Abstract
Apurinic/apyrimidinic endonuclease1/redox factor-1 (APE1/ Ref-1) is a multifunctional protein involved in base excision DNA repair and transcriptional regulation of gene expression. APE1/Ref-1 is mainly localized in the nucleus, but cytoplasmic localization has also been reported. However, the functional role of cytoplasmic APE1/Ref-1 and its redox cysteine residue are still unknown. We investigated the role of cytoplasmic APE1/Ref-1 on tumor necrosis factor-α (TNF-α)-induced vascular cell adhesion molecule-1 (VCAM-1) expressions in endothelial cells. Endogenous APE1/Ref-1 was mainly observed in the nucleus, however, cytoplasmic APE1/Ref-1 was increased by TNF-α. Cytoplasmic APE1/ Ref-1 expression was not blunted by cycloheximide, a protein synthesis inhibitor, suggesting cytoplasmic translocation of APE1/Ref-1. Transfection of an N-terminus deletion mutant APE1/Ref-1(29-318) inhibited TNF-α-induced VCAM-1 expression, indicating an anti-inflammatory role for APE1/Ref-1 in the cytoplasm. In contrast, redox mutant of APE1/Ref-1 (C65A/C93A) transfection led to increased TNF-α-induced VCAM-1 expression. Our findings suggest cytoplasmic APE1/Ref-1 localization and redox cysteine residues of APE1/Ref-1 are associated with its anti-inflammatory activity in endothelial cells.
Keywords: APE1/Ref-1, endothelial cells, TNF-α, VCAM-1
INTRODUCTION
Apurinic/apyrimidinic endonuclease1/redox factor-1 (APE1/Ref-1) is a multifunctional protein involved in base excision DNA repair and transcriptional regulation of gene expression. The multifunctional nature of APE1/Ref-1 has been uncovered through extensive study of its cellular response to oxidative stress (Jeon and Irani, 2009). Redox and DNA repair functions of APE1/Ref-1 are completely independent (Xanthoudakis et al., 1994), and analysis of truncated APE1/Ref-1 proteins in vitro has revealed that redox and repair activities are encoded by distinct regions. Specifically, the N-terminal region contains the redox regulatory domain characterized by 2 critical cysteine residues, C65 and C93 (Xanthoudakis et al., 1992; 1994). Nuclear localization of APE1/Ref-1 is controlled by the first 20–35 amino acids at the N-terminal sequence (Jackson et al., 2005; Jeon et al., 2004). It also has been reported that nitrosation of APE1/Ref-1 leads to cytoplasmic localization in a chromosome region maintenance 1 (CRM1)-independent process (Qu et al., 2007). Thus, nuclear import and export systems may control subcellular distribution of APE1/Ref-1 (Qu et al., 2007).
APE1/Ref-1 suppresses oxidative stress through modulation of cytoplasmic Rac1-regulated generation of reactive oxygen species (Angkeow et al., 2002; Ozaki et al., 2002) and stimulates nitric oxide production by activating endothelial nitric oxide synthase (Jeon et al., 2004). In endothelial cells, APE1/Ref-1 mitigates TNF-α-induced monocyte adhesion and suppresses the expression of vascular cell adhesion molecules (Kim et al., 2006). Recently, it was reported that APE1/Ref-1 suppresses oxidized LDL-induced p66shc activation in endothelial cells by inhibiting PKCβII-mediated serine phosphorylation of p66shc (Lee et al., 2011). Therefore, a possible protective role of APE1/Ref-1 in vascular inflammatory disorders was proposed.
Although APE1/Ref-1 is mainly observed in the nucleus, cytoplasmic APE1/Ref-1 has been reported in response to various stimuli (Choi et al., 2013; Lee et al., 2011; Tell et al., 2005). However, the functional role of cytoplasmic APE1/Ref-1 has not been experimentally defined. To date, the specific domain of APE1/Ref-1 responsible for the coordinated control of vascular endothelial activations has not been elucidated. In this study, we have been used a mutant with a N-terminus deletion of 28 amino acids (29–318) and a double redox point mutant (C65A/ C93A) to investigate the functional role of cytoplasmic APE1/ Ref-1 to determine the critical domain for anti-inflammatory activity of APE1/Ref-1 in endothelial cells.
MATERIALS AND METHODS
Cells culture and reagents
Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (USA). Endothelial growth medium, fetal bovine serum and antibiotics were purchased from Gibco (USA). Human TNF-α, diphenyleneiodonium (DPI), leptomycin B (LMB) and cycloheximide (CHX) were purchased from Sigma (USA). Antibodies against VCAM-1, ICAM-1, PARP, α-tubulin and β-actin were obtained from Santa Cruz Biotechnology (USA).
Construction of APE1/Ref-1 plasmids and transfection
N-terminus deletion mutants (putative nuclear localization signals, NLS) of APE1/Ref-1 were generated by deleting 28 amino acids of the N-terminus (29–318) using recombinant DNA technology on the pEGFP-APE1/Ref-1 vector containing the full-length APE1/Ref-1 cDNA. Forward primers were added as initiation codons of ATG with KpnI sites, and all reverse primers were added as termination codons of TGA with BamHI sites at their 5′-ends. PCR reactions were performed using high fidelity DNA polymerase according to the manufacturer’s instruction (Intron, Korea). HA-APE1/Ref-1 (C65A/C93A) was generated by site-directed mutagenesis as previously described (Jeon et al., 2004). All deletions and mutations were validated by DNA sequencing. HUVECs were transfected with 0.5–2 μg of APE1/Ref-1 plasmid using Lipofectamine™ 2000 reagent according to manufacturer’s instruction, showed 20–30% transfection efficiency (Joo et al., 2009). After 18 h of TNF-α treatment, Western blot analysis was performed using prepared cell lysates.
Measurement of reactive oxygen species (ROS)
Intracellular ROS generation was measured by fluorometric examination with dichlorofluoresceindiacetate (H2DCFDA). H2DCFDA dye is cleaved by nonspecific esterases, which is oxidized by peroxide, yielding fluorescent 2′, 7′-dichlorofluo-rescein (DCF). The 1 × 104 cells were plated and incubated in a 96-well plate for 24 h. The cells were activated with 15 ng/ml TNF-α in the absence or presence DPI. Subsequently, the cells were stained with 5 μM H2DCFDA for 30 min at 37°C. Fluorescence was observed under fluorescent microscope (Carl Zeiss) and the intensity was measured using a Fluorskan (Thermo Scientific, USA) with a 485 nm excitation and a 530 nm emission filter set.
Western blotting
Cells were harvested on ice using lysis buffer containing 20 mM Tris-Cl, pH 7.5, 100 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM Na3VO3, 1 mM beta-glycerophosphate, 4 mM Na pyrophosphate, 5 mM NaF, 1% Triton X-100, and a protease inhibitor cocktail as previously described (Lee et al., 2012). The cell lysate was cleared by centrifugation at 12,000 × g for 15 min, and supernatant was used for immunoblotting. Proteins were resolved on 10% SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane. After blocking with 5% skim milk for 1 h at room temperature, blots were incubated overnight at 4°C with specific primary antibody (1:1000) and subsequently with horseradish peroxidase (HRP)-conjugated secondary antibody. The immunoreactive bands were visualized using an enhanced chemiluminescence method (Pierce Biotechnology, USA). As a protein loading control, each membrane was stripped and reacted with an anti-β-actin antibody to normalize for amount of protein. Nuclear and cytoplasmic extracts were prepared using nuclear and cytoplasmic extraction reagent kits according to instructions provided by the manufacturer (Pierce).
Immunocytochemistry for localization of APE1/Ref-1
For immunofluorescent staining, endothelial cells were grown on glass coverslips and treated with TNF-α. To observe the endogenous APE1/Ref-1, cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100, and blocked with 5% bovine serum albumin (BSA) for 1 h as previously described (Lee et al., 2011; 2012; 2013). Coverslips were then incubated for 1 h at 25°C in anti-APE1/Ref-1 (dilution 1:200) primary antibody in 1% BSA. Cells were washed and incubated in FITC-labeled secondary antibodies for 1 h. Mitotracker Red (Molecular probes) and DAPI were used for 15 min to stain mitochondria and nucleus, respectively. In some experiments, a subcellular localization of APE1/Ref-1 mutant protein was observed in cells transfected with pEGFP-APE1/Ref-1 plasmids (1 μg). Coverslips were mounted on microscope slides, and fluorescence signals were visualized with a confocal microscope (Carl Zeiss).
Statistical analysis
All data are expressed as the mean ± S.E.M. Statistical significance of differences in measured variables was determined using one-way ANOVA followed by Dunnett’s or Bonferroni’s test for multiple comparisons. Differences were considered significant at P < 0.05.
RESULTS
Ectopic overexpression of APE1/Ref-1 inhibited TNF-α- induced VCAM-1 expression
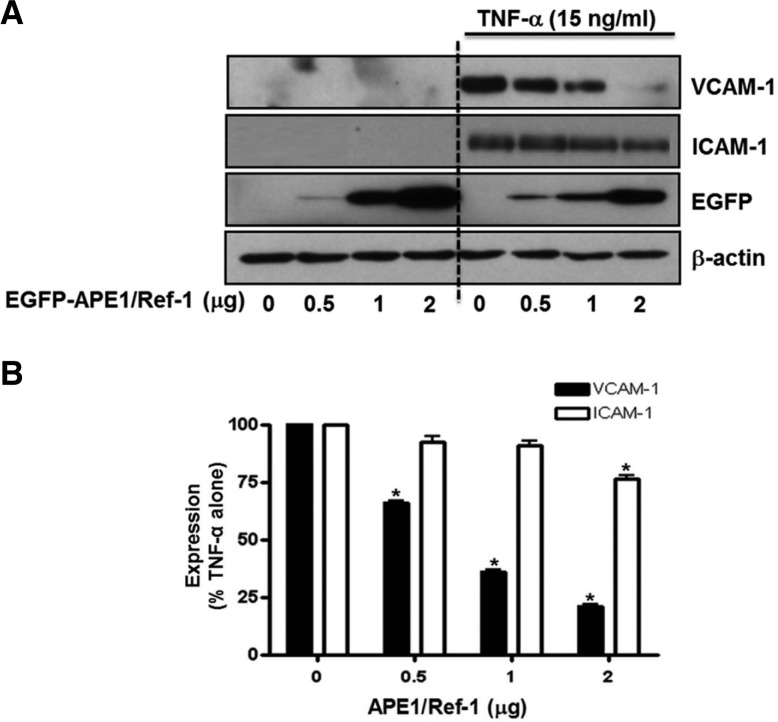
Firstly, we determined whether the expression of inflammatory cell adhesion molecules, VCAM-1 and ICAM, in response to TNF-α is regulated by APE1/Ref-1 level in human endothelial cells. To explore this possibility, cells were transfected with APE1/Ref-1 gene and determined changes in level of VCAM-1 and ICAM-1 expression. Twenty-four hours after APE1/Ref-1 plasmid transfection, Western blot analysis against adhesion molecules was performed using cell lysates. The expression level of adhesion molecules was not detected under basal conditions; however, TNF-α (15 ng/ml) treatment for 18 h resulted in markedly induced expression of VCAM-1 and ICAM-1 in HUVECs. Plasmid transfection with pEGFP-APE1/Ref-1 (0.5–2 μg) did not induce by itself in expression of adhesion molecules; however, it significantly inhibited TNF-α-induced VCAM-1 expression in a dose-dependent manner (Fig. 1). It was also observed the expression level of ICAM-1 was statistically significantly decreesed by 21%, compared to only TNF-α stimulated cell. These data indicate that the expression of TNF-α-induced cell adhesion molecules can be modulated by overexpression of APE1/ Ref-1 in endothelial cells.
Fig. 1.
APE1/Ref-1 overexpression inhibited TNF-α-induced VCAM-1 and ICAM-1 expression in endo-thelial cells. (A) Western blot analysis for APE1/Ref-1 VCAM-1, ICAM-1 was performed in EGFP-APE1/ Ref-1-transfected endothelial cells. Ectopic expression of APE1/Ref-1 using EGFP-APE1/Ref-1 plasmid inhibited TNF-α-stimulated VCAM-1 and ICAM-1 expression (B) Summarized data from Fig. 1A. Each bar shows the mean ± SE (n = 4). *p < 0.05 vs. non-transfected control cells by one-way ANOVA followed by Dunnett’s test.
Subcellular localization of APE1/Ref-1
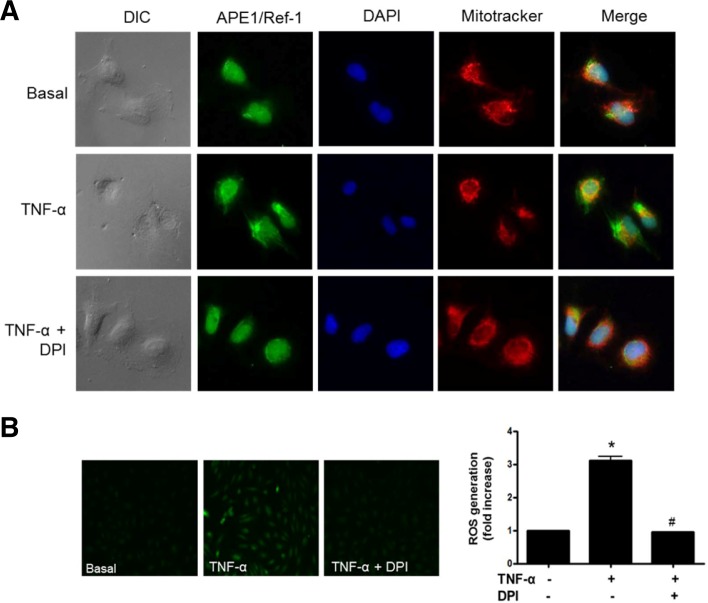
Next, we determined whether the subcellular localization of endogenous APE1/Ref-1 was affected by TNF-α, an inflammatory cytokine. Immunocytochemistry of endogenous APE1/Ref-1 was performed in cells permeabilized with Triton X-100. In basal cells, APE1/Ref-1 was mainly localized to the nucleus. In TNF-α-treated endothelial cells, immunofluorescent signal of APE1/Ref-1 was detected in cytoplasm as well as the nucleus, indicating cytoplasmic translocation and/or cytoplasmic expression.
ROS generation by TNF-α treatment in HUVECs was measured using DCFDA fluorescent dye. HUVECs treated with TNF-α for 6 h were brightly stained with DCF, suggesting generation of ROS as shown in Fig. 2B fluorescent image. We observed that the TNF-α-induced ROS generation were completely protected by 10 μM DPI pretreatment, a NADPH oxidase inhibitor. In accordance with block of TNF-α-stimulated ROS generation by DPI, cytoplasmic translocalization of APE1/Ref-1 was attenuated and the APE1/Ref-1 was mainly localized in the nucleus, similar to the basal condition (Fig. 2A). These results indicate that NADPH oxidase is involved in translocation of APE1/Ref-1 in endothelial cells.
Fig. 2.
Intracellular localization of endogenous APE1/Ref-1 in endothelial cells. (A) Endogenous APE1/Ref-1 is mainly localized in the nucleus. Tumor necrosis factor-α (TNF-α) treatment caused cytoplasmic translocation of APE1/Ref-1 in endothelial cells but not in cells pretreated with diphenyleneiodonium (DPI, 10 μM), an inhibitor of NADPH oxidase. Cells were grown on glass coverslips and treated with TNF-α (15 ng/ml) for 30 min in the absence or presence of DPI. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. Cells were immunostained with anti-APE1/Ref-1 (Green, Alexa 488), mitotracker red (red fluorescence) for mitochondrial staining, and 4′,6-diamidino-2-phenylindole (DAPI, blue fluorescence) for nuclear staining. Magnification, 400×. Similar results were observed in experiments run in triplicate. (B) ROS generation in TNF-α-stimulated HUVECs was observed. Representative fluorescent images show ROS levels in basal and HUVECs stimulated with TNF-α in the absence or presence of DPI. The fluorescent intensity of ROS was measured by fluorometer. Data are presented as means ± SEMs (n = 3). Significantly different compared with basal (*p < 0.05) and TNF-α-stimulated cells with DPI (#p < 0.05) by one-way analysis of variance followed by Dunnett’s test.
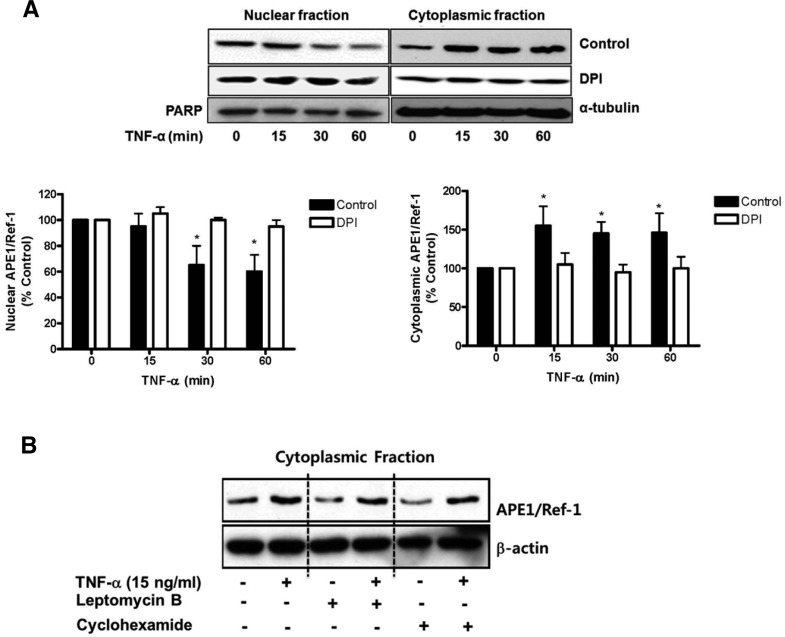
NADPH oxidase inhibition suppressed the cytoplasmic translocation of APE1/Ref-1
Since cytoplasmic expression of APE1/Ref-1 was detected by immunocytochemistry, we further investigated whether exposure to TNF-α induces cytoplasmic translocation of APE1/Ref-1 in endothelial cells. As shown in Fig. 3A, the nuclear APE1/Ref-1 was reduced by treatment of TNF-α within 15 min. In contrast, the cytoplasmic APE1/Ref-1 increased in a time-dependent manner. However, cytoplasmic translocation in response to TNF-α was blunted by the pretreatment of DPI, suggesting involvement of NADPH oxidase (Fig. 2). Subsequently, we investigated whether cytoplasmic APE1/Ref-1 was the result of de novo protein synthesis or nuclear export. The level of cytoplasmic APE1/Ref-1 was measured in cells treated with cycloheximide (CHX, 10 ng/ml for 1 h), a protein synthesis inhibitor, or leptomycin B (LMB, 10 ng/ml for 1 h), an inhibitor of the nuclear export factor CRM-1. As shown in Fig. 3B, neither CHX nor LMB pretreatment had an effect on the level of cytoplasmic APE1/Ref-1 in response to TNF-α-treatment. Collectively, these data indicate that increased cytoplasmic APE1/Ref-1 in response to TNF-α may be the result of subcellular translocation.
Fig. 3.
Tumor necrosis factor-α (TNF-α) induced cytoplasmic translocation of APE1/Ref-1 in endothelial cells. (A) TNF-α treatment caused rapid translocation of APE1/Ref-1 into cytosol in endothelial cells. Immunoblotting for APE1/Ref-1 using cytosolic and nuclear fractions was performed. Each fraction was prepared from cells following treatment of TNF-α (15 ng/ml) for the indicated time. PARP and α-tubulin were used as nuclear and cytoplasmic marker, respectively. Each bar shows the mean ± SE (n = 3). *p < 0.05 vs. compared with basal. (B) Effect of leptomycin B or cyclohexamide on TNF-α-induced cytoplasmic translocation in endothelial cells. Cells were pre-incubated with leptomycin B (LMB, 10 ng/ml for 1 h) or cyclohexamide (CHX, 10 ng/ml for 1 h) and then stimulated with TNF-α (15 ng/ml) for 30 min. Similar results were observed in experiments run in triplicate.
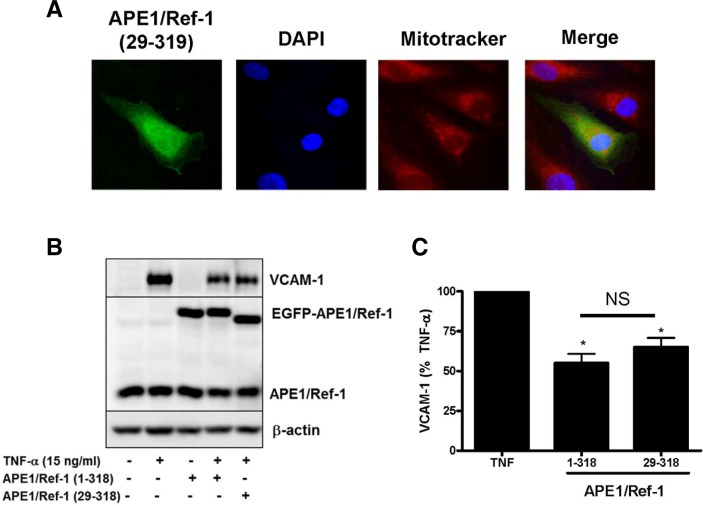
NLS-deleted APE1/Ref-1 inhibited TNF-α-induced VCAM-1 expression
To understand the functional role of cytoplasmic APE1/Ref-1 in endothelial activation, the NLS-deletion mutant of APE1/Ref-1 was generated by deleting 28 amino acids from the N-terminus to produce APE1/Ref-1(29–318). The effect of NLS-deleted APE1/Ref-1 mutant on TNF-α-induced VCAM-1 expression was determined. As observed by immunocytochemistry (Fig. 4A), the NLS-deletion mutant of APE1/Ref-1 (29–318) localized to the diffuse cytoplasm. The subcellular distribution of ectopically expressed EGFP-APE/Ref-1 or EGFP-APE1/Ref-1 (29–318) proteins was also confirmed by nuclear/ cytoplasmic fractionation. As shown in Fig. 4A, EGFP-APE/Ref-1 was mainly located in the nucleus, the EGFP-APE1/Ref-1 was dramatically translocated into the cytoplasm after TNF-α stimulation. In contrast, the EGFP-APE1/Ref-1(29–318) was consistently detected in cytoplasmic fraction, regardless of TNF-α treatment.
Fig. 4.
N-terminus deletion mutant APE1/Ref-1 (29–318) inhibited TNF-α-induced VCAM-1 expression in endothelial cells. (A) Immunocytochemistry for analysis of N-terminus deletion mutant EGFP-APE1/ Ref-1 (29-318) in endothelial cells. Cells were stained with mitotracker red (red fluorescence) for mitochondria and 4′,6-diami-dino-2-phenylindole (DAPI, blue fluorescence) for nucleus. Merged images show green fluorescent staining in the cytoplasm due to cytoplasmic localization of EGFP-APE1/Ref-1 (29–318). The subcellular localization of EGFP-APE1/Ref-1 (29–318) fractions in response to TNF-α was confirmed in Western blot using cytosolic and nuclear fractions. (B) The expression of EGFP-APE1/Ref-1 (1–318) and EGFP-APE1/Ref-1 (29–318) by plasmid transfection significantly inhibited TNF-α-induced VCAM-1 expression at 15 ng/ml for 18 h. (C) Summarized data for Fig. 4B. Each bar shows the mean ± SE (n = 4). *p < 0.05 vs. compared with TNF-α alone. NS, non-significant.
Interestingly, TNF-α-induced VCAM-1 expression was inhibited by wild type of APE1/Ref-1 (1–318) and it was also inhibited by the transfection of NLS-deletion mutant APE1/Ref-1 (29–318) which was showing cytoplasmic localization, suggesting anti-inflammatory action of cytoplasmic APE1/Ref-1 (Figs. 4B and 4C).
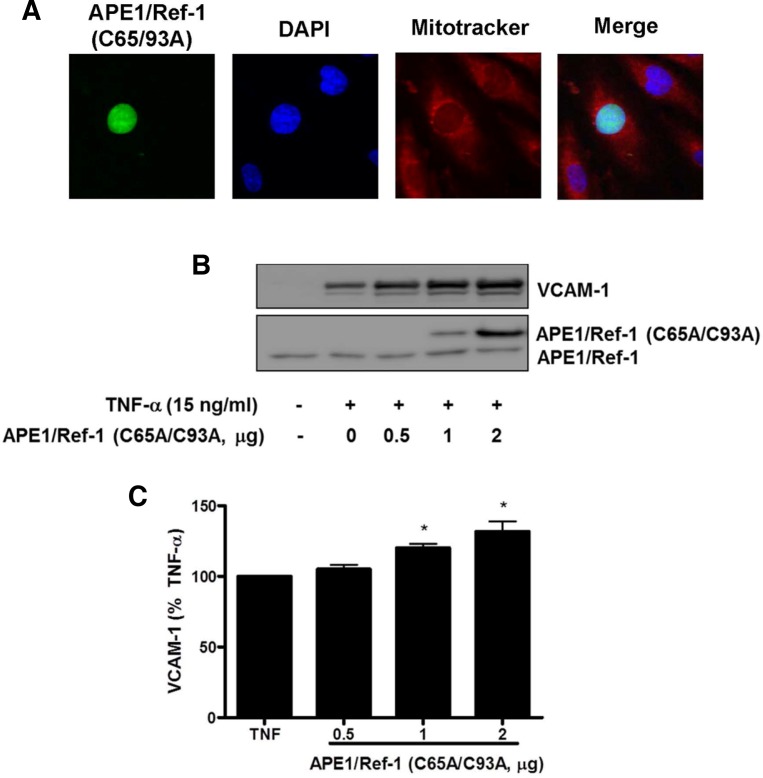
Redox cysteine mutant of APE1/Ref-1 increased TNF-α-induced VCAM-1 expression
The role of APE1/Ref-1 redox function was further investigated using the redox mutant APE1/Ref-1 (C65A/C93A) in TNF-α-treated endothelial cells. The redox mutant APE1/Ref-1 (C65A/ C93A) was generated by substitution of cysteine 65 and 93 with alanine in APE1/Ref-1 by point mutation. As observed by immunocytochemistry (Fig. 5A), the redox mutant APE1/Ref-1 (C65A/C93A) was localized to nucleus which was co-localized with DAPI as nucleus marker. The nuclear localization of EGFP-APE1/Ref-1 (C65/93A) protein was also confirmed by nuclear/cytoplasmic fractionation. Plasmid transfection with redox mutant APE1/Ref-1 (C65A/C93A) (0.5–2 μg) augmented TNF-α-induced VCAM-1 expression in a dose-dependent manner as shown in Figs. 5B and 5C. This data suggests that redox active site cysteine 65 and 93 of APE1/Ref-1 contribute to the anti-inflammatory action of APE1/Ref-1 in TNF-α-activated endothelial cells.
Fig. 5.
Double redox mutant APE1/Ref-1 (C65/93A) augmented TNF-α-induced VCAM-1 expression in endothelial cells. (A) Immunocytochemistry for analysis of double redox mutant APE1/ Ref-1 (C65A/C93A) in endothelial cells. Cells were stained with mitotracker red (red fluorescence) for mitochondria and 4′,6-diamidino-2-phenylindole (DAPI, blue fluorescence) for nucleus. Merged images show green fluorescent staining in the cytoplasm due to cytoplasmic localization of EGFP-APE1/Ref-1 (29–318). The subcellular localization of EGFP- APE1/Ref-1 (C65/93A) fractions in response to TNF-α was confirmed in Western blot using cytosolic and nuclear fractions. (B) The expression of HA-APE1/Ref-1 (C65A/C93A) by plasmid transfection significantly augmentted TNF-α-induced VCAM-1 expression at 15 ng/ml for 18 h. (C) Summarized data for Fig. 5B. Each bar shows the mean ± SE (n = 4). *p < 0.05 vs. compared with TNF-α alone.
DISCUSSION
As an accumulation of evidence has shown, APE1/Ref-1 is not only involved in DNA repair and transcriptional regulation in the nucleus but also has a pleiotropic role in controlling cellular responses to oxidative stress (Jeon and Irani, 2009; Tell et al., 2009). Furthermore, extra-nuclear functions of APE1/Ref-1 have been revealed (Jeon and Irani, 2009). APE1/Ref-1 reduces intracellular ROS production via inhibition of Rac1 activation in NADPH oxidase (Angkeow et al., 2002; Guo et al., 2008; Ozaki et al., 2002). It is well known that mitogen-activated protein kinase (MAPK)s are activated by growth factors, environmental stresses and inflammatory cytokine (Chen et al., 2001). We previously reported that the overexpression of APE1/Ref-1 using the adenoviral gene transfer system inhibits TNF-α-induced ROS production and VCAM-1 in endothelial cells (Angkeow et al., 2002; Kim et al., 2006; Lee et al., 2009). In addition, p38 MAPK activation by TNF-α was blocked by overexpression of APE1/Ref-1 resulting in inhibition of TNF-α-induced VCAM-1 in endothelial cell (Kim et al., 2006). TNF-α-induced VCAM-1 is closely associated with NADPH oxidase and mitochondrial ROS in endothelial cells (Bae et al., 2011; Yu et al., 2006). In the present study, our data showed plasmid transfection of APE1/Ref-1 inhibits TNF-α-induced VCAM-1 expression in endothelial cells, suggesting a biological function for APE1/Ref-1 against cytokine-induced endothelial inflammation.
APE1/Ref-1 is a ubiquitous protein in cells, but its expression pattern differs based on cell type. The APE1/Ref-1 subcellular localization is mainly nuclear, but cytoplasmic identification has also been reported, especially during high metabolic or proliferative states (Duguid et al., 1995; Kakolyris et al., 1998; Tell et al., 2005). Some evidence suggests that APE1/Ref-1 expression and subcellular localization are finely tuned. The subcellular distribution of APE1/Ref-1 may be regulated by both nuclear import and export systems (Jackson et al., 2005). APE1/Ref-1 undergoes active shuttling between the cytoplasm and nucleus in response to oxidative (Angkeow et al., 2002; Bhakat et al., 2003; Tell et al., 2000a) and nitrosative stress (Qu et al., 2007). In endothelial cells, the translocation of APE1/Ref-1 into the cytoplasm has been previously reported. Protein kinase C activation causes an increase of APE1/Ref-1 cytoplasmic translocation in endothelial cells (Lee et al., 2011). In this study, we confirmed that cytoplasmic APE1/Ref-1 is increased in response to TNF-α, and is not blocked by cycloheximide, a de novo protein synthesis inhibitor or leptomycin B, an inhibitor of nuclear exportin. This data indicates that the increased amount of APE1/Ref-1 in the cytoplasm after TNF-α-stimulation is most likely due to cytoplasmic translocation of APE1/Ref-1 in endothelial cells (Fig. 2).
In highly metabolically active cells, APE1/Ref-1 undergoes translocation from cytoplasm to nucleus in different cell types upon ROS exposure. The addition of thyrotropin to the culture medium increases the cytoplasm-to-nucleus translocation of APE1/Ref-1 in thyroid cells (Tell et al., 2000a). Exposure of B-lymphocytes to hydrogen peroxide induces a rapid and sustained increase in APE1/Ref-1 protein levels of the nucleus (Tell et al., 2000b). In the present study, we observed that APE1/Ref-1 is mainly localized in the nucleus of endothelial cells and exposure to TNF-α causes an increase of cytoplasmic translocation. However, the cell mechanism that regulates this re-localization of APE1/Ref-1 remains to be defined in endothelial cells. Our data shows that nucleus-to-cytoplasm translocation of APE1/Ref-1 in response to TNF-α is blocked by a NADPH oxidase inhibitor. This suggests that the presence of APE1/Ref-1 in the cytoplasm may reflect its involvement in cellular responses to oxidative stress in endothelial cells.
Analysis using deletion mutant of APE1/Ref-1 revealed that N-terminus 28 amino acids deletion of the APE1/Ref-1 which includes the putative NLS is biologically active and shows cytoplasmic localization. Since NLS-deletion mutant of APE1/Ref-1 is mainly located in the cytoplasm, inhibitory action of NLS-deletion mutant of APE1/Ref-1 on VCAM-1 is not mediated by nuclear function of APE1/Ref-1 which could modulate several nuclear transcriptional factors such as NF-κB and AP-1 (Evans et al., 2000). Plasmids transfection of NLS-deletion mutant APE1/Ref-1 (29–318) inhibits TNF-α-induced expression of VCAM-1, similar to wild type APE1/Ref-1. This suggests that the cytoplasmic localization of APE1/Ref-1 contributes to anti-inflammatory action in endothelial cells.
Functional activity of the redox domain in endothelial cells is poorly understood, Several studies of APE1/Ref-1 deletion mutants have revealed that the N-terminal of 1-127 amino acid residues are sufficient for its redox activation of c-Jun binding repair in vitro, and redox activities of APE1/Ref-1 are encoded by two distinct regions of APE1/Ref-1 (Xanthoudakis et al., 1994). Therefore, the N-terminal 1–127 amino acid residues of APE1/ Ref-1 are the putative redox domain. In the present study, transfection of double cysteine mutant, APE1/Ref-1 (C65A/C93A) resulted in an increase of TNF-α-induced VCAM-1 expression. This suggests that the redox active sites at cysteine 65 and 93 of the redox domain of APE1/Ref-1 contribute to inhibition of APE1/Ref-1 in TNF-α-induced VCAM-1 expression of endothelial cells.
In conclusion, our results suggest that cytoplasmic localization of APE1/Ref-1 contributes to the anti-inflammatory activity observed in endothelial cells. Specifically, cysteine of 65 and 93 residues play an important role in the anti-inflammatory action of APE1/Ref-1 in endothelial cells.
Acknowledgments
This study was financially supported by a research fund of Chungnam National University in 2011.
REFERENCES
- Angkeow P, Deshpande SS, Qi B, Liu YX, Park YC, Jeon BH, Ozaki M, Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002;9:717–725. doi: 10.1038/sj.cdd.4401025. [DOI] [PubMed] [Google Scholar]
- Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakat KK, Izumi T, Yang SH, Hazra TK, Mitra S. Role of acetylated human AP-endonuclease (APE1/Ref-1) in regulation of the parathyroid hormone gene. EMBO J. 2003;22:6299–6309. doi: 10.1093/emboj/cdg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Choi S, Lee YR, Park MS, Joo HK, Cho EJ, Kim HS, Kim CS, Park JB, Irani K, Jeon BH. Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. Biochem Biophys Res Commun. 2013;435:403–407. doi: 10.1016/j.bbrc.2013.04.101. [DOI] [PubMed] [Google Scholar]
- Duguid JR, Eble JN, Wilson TM, Kelley MR. Differential cellular and subcellular expression of the human multifunctional apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme. Cancer Res. 1995;55:6097–6102. [PubMed] [Google Scholar]
- Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- Guo Y, Chen J, Zhao T, Fan Z. Granzyme K degrades the redox/DNA repair enzyme Ape1 to trigger oxidative stress of target cells leading to cytotoxicity. Mol Immunol. 2008;45:2225–2235. doi: 10.1016/j.molimm.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Jackson EB, Theriot CA, Chattopadhyay R, Mitra S, Izumi T. Analysis of nuclear transport signals in the human apurinic/apyrimidinic endonuclease (APE1/Ref1) Nucleic Acids Res. 2005;33:3303–3312. doi: 10.1093/nar/gki641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BH, Irani K. APE1/Ref-1: versatility in progress. Antioxid Redox Signal. 2009;11:571–573. doi: 10.1089/ars.2008.2223. [DOI] [PubMed] [Google Scholar]
- Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, Liu YX, Kim JM, Ozaki M, White AR, et al. Apurinic/apyrmidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004;95:902–910. doi: 10.1161/01.RES.0000146947.84294.4c. [DOI] [PubMed] [Google Scholar]
- Joo HK, Oh SC, Cho EJ, Park KS, Lee JY, Lee EJ, Lee SK, Kim HS, Park JB, Jeon BH. Midazolam inhibits tumor necrosis factor-alpha-induced endothelial activation involvement of the peripheral benzodiazepine receptor. Anesthesiology. 2009;110:106–112. doi: 10.1097/ALN.0b013e318190bc69. [DOI] [PubMed] [Google Scholar]
- Kakolyris S, Kaklamanis L, Giatromanolaki A, Koukourakis M, Hickson ID, Barzilay G, Turley H, Leek RD, Kanavaros P, Georgoulias V, et al. Expression and subcellular localization of human AP endonuclease 1 (HAP1/Ref-1) protein: a basis for its role in human disease. Histopathology. 1998;33:561–569. doi: 10.1046/j.1365-2559.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- Kim CS, Son SJ, Kim EK, Kim SN, Yoo DG, Kim HS, Ryoo SW, Lee SD, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc Res. 2006;69:520–526. doi: 10.1016/j.cardiores.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, Bae YM, Kim HS, Lee SK, Park SH, et al. Gene transfer of redox factor-1 inhibits neointimal formation involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated syk pathway. Circ Res. 2009;104:219–U162. doi: 10.1161/CIRCRESAHA.108.178699. [DOI] [PubMed] [Google Scholar]
- Lee SK, Chung JI, Park MS, Joo HK, Lee EJ, Cho EJ, Park JB, Ryoo S, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease 1 inhibits protein kinase C-mediated p66shc phosphorylation and vasoconstriction. Cardiovasc Res. 2011;91:502–509. doi: 10.1093/cvr/cvr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Lee JY, Joo HK, Cho EJ, Kim CS, Lee SD, Park JB, Jeon BH. Tat-mediated p66shc transduction decreased phosphorylation of endothelial nitric oxide synthase in endothelial cells Korean. J Physiol Pharmacol. 2012;16:199–204. doi: 10.4196/kjpp.2012.16.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Lee YR, Joo HK, Cho EJ, Choi S, Sohn KC, Lee SD, Park JB, Jeon BH. Arginase II inhibited lipopolysaccharide-induced cell death by regulation of iNOS and Bcl-2 family proteins in macrophages. Mol. Cells. 2013;35:396–401. doi: 10.1007/s10059-013-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M, Suzuki S, Irani K. Redox factor-1/APE suppresses oxidative stress by inhibiting activity of the rac1 GTPase. FASEB J. 2002;16:889–890. doi: 10.1096/fj.01-0664fje. [DOI] [PubMed] [Google Scholar]
- Qu J, Liu GH, Huang B, Chen C. Nitric oxide controls nuclear export of APE1/Ref-1 through S-nitrosation of Cysteines 93 and 310. Nucleic Acids Res. 2007;35:2522–2532. doi: 10.1093/nar/gkl1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell G, Pellizzari L, Pucillo C, Puglisi F, Cesselli D, Kelley MR, Di Loreto C, Damante G. TSH controls Ref-1 nuclear translocation in thyroid cells. J Mol Endocrinol. 2000a;24:383–390. doi: 10.1677/jme.0.0240383. [DOI] [PubMed] [Google Scholar]
- Tell G, Zecca A, Pellizzari L, Spessotto P, Colombatti A, Kelley MR, Damante G, Pucillo C. An ‘environment to nucleus’ signaling system operates in B lymphocytes: redox status modulates BSAP/Pax-5 activation through Ref-1 nuclear translocation. Nucleic Acids Res. 2000b;28:1099–1105. doi: 10.1093/nar/28.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7:367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11:601–619. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S, Miao G, Wang F, Pan YCE, Curran T. Redox activation of Fos Jun DNA-binding activity is mediated by a DNA-repair enzyme. EMBO J. 1992;11:3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S, Miao GG, Curran T. The redox and DNA-repair activities of Ref-1 are encoded by nonover-lapping domains. Proc. Natl. Acad. Sci. USA. 1994;91:23–27. doi: 10.1073/pnas.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JH, Kim CS, Yoo DG, Song YJ, Joo HK, Kang G, Jo JY, Park JB, Jeon BH. NADPH oxidase and mitochondrial ROS are involved in the TNF-alpha-induced vascular cell adhesion molecule-1 and monocyte adhesion in cultured endothelial cells Korean. J Physiol Pharmacol. 2006;10:217–222. [Google Scholar]