span in lower organisms and protects against age-related diseases in mammals (Finkel et al., 2009; Guarente, 2006; 2011; Haigis and Sinclair, 2010; Houtkooper et al., 2012; Imai et al., 2000). SIRT1 senses cellular energy levels through the level of NAD+ and conveys the information into transcriptional outputs to maintain homeostasis. In response to energy-deprived conditions like fasting, exercise, and calorie restriction, SIRT1 deacetylates and modulates activities of many transcriptional regulators, including PGC-1α, SREBP-1, FXR, FOXOs, NF-κB, and PPARγ (Kemper et al., 2009; 2013; Kitamura et al., 2005; Ponugoti et al., 2010; Rodgers et al., 2005; Qiang et al., 2012; Walker et al., 2010; Yoshizaki et al., 2009), which results in beneficial metabolic outcomes. Expression and activity of SIRT1 are dynamically regulated in response to different energy/nutrient status under physiological conditions, but they are constitutively depressed in obesity and the aged (Canto et al., 2012; Choi et al., 2013; Houtkooper et al., 2012; Lee et al., 2010). In this regard, understanding of how SIRT1 levels and activities are regulated is important for the development of therapeutic agents for treating age-related diseases involving SIRT1 dysfunction, such as obesity, diabetes, cardiovascular diseases, and neurodegenerative disease.
MicroRNAs (miRs) are small (19–23 nt) non-coding RNAs that act as powerful cellular regulatory molecules (Hobert, 2008; Neilson and Sharp, 2008). MiRs have been intensively studied because of their crucial functions in diverse biological pathways, including development, differentiation, cell proliferation, and metabolism (Baroukh et al., 2007; Esau et al., 2006; Lee at al., 2010; Lovis et al., 2008; Najafi-Shoushtari et al., 2010; Rayner et al., 2010). miRs are transcribed as precursor, often polycistronic, RNAs from intragenic and intergenic positions, and mature miRs are generated by a series of enzymatic process-sing steps in the nucleus and cytoplasm (Hobert, 2008; Neilson and Sharp., 2008; Yang and Lai., 2011). Mature miRs bind to the 3′ untranslated regions (UTRs) of target mRNAs and inhibit protein translation and/or mRNA stability (Lewis et al., 2003). Emerging evidence indicates that miRs suppress expression and/or deacetylase activity of SIRT1 by directly targeting SIRT1 and NAMPT, a key NAD+ biosynthetic enzyme (Choi et al., 2013; Lee et al., 2010; Yamakuchi et al., 2008; Zhang et al., 2013). Remarkably, miRs are aberrantly expressed in metabolic disease, cancer, and other human diseases (Lee and Kemper, 2010; Rottiers et al., 2011; Zhang et al., 2013), revealing the potential of such miRs as therapeutic targets for treating these diseases associated with SIRT1 dysfunction.
In this review, we will discuss how the key metabolic regulator SIRT1 and other sirutin family proteins are post-transcriptionally regulated by miRs. We will focus on miR-34a, which is highly elevated in obesity and inhibits SIRT1 expression and also the deacetylase activity of SIRT1 and possibly other sirtuins by reducing cellular NAD+ levels (Choi et al., 2013; Lee and Kemper., 2010; Lee et al., 2010; Zhang et al., 2013). We will also discuss the potential and advantages of downregulating sirtuin-inhibiting miRs for treating human disease, focusing on obesity-related metabolic diseases.
REGULATION OF SIRT1 EXPRESSION BY MIRS
miR-34a regulation of SIRT1 expression
Expression of SIRT1 is controlled at multiple levels by transcriptional, post-transcriptional, and post-translational mechanisms under physiological and pathological conditions. In response to fluctuating nutrient/energy levels during fasting/feeding cycles under physiological conditions, expression of SIRT1 is dynamically controlled to maintain energy homeostasis and metabolic adaptation (Finkel et al., 2009; Guarente, 2006; 2011; Haigis and Sinclair, 2010; Houtkooper et al., 2012; Imai et al., 2000). In contrast, SIRT1 expression levels are constitutively depress-sed in pathological conditions like obesity and diabetes and in aged animals (Guarente, 2011; Lee et al., 2010; Rodgers and Puigserver, 2007; Yamamoto et al., 2007).
Emerging evidence indicates that miRs are important regulators of SIRT1 expression (Ito et al., 2010; Lee et al., 2010; Lovis et al., 2008; Ortega et al., 2010; Zovoilis et al., 2011). miRs that directly target SIRT1 are summarized in Table 1. Our group and others showed that miR-34a directly binds to the 3′ untranslated region (UTR) of SIRT1 mRNA and reduces its expression (Lee et al., 2010; Yamakuchi et al., 2008). We further showed that hepatic miR-34a levels are highly elevated in high fat diet-induced obese mice and leptin-deficient genetic obese mice (Lee et al., 2010). Interestingly, miR-34a was the miR most highly aberrantly elevated in metabolic disease-prone mice lacking the nuclear receptor FXR (Lee et al., 2010). Consistent with our initial findings, miR-34a was indeed identified as the most highly elevated hepatic miR in both dietary and genetic obese mice based on miR microarray analysis (Trajkovski et al., 2011). Remarkably, hepatic miR-34a levels are also dramatically elevated in obesity-induced liver steatosis patients (Cheung et al., 2008).
Table 1.
miRNAs that target SIRT1
| MicroRNA | Tissue | Function (Reference) |
|---|---|---|
| miR-34a | Liver, Pancreas Adipose Brain Vascular endothelial cell |
Lipid metabolism, promote fatty liver (Lee et al., 2010) |
| Insulin seceretion, beta cell apoptosis (Lovis et al., 2008) | ||
| Adipocyte differentiation (Ortega et al., 2010) | ||
| Neurodegenerative age-associated disease (Zovoilis et al., 2011) | ||
| Endothelial cell senescence (Ito et al., 2010) | ||
| miR-181a | Liver | Hepatic insulin signaling, Glucose homeostasis (Zhou et al., 2012) |
| miR-9 | Pancreas | Insulin secretion (Ramachandran et al., 2011) |
| miR-146 | Beta cell apoptosis (Lovis et al., 2008) | |
| miR-143 | Adipose tissue | Adipocyte differentiation, triglyceride accumulation (Pramanik et al., 2011; Xie et al., 2009) |
| miR-132 | ||
| Induction of inflammatory cytokine (Strum et al., 2009) | ||
| miR-34c | Brain | Memory impairment (Khanna et al., 2011; Zovoilis et al., 2011) |
| miR-217 | Vascular endothelial cell | Endothelial cell senescence (Menghini et al., 2009) |
Our group recently showed that downregulation of miR-34a by in vivo treatment with a miR-34a antisense oligonucleotide restored SIRT1 levels in fatty livers of diet-induced obese mice, resulting in beneficial transcriptional and metabolic responses, including decreased liver fat and increased glucose tolerance and insulin sensitivity (Choi et al., 2013; Fu et al., 2012). In line with the role of miR-34a in the inhibition of SIRT1 in age-related metabolic diseases, recent studies have shown that expression of miR-34a is increased during aging in rat liver (Li et al., 2011), miR-34 levels are increased with aging in drosophila and increased neurodegenerative disorders (Liu et al., 2012), and that miR-34a levels are also elavated in aged heart and down-regulation of miR-34a reduced age-related cardiomyocyte cell death (Boon et al., 2013).
Regulation of SIRT1 expression by other miRs
SIRT1 is also inhibited by other miRs in peripheral metabolic tissues and brain (Table 1). miR-181a inhibits expression of SIRT1 by directly binding to the 3′ UTR of SIRT1 mRNA and miR-181a levels are highly elevated in insulin-resistant hepatocytes and also in the serum of diabetes patients (Zhou et al., 2012). Indeed, overexpression of miR-181a attenuated hepatic insulin signaling and conversely, the downregulation of miR-181a improved glucose homeostasis in diet-induced obese mice (Zhou et al., 2012). miR-9 and miR-146, as well as miR-34a, were also shown to directly target SIRT1 in pancreatic cells, which resulted in attenuated insulin secretion as a result of decreased exocytosis and in β-cell apoptosis (Lovis et al., 2008; Ramachandran, 2011). miR-143 was shown to inhibit expression of SIRT1 in adipose tissue by directly targeting its 3-UTR, resulting in stimulation of adipogenesis and decreased glucose uptake and glucose intolerance (Pramanik et al., 2011; Xie et al., 2009). Further, miR-132 directly targets SIRT1 and increases the acetylation levels of a SIRT1 target gene, the inflammatory gene activator, NF-κB, and the production of the chemokines, IL-8 and MCP-1 (Strum et al., 2009).
Expression levels of several miRs, including miR-34a/c, miR-217, and miR-22, that directly target SIRT1 increase during aging and accelerate cellular senescence in liver, heart, and neurons, which is associated with reduced SIRT1 levels (Liu et al., 2012; Zovoilis et al., 2011). Notably, miR-34c is highly elevated in brains of mice that are models of Alzheimer disease and also human patients (Zovoilis et al., 2011). miR-217 and miR-34a are inversely correlated with SIRT1 expression and significantly upregulated in human umbilical vein endothelial cells in old compared to young individuals (Ito et al., 2010; Menghini et al., 2009). miR-217 was also shown to be important in senescence for the development of atherosclerosis by inhibiting SIRT1, reducing nitric oxide availability, and deacetylating FoxO1 (Menghini et al., 2009).
Regulation of expression of sirtuin members by miRs
The mammalian sirtuin family is comprised of seven members (SIRT1–7). All are NAD+-dependent deacetylases, SIRT4 and SIRT6 have additional ADP-ribosyl transferase activities (Nakagawa et al., 2009), and SIRT5 catalyzes desuccininylation and demalonylation in mitochondria (Du et al., 2011). Sirtuin proteins have distinct subcellular localizations, target proteins, and tissue distributions (Table 2). SIRT1, SIRT2, SIRT6, and SIRT7 are nuclear proteins and SIRT1 shuttles between the nucleus and cytoplasm. SIRT3, SIRT4, and SIRT5 are mitochondrial proteins although SIRT3 was also shown to deacetylate histones in the nucleus (Gurd et al., 2012; Hout-kooper et al., 2012; Iwahara et al., 2012; Pirinen et al., 2012). Regarding cellular functions, SIRT1 has been most intensively studied and as noted above has beneficial impacts on numerous diseases. SIRT3 was shown to repress reactive oxygen species in mitochondria and interestingly, genetic polymorphism in the SIRT3 promoter is associated with extreme longevity of life in an Italian population (Bellizzi et al., 2005; Guarente, 2011; Kong et al., 2010; Rose et al., 2003). SIRT6 plays an important role in the epigenomic regulation of metabolic path-ways and possible the aging process by deacetylating histone H3K9 and H3K56, and SIRT6-null mice show severe metabolic defects early in life and develop other age-related metabolic abnormalities (Mostoslavsky et al., 2006). Functions of SIRT7 are not clear, although it may have a role in preventing cardiac hypertrophy through p53 deacetylation (Vakhrusheva et al., 2008).
Table 2.
Sirtuins regulated by miRNAs
| Sirtuin | Localization | Activity | Target | Function | miRNAs |
|---|---|---|---|---|---|
| SIRT1 | Nucleus, Cytosol | Deacetylation | PGC-1α, FOXO, p53, HIF1a, SREBP-1c, CREB, NF-κb, FXR, LXR, and more | Metabolism, Inflammation, Cell cycle/apoptosis, Stress response | miR-9, miR-22, miR-34a, miR-34c, miR-132, miR-143, miR-146, miR-181, miR-217 and more |
| SIRT2 | Cytosol, Nucleus | Deacetylation | PEPCK, FOXO1, PAR3, Tubulin | Cell cycle Tumorigenesis | Not known |
| SIRT3 | Mitochondria, Nucleus | Deacetylation | LCAD, HMGCS2, GDH, IDH2 and more | Metabolism | Not known |
| SIRT4 | Mitochondria | ADP-ribosylation | GDH | Insulin secretion, Fatty acid oxidation | Not known |
| SIRT5 | Mitochondria | Deacetylation,Demalonylation, Desuccinylation | CPS1 | Urea cycle | Not known |
| SIRT6 | Nucleus | Deacetylation, ADP-ribosylation | H3K9, H3K56, SIRT6 | DNA repair, Metabolism | miR-33a/33b, miR-766 |
| SIRT7 | Nucleus | Not Known | Not Known | rDNA transcription | miR-125a, miR-125b |
While regulation of SIRT1 by miRs is well known, miR regulation of other sirutins has just begun to be understood. Only three sirtuins, SIRT1, SIRT6 and SIRT7, are known to be direct targets of miR regulation (Table 2). Inhibition of SIRT6 expression by miR-33 resulted in increased histone acetylation at lipid metabolic target genes and derepression of SREBP-dependent lipogenesis (Davalos et al., 2011). Notably, miR-33a and miR-33b, transcribed from the introns of SREBPs, together with SREBPs have crucial roles in the regulation of cholesterol and lipid metabolism (Davalos et al., 2011; Najafi-Shoushtari et al., 2010; Rayner et al., 2010). A recent study showed that there is an inverse correlation between expression of SIRT6 and miR-766 and that miR-766 inhibits expression of SIRT6 in dermal fibroblasts from different aged groups while in turn, SIRT6 inhibits transcription of miR-766, suggesting a feedback regulatory loop in reprogramming of aging cells (Sharma et al., 2013). Levels of SIRT7 are high in metabolically active tissues, such as liver and spleen, but very low in non-proliferating tissues, including heart and brain (Barber et al., 2012; Ford et al., 2006; Yamamoto et al., 2007). Notably, expression of SIRT7 is highly elevated in hepatocellular carcinoma patients. In line with these findings suggesting that SIRT7 increases cellular proliferation, a recent study reported that miR-125a and miR-125b directly target SIRT7, reducing SIRT7 levels, which results in the inhibition of cyclin D1 expression and induction of G1 cell cycle arrest (Kim et al., 2013).
REGULATION OF NAD+-DEPENDENT SIRT1 ACTIVITY BY MIRS
Regulation of cellular NAD+ levels
The NAD+/NADH redox state reflects cellular energy levels. SIRT1 and other sirutin proteins are NAD+-dependent deacetylases and their enzymatic activities are increased in response to elevated NAD+ levels under energy-deprived conditions (Imai et al., 2000). Since NAD+ acts as an essential cofactor for sirtuins and also for other NAD+-consuming enzymes, we will briefly summarize how the production and consumption of NAD+ modulates its intracellular content.
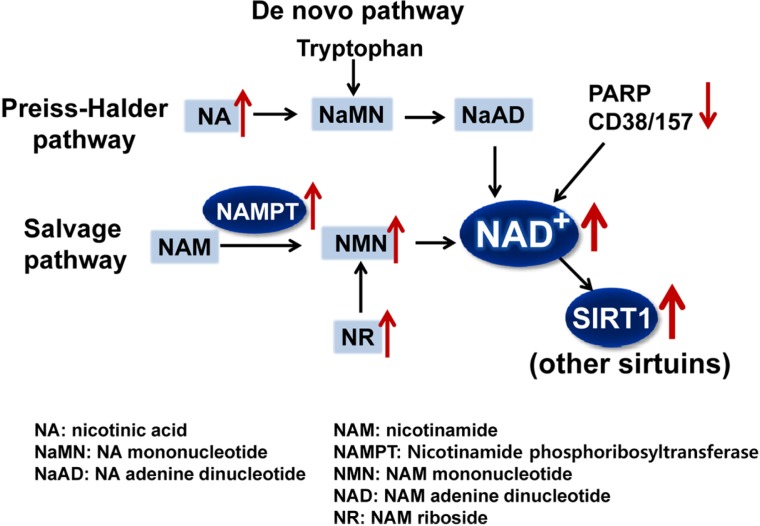
Cellular NAD+ levels are increased via three pathways: a de novo biosynthetic pathway from tryptophan, the Preiss-Halder pathway from nicotinic acid, and a salvage pathway from nicotinamide (Fig. 1). The salvage pathway is the main mechanism for increasing cellular NAD+ levels (Belenky et al., 2007), and nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme in this pathway (Houtkooper et al., 2010). Recent studies have demonstrated the therapeutic potential of increasing the bioavailability of NAD+ for treating obesity-related metabolic disorders. Nicotinamide mononucleotide (NMN), a key NAD+ intermediate, ameliorates glucose intolerance by restoring NAD+ levels in obesity-induced diabetic mice, and nicotinamide riboside (NR) supplementation protects against diet-induced metabolic abnormalities by increasing NAD+ levels and thus, activating SIRT1 and SIRT3 (Canto et al., 2012). Conversely, cellular NAD+ levels are reduced by NAD+-consumers, such as, poly (ADP-ribose) polymerase (PARP) and cADP ribose synthase 38 (CD38) (Fig. 1). PARPs, upon activation by DNA damage and oxidative stress, rapidly consume NAD+, resulting in decreased SIRT1 activity (Bai et al., 2011; Pillai et al., 2005). Consistent with these results, deletion of CD38 significantly activates SIRT1 and results in functional outcomes similar to those of SIRT1 activation, including decreased acetylation levels of SIRT1 target proteins and protection against diet-induced obesity (Aksoy et al., 2006). These findings suggest that inhibition of the NAD+ consumers, PARPs or CD38, or metabolic supplementation that increases cellular NAD+ levels have beneficial metabolic effects by increasing the deacetylase activities of SIRT1 and other sirtuins.
Fig. 1.
Therapeutic approaches to increase cellular NAD+ levels. The three major pathways for the biosynthesis of NAD+ are shown. Approaches to increase NAD+ levels include administering the metabolites, NA, NR, or NMN to increase their levels or increasing NAMPT activity (up arrowheads). In addition, down-regulation (down arrowhead) of the NAD+ consumers, PARP and CD38/157, will result in increased cellular NAD+ levels. The increased NAD+ levels will increase the levels and activity of NAD+-dependent SIRT1 and other sirtuins with beneficial metabolic outcomes.
MiR regulation of NAD+ levels by directly targeting NAMPT
NAD+ levels and SIRT1 activity are decreased in obesity and in the aged (Choi et al, 2013; Finkel et al., 2009; Haigis and Sinclair, 2010; Houtkooper et al., 2012; Lee et al., 2010; Yoshino et al., 2011), but the underlying mechanisms are unclear. Recent studies have indicated that miRs, highly elevated in obesity and the aged (Boon et al., 2013; Cheung et al., 2008; Khanna et al 2011; Lee et al., 2010; Liu et al., 2012), reduce NAD+ levels and SIRT1 activity by directly targeting NAMPT. Our group recently discovered a surprising functional link between decreased NAD+ levels and elevated miR-34a in obesity (Choi et al., 2013). As noted above, miR-34a is the most highly elevated hepatic miR in both diet-induced and genetic obese mice and also substantially elevated in liver steatosis patients (Carmelli et al., 2011; Cheung et al., 2008; Lee et al., 2010; Li et al., 2009; Trajkovski et al., 2011). miR-34a inhibits NAMPT expression by directly binding to the 3′UTRs of NAMPT mRNA.
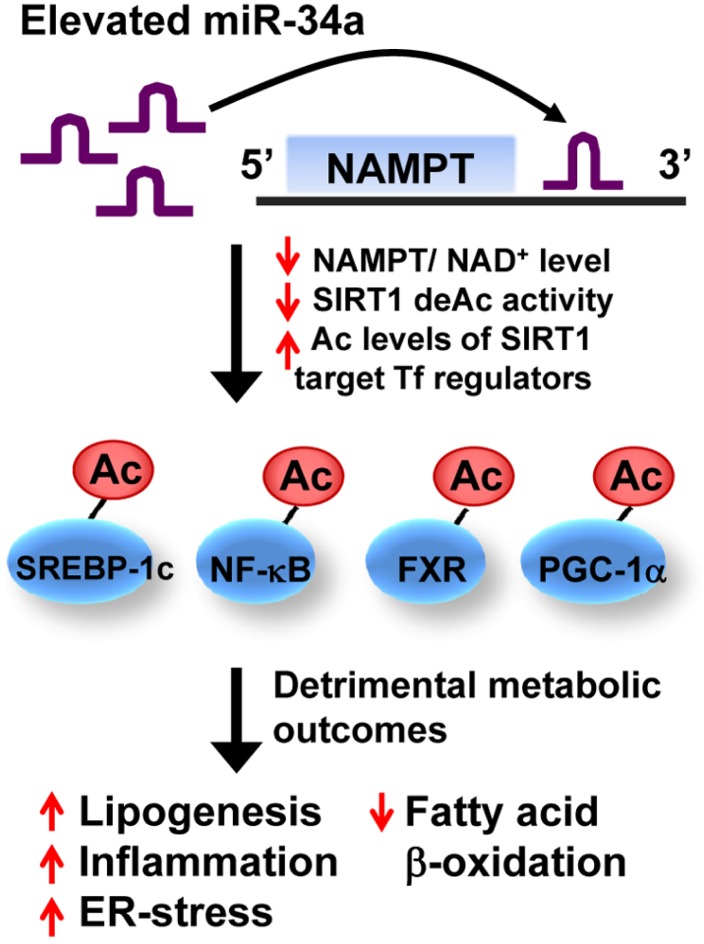
Adenoviral-mediated hepatic overexpression of miR-34a in mice in vivo reduced NAMPT/NAD+ levels, and consequently SIRT1 deacetylase activity, and increased acetylation of the SIRT1 target transcriptional regulators, a key transcriptional coactivator for mitochondrial function and fat oxidation PGC-1α, the bile acid nuclear receptor showing beneficial metabolic outcomes FXR, a key lipogenic transcriptional activator SREBP-1c, and a key inflammatory gene activator, NF-κB (Choi et al., 2013; Kemper et al., 2013). Increased protein acetylation levels increase transcriptional activities of SREBP-1c and NF-κB, whereas decrease those of PGC-1α and FXR (Kemper et al., 2009; 2013; Ponugoti et al., 2010; Rodgers et al., 2005; Walker et al., 2010; Yoshizaki et al., 2009), resulting in obesity-mimetic detrimental transcriptional and metabolic outcomes (Fig. 2). Remarkably, in vivo downregulation of elevated miR-34a in diet-induced obese mice restored hepatic NAD+ levels and SIRT1 deacetylase activity, ameliorated liver steatosis, and improved insulin sensitivity (Choi et al., 2013). These recent findings collectively indicate that elevated miR-34a in obesity inhibits deacetylase activity of SIRT1 as well as its expression.
Fig. 2.
Effects of miR-34a on NAMPT/NAD+ levels, SIRT1 activity, and metabolic outcomes. Adenoviral-mediated hepatic overexpression of miR-34a decreases expression of NAMPT/NAD+ levels by directly binding to the 3′UTR of Nampt transcript and consequently, reduces NAD+-dependent SIRT1 deacetylase activities. Decreased SIRT1 activity increases acetylation levels of SIRT1-target transcritpional (Tf) regulators, inclduing SREBP-1c, NF-κB, FXR, and PGC-1α, which results in detrimental transcirptional and mebaolic outcomes that promote hepatic lipogenesis, inflammation, and ER-stress, and reduced fatty acid β-oxidation.
In addition to miR-34a, miR-26b was also shown to decrease NAD+ levels by directly targeting the Nampt 3′ UTR. Notably, miR-26b levels were decreased in cancer tissues relative to adjacent normal tissues in 18 colorectal cancer patients. Over-expression of miR-26b indeed strongly inhibits survival and invasion of LoVo colon cancer-derived cells and these effects were partially abrogated by the addition of NAD+, suggesting that this miR functions as a tumor suppressor (Zhang et al., 2013). Intriguingly, some PARP members that also utilize cellular NAD+ form a complex with miRNA-binding Argonaute family members (Ago-1–4) under stressful conditions to relieve miR-mediated gene repression (Leung et al., 2011).
A SIRT1/NAMPT regulatory loop in physiology and disease
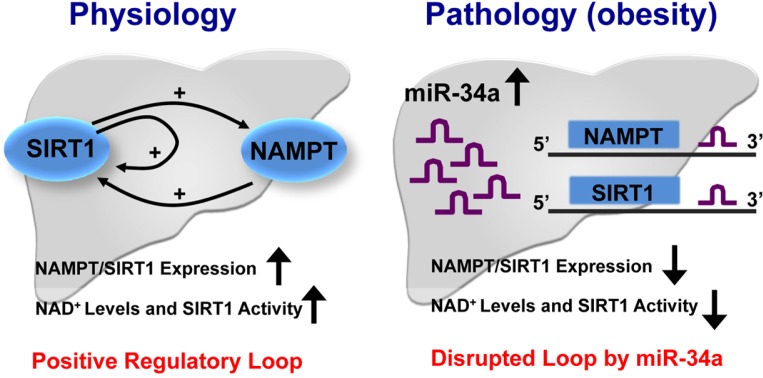
Previous studies have suggested that both SIRT1 and the key NAD+ biosynthetic enzyme NAMPT work together in the regulation of metabolic pathways, including circadian regulation of metabolism in liver and insulin secretion by β-cells (Imai and Kiess, 2009; Nakahata et al., 2009; Ramsey et al., 2009). In line with these studies, we recently reported a positive regulatory loop between SIRT1 and NAMPT that was disrupted in obesity by elevated miR-34a (Choi et al., 2013). In response to fasting under physiological conditions, SIRT1 occupancy is substantially elevated at the Nampt and Sirt1 genes during fasting, which is associated with increased levels of the gene-activating histone mark, H3K4 methylation, and decreased levels of the gene-repressing mark, H3K9 methylation, leading to induction of these genes. The resulting induction of NAMPT, in turn, raises cellular NAD+ levels, which results in increased SIRT1 activity (Fig. 3, left). Since SIRT1 is known as a gene silencer by deacetylating histones at target genes, these recent findings that SIRT1 acts as a positive regulator of Nampt and Sirt1 genes are intriguing. It will be important to see how SIRT1 functions as a positive gene regulator.
Fig. 3.
Model of the SIRT1/NAMPT regulatory loop and its inhibition by miR-34a in disease. In the fed state, NAD+ levels and SIRT1 activity are low. During energy-deprived conditions like fasting, NAD+ levels are high resulting in increased SIRT1 activity. SIRT1 is recruited to the Nampt and Sirt1 genes, resulting in epigenomic activation of these genes. Induced NAMPT, in turn, increases cellular NAD+ levels and SIRT1 deacetylase activity, thus completing a positive regulatory loop. The increased SIRT1 activity results in beneficial outcomes, such as increased lipid oxidation and reduced lipogenesis and inflammation (left panel). In fatty liver in obesity, however, miR-34a is elevated in both the fasting and fed states and inhibits both NAMPT and SIRT1 expression by binding to the 3′ UTRs of these mRNAs, which effectively disrupts the positive regulatory loop. The decreased SIRT1 expression and activity results in detrimental metabolic outcomes (right panel).
In sharp contrast, however, in diet-induced obese mice, these fasting-mediated effects on SIRT1 occupancy and histone modifications are absent. Abnormal cellular signaling and many other factors may contribute to the dysregulated SIRT1/NAMPT loop in obesity, but elevated miR-34a alone, which inhibits both NAMPT and SIRT1 expression, should effectively disrupt this positive regulatory loop. This disruption by miR-34a in obesity contributes to reduced NAD+ levels and SIRT1 deacetylase activity, resulting in detrimental outcomes of decreased lipid oxidation and increased lipogenesis and inflammation (Fig. 3, right). These findings identify a novel function for miR-34a in reducing NAMPT/NAD+ levels and SIRT1 activity, revealing the potential therapeutic value of targeting miR-34a to increase SIRT1 activity and possibly other NAD+-dependent sirtuin deacetylases.
Therapeutic potential of targeting sirtuin-inhibiting miRs
miRs were first identified in C. elegans about 20 years ago and since then, miRs have received increasing and substantial interest because of their potential as powerful therapeutic targets. Gain- or loss-of-functional approaches have been used to restore aberrant expression of miRs toward normal utilizing downregulation or miR-mimetics. The levels of nearly all of the known sirtuin-inhibiting miRs, including miR-34a, are highly elevated in metabolic and neurodegenerative disease (Boon et al., 2013; Carmelli et al., 2011; Cheung et al., 2008; Lee et al., 2010; Li et al., 2009; Liu et al., 2012; Trajkovski et al., 2011; Zovoilis et al., 2011). To downregulate miRs in vivo, several antisense approaches have been used: antagomiRs, which are conjugated to cholesterol to facilitate cellular uptake; locked nucleic acid (LNA) phosphorothioate chemistry; and chemical modification of the oligonucleotide at the 2′-sugar and phosphate backbone moiety with MOE (2′-O-methoxyethylphosphorothioate). All of these modifications are designed to facilitate cellular uptake of miRs and to protect them from nuclease digestion.
miRs may target multiple genes involved in the same functional pathway (Van Rooji, 2011), as shown by the regulation of SIRT1 by miR-34a which directly targets SIRT1 and NAMPT (Choi et al., 2013). In addition, miR-34a also targets β-Klotho which is a coreceptor for the recently emerging improtant metabolic hormones, FGF19 (Fu et al., 2012) and FGF21. In particular, FGF21 mediates fasting responses and regulates energy metabolism in part by activating an AMPK/SIRT1 regulatory axis (Chau et al., 2010), although whether miR-34a attenuates FGF21 signaling by directly targeting bKL in adipsoe tissues has not been shown. Therefore, downregulation of elevated miR-34a in obesity should have beneficial effects by restoring functions of multiple targets in metabolic tissues, which may provide a therapeutic advantage compared to the classical therapeutic approaches that target a single protein. Indeed, downregulation of miR-34a by treatment with the MOE-modified antisense-miR34a in dietary obese mice restored SIRT1 activity and expression levels, resulting in dramatic improvement in metabolic outcomes (Choi et al., 2013). However, since individual miRs regulate multiple genes, targeting miRs could also result in detrimental non-specific side effects. Therefore, it will be important to understand the global functions and tissue-specific expression of miRs in vivo in order to understand the possible consequences of their inhibition or increased expression.
Another important issue is that miRs that are aberrantly elevated in obesity may also function as tumor suppressors. One such an example is miR-34a. miR-34a expression is regulated by the tumor suppressor p53 and induces apoptosis, cell cycle arrest, and senescence when miR-34a is overexpressed in cancer cells (Chang et al., 2007). Indeed, recent in vivo studies using gain- and loss-of-function approaches have shown that miR-34a suppresses tumor growth and metastasis in liver cancer and prostate cancer (He et al., 2007; Kota et al., 2009; Liu et al., 2011). Therefore, targeting miRs for treating obesity-related metabolic diseases will require careful modulation of miRs levels to optimally repress miRs and correct metabolic abnormalities without causing carcinogenic side effects. In this regard, the treatment time and dose of the antisense oligonucleotide used to downregulate miRs will be critical.
CONCLUSION
Aging is the single most important risk factor for many human diseases. While there has been a continuous debate on the role of SIRT1 as an anti-aging protein, it is more certain that SIRT1 prevents the onset of many age-related diseases and slows down disease progression. Therefore, identification of molecular strategies to increase and restore SIRT1 activity and expression levels, which are often downregulated in age-related metabolic disease, will be important for the development of therapeutic agents. An attractive strategy is targeting miRs that both directly inhibit SIRT1 expression and inhibit SIRT1 activity by decreasing cellular NAD+ levels.
Acknowledgments
The published work in the laboratory of authors at the Univrsity of Illinois at Urbana-Champaign, USA, was supported by grants from NIH DK95842 and DK62777 to JK. SC is currently at the Ajou University, Korea, and supported by the National Research Foundation, Korea, NRF-2013-013774. We thank Byron Kemper for reading the manuscript.
REFERENCES
- Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber MF, Michishita-Kioi E, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL, Chen K, et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012;487:114–118. doi: 10.1038/nature11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroukh N, Ravier MA, Loder MK, Hill EV, Bounacer A, Scharfmann R, Rutter GA, Van Obberghen E. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic β-cell lines. J Biol Chem. 2007;282:19575–19588. doi: 10.1074/jbc.M611841200. [DOI] [PubMed] [Google Scholar]
- Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85:258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide ribosideenhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6:e23937. doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc. Natl. Acad. Sci. USA. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ. Nonalcoholic steatohepatitis is associated with altered hepatic microRNA expression. Hepatology. 2008;48:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, Kang Y, Li X, Kemper B, Kemper JK. Elevated micro-RNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013 doi: 10.1111/acel.12135. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávalos A, Goedeke L, Smibert P, Ramírez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, et al. miR-122 regulation of lipid metabolism revealed by in vivo anti-sense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20:1075–1080. doi: 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T, Choi SE, Kim DH, Seok S, Suino-Powell KM, Xu HE, Kemper JK. Aberrantly elevated microRNA-34a in obesity attenuates hepatic responses to FGF19 by targeting a membrane coreceptor beta-Klotho. Proc. Natl. Acad. Sci. USA. 2012;109:16137–16142. doi: 10.1073/pnas.1205951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:81–90. doi: 10.1101/sqb.2011.76.010629. [DOI] [PubMed] [Google Scholar]
- Gurd BJ, Holloway GP, Yoshida Y, Bonen A. In mammalian muscle, SIRT3 is present in mitochondria and not in the nucleus; and SIRT3 is upregulated by chronic muscle contraction in an adenosine monophosphate-activated protein kinase-independent manner. Metabolism. 2012;61:733–741. doi: 10.1016/j.metabol.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al. A micro-RNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Cantó C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Kiess W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front Biosci. 2009;14:2983–95. doi: 10.2741/3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Bonasio R, Narendra V, Reinberg D. SIRT3 functions in the nucleus in the control of stress-related gene expression. Mol Cell Biol. 2012;32:5022–5034. doi: 10.1128/MCB.00822-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu S, Chiang CM, Veenstra TD. FXR acetylation is normally dynamically regulated by p300 and SIRT1. but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper JK, Choi SE, Kim DH. Sirtuin 1 deacetylase: a key regulator of hepatic lipid metabolism. Vitam Horm. 2013;91:385–404. doi: 10.1016/B978-0-12-407766-9.00016-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Muthusamy S, Liang R, Sarojini H, Wang E. Gain of survival signaling by downregulation of three key miRNAs in brain of calorie-restricted mice. Aging. 2011;3:223–236. doi: 10.18632/aging.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Noh JH, Jung KH, Eun JW, Bae HJ, Kim MG, Chang YG, Shen Q, Park WS, Lee JY, et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology. 2013;57:1055–1067. doi: 10.1002/hep.26101. [DOI] [PubMed] [Google Scholar]
- Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. Sirtuin 3, a new target of PGC-1α, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kemper JK. Controlling SIRT1 expression by microRNAs in health and metabolic disease. Aging. 2010;2:527–534. doi: 10.18632/aging.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Padhye A, Sharma A, Song G, Miao J, Mo YY, Wang L, Kemper JK. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem. 2010;285:12604–126011. doi: 10.1074/jbc.M109.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. Poly(ADP-Ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol. Cell. 2011;42:489–499. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Li S, Chen X, Zhang H, Liang X, Xiang Y, Yu C, Zen K, Li Y, Zhang CY. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J Lipid Res. 2009;50:1756–1765. doi: 10.1194/jlr.M800509-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Muthusamy S, Liang R, Sarojini H, Wang E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mech Ageing Dev. 2011;132:75–85. doi: 10.1016/j.mad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Landreh M, Cao K, Abe M, Hendriks GJ, Kennerdell JR, Zhu Y, Wang LS, Bonini NM. The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature. 2012;482:519–523. doi: 10.1038/nature10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovis P, Roggli E, Laybutt DR, Gattesco S, Yang JY, Widmann C, Abderrahmani A, Regazzi R. Alterations in microRNA expression contribute tofatty acid-induced pancreatic beta-cell dysfunction. Diabetes. 2008;57:2728–2736. doi: 10.2337/db07-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson JR, Sharp PA. Small RNA regulators of gene expression. Cell. 2008;134:899–902. doi: 10.1016/j.cell.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Ortega FJ, Moreno-Navarrete JM, Pardo G, Sabater M, Hummel M, Ferrer A, Rodriguez-Hermosa JI, Ruiz B, Ricart W, Peral B, et al. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One. 2010;5:e9022. doi: 10.1371/journal.pone.0009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- Pirinen E, Lo, Sasso G, Auwerx J. Mitochondrial sirtuins and metabolic homeostasis. Best Pract Res Clin Endocrinol Metab. 2012;26:759–770. doi: 10.1016/j.beem.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik D, Campbell NR, Karikari C, Chivukula R, Kent OA, Mendell JT, Maitra A. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther. 2011;10:1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran D, Roy U, Garg S, Ghosh S, Pathak S, Kolthur-Seetharam U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic β-islets. FEBS J. 2011;278:1167–1174. doi: 10.1111/j.1742-4658.2011.08042.x. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. USA. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rottiers V, Najafi-Shoushtari SH, Kristo F, Gurumurthy S, Zhong L, Li Y, Cohen DE, Gerszten RE, Bardeesy N, Mostoslavsky R, et al. MicroRNAs in metabolism and metabolic diseases. Cold Spring Harb Symp Quant Biol. 2011;76:225–233. doi: 10.1101/sqb.2011.76.011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38:1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- Sharma A, Diecke S, Zhang WY, Lan F, He C, Mordwinkin NM, Chua KF, Wu JC. The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J Biol Chem. 2013;288:18439–18447. doi: 10.1074/jbc.M112.405928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum JC, Johnson JH, Ward J, Xie H, Field J, Hester A, Alford A, Waters KM. MicroRNA 132 regulates nutritional stressinduced chemokine production through repression of SirT1. Mol Endocrinol. 2009;23:1876–1884. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- Van Rooji E. The art of microRNA research. Circ Res. 2011;108:219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are down-regulated in obesity. Diabetes. 2009;58:1050–1057. doi: 10.2337/db08-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. USA. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21:1745–1755. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol. Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. sirT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Tong J, Huang G. Nicotinamide phosphoribosyl transferase (Nampt) is a target of MicroRNA-26b in colorectal cancer cells. PLoS One. 2013;8:e69963. doi: 10.1371/journal.pone.0069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Li C, Qi W, Zhang Y, Zhang F, Wu JX, Hu YN, Wu DM, Liu Y, Yan TT, et al. Downregulation of miR-181a upregulates sirtuin-1 (SIRT1) and improves hepatic insulin sensitivity. Diabetologia. 2012;55:2032–2043. doi: 10.1007/s00125-012-2539-8. [DOI] [PubMed] [Google Scholar]
- Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P, Farinelli L, Delalle I, Schmitt A, Falkai P, et al. microRNA-34c is a novel target to treat dementias. EMBO J. 2011;30:4299–4308. doi: 10.1038/emboj.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]