Abstract
Drug repositioning can identify new therapeutic applications for existing drugs, thus mitigating high R&D costs. The Protein kinase 2 (CK2) inhibitor CX-4945 regulates human cancer cell survival and angiogenesis. Here we found that CX-4945 significantly inhibited the RANKL-induced osteoclast differentiation, but enhanced the BMP2-induced osteoblast differentiation in a cell culture model. CX-4945 inhibited the RANKL-induced activation of TRAP and NFATc1 expression accompanied with suppression of Akt phosphorylation, but, in contrast, it enhanced the BMP2-mediated ALP induction and MAPK ERK1/2 phosphorylation. CX-4945 is thus a novel drug candidate for bone-related disorders such as osteoporosis.
Keywords: CK2, CX-4945, differentiation, osteoblast, osteoclast
INTRODUCTION
In recent years, the biopharmaceutical industry has grown and there has been increased investment in the research and development (R&D) of new drugs. However, the approval of new drugs has not kept pace with the steady and dramatic increase in R&D spending. For example, most pharmaceutical companies invest in facets of R&D, such as assay development, high-throughput screening (HTS), and pharmacokinetic studies, but this investment does not always result in the successful development of new drugs. To reduce the gap between increased spending and the relative scarcity of new drugs, many companies are screening existing drugs in order to reposition them as drug candidates for other indications.
Drug repositioning, also known as redirecting, repurposing and reprofiling, is defined as the process of searching for new applications for existing drugs that are outside the scope of the original medical indication (Longman, 2004; Stuart, 2004). This allows companies a way to develop drugs that is faster and that reduces the risks of failure in drug discovery and development. Sildenafil (Viagra) is an example of drug repositioning by Pfizer. Sildenafil was originally intended to relax coronary blood flow, but the expected effects were not observed in clinical trials. However, this drug was effective for erectile dysfunction and was rapidly developed for this indication via the drug repositioning process (Haltmaier, 1998).
Moreover, many companies are starting to use ‘crowdsourcing’ to assist the process of drug repositioning. Crowdsourcing is a way to gather information through a distributed network such as a web-based business and can provide a company with novel targets and indications after investigators post information about the existing drug online. Crowdsourcing thus allows a company to gather key information without additional spending (Parvanta et al., 2013).
Protein kinase 2 (formerly casein kinase 2 or II) is a serine/threonine protein kinase that is ubiquitously expressed in most types of cells. CK2 is overexpressed in many types of cancer; notably, it affects the proliferation and helps maintain the phenotype of cancer (Ahmed, 1999; Ahmed et al., 2002; Allende and Allende, 1995; Guerra and Issinger, 1999). Recent reviews provide a potent rationale for considering CK2 as a therapeutic target for human cancer, and several CK2 inhibitors have been developed and are being tested in ongoing clinical trials (Cozza et al., 2012; Kim and Kim, 2012; Sarno et al., 2011).
CX-4945 (Fig. 1A) was developed and optimized as a selective inhibitor of CK2 by Cylene pharmaceuticals and is in ongoing clinical trials (clinicaltrials.gov; identifier: NCT00891280 & NCT01199718). CX-4945 was developed with the intention of using it in combination with diverse anti-cancer drugs, and it has been investigated for various cancer indications. Preliminary reports showed that CX-4945 has potent anti-proliferative, anti-angiogenic and anti-inflammatory activity via inhibition of CK2-Akt mediated signaling pathways in human cancer cells (Pierre et al., 2011a; 2011b; Siddiqui-Jain et al., 2010). A recent study showed that activation of Akt induces osteoclastogenesis via GSK3β/NFATc1 signaling (Moon et al., 2012) and that bone morphogenetic protein (BMP)-2-induced CK2 regulates osteoblast differentiation via Smad1/5/8 signaling (Bragdon et al., 2010). Recently, we reported the in vitro and in vivo pharmacokinetic profiles of CX-4945 to confirm the metabolic stability and bio-availability as a potent drug candidate (Son et al., 2013). Our report represented that CX-4945 shows good pharmacokinetics profiles such as long half-life, high oral bioavailability and non-cardiac toxicity. Based on this information and since there are no data related to the effect of CX-4945 on bone remodeling, we investigated the effect of CX-4945 on the receptor activator of NF-κB ligand (RANKL)-induced osteoclast differentiation and on BMP2-induced osteoblast differentiation.
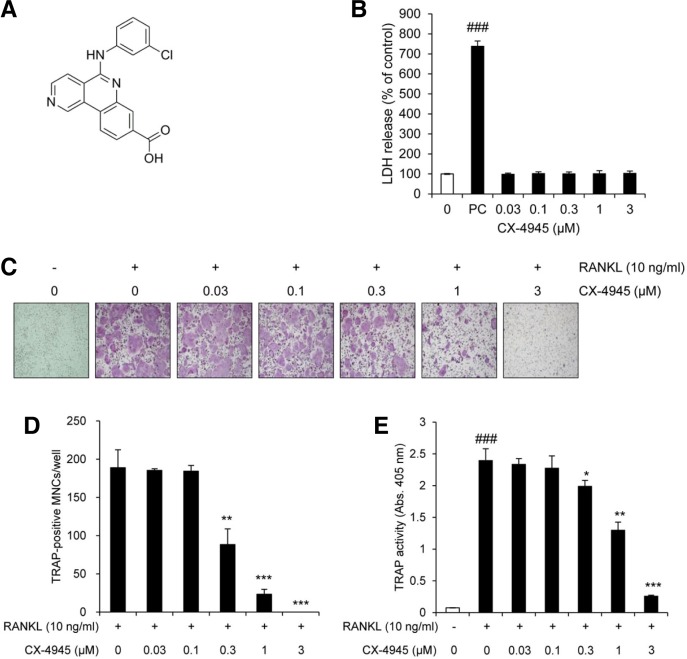
Fig. 1.
CX-4945 inhibits osteoclast differentiation. (A) The chemical structure of CX-4945. (B) The cytotoxic activity of CX-4945 in BMMs was evaluated by measuring the level of released LDH using the CytoTox 96 NonRadioactive Cytotoxicity Assay kit. Tween-20 treatment was served as a positive control (PC). The effect of CX-4945 on the RANKL-induced osteoclast differentiation was evaluated by visualized TRAP (C), counting the number of TRAP-positive multinucleated cells (MNCs) containing more than 5 nuclei (D), and measuring the activity of TRAP (E). The data are representative of at least three independent experiments. ###p < 0.001 (versus the control); *p < 0.05; **p < 0.01; ***p < 0.001 (versus cells treated with RANKL alone).
MATERIALS AND METHODS
Materials
CX-4945 was purchased from Sequoia Research Products (Pangbourne, UK). Recombinant human BMP-2, macrophage colony stimulating factor (M-CSF) and RANKL were obtained from R&D systems, Inc. (USA). Fetal bovine serum (FBS), DMEM, α-MEM and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) were purchased from Invitrogen (USA). Antibodies against Smad1/5/8, phosphorylated (p)-ERK1/2, ERK1/2, p-p38, p38, p-Akt (S473), Akt and c-Fos were purchased from Cell Signaling Technology, Inc. (USA). Antibodies against actin and nuclear factor of activated T cells c1 (NFATc1) were purchased from Santa Cruz Biotechnology, Inc. (USA).
Cell culture and differentiation
Murine bi-potential mesenchymal precursor C2C12 cells were maintained in DMEM containing 10% heat-inactivated FBS and 1% antibiotics in humidified atmosphere of 5% CO2 at 37°C. For differentiation, C2C12 cells were seeded in a 96-well plate for 48 h and then treated with recombinant human BMP-2 (25 ng/ml) in media containing 5% FBS. The medium was changed every 3 days. For osteoclast differentiation, bone marrow-derived macrophages (BMMs) were obtained as described in a previous study (Choi et al., 2012) Isolated BMMs (1 × 104 cells/well) were differentiated with M-CSF (30 ng/ml) and RANKL (10 ng/ml) in media containing 10% FBS for 4 days.
Lactate dehydrogenase (LDH) assay
BMMs (1 × 104 cells/well) were seeded in a 96-well plate and incubated for 24 h. BMMs were treated with M-CSF (30 ng/ml) or with M-CSF plus CX-4945 in media containing 10% FBS for 3 days. The released level of LDH was detected using the CytoTox 96 NonRadioactive Cytotoxicity Assay kit (Promega, USA) according to the manufacturer’s instruction. The absorbance was measured using a Wallac EnVision microplate reader (PerkinElmer, Finland). All experiments were performed in triplicate.
Tartrate-resistant acid phosphatase (TRAP) staining and activity assay
BMMs (1 × 104 cells/well) were seeded in a 96-well plate and incubated for 24 h. Cells were treated with M-CSF (30 ng/ml), M-CSF plus RANKL (10 ng/ml), or M-CSF and RANKL in combination with CX-4945 in media containing 10% FBS for 4 days. After incubation, cells were fixed with 3.7% formaldehyde, permeabilized with 0.1% Triton X-100 and incubated with reagents from the Leukocyte Acid Phosphatase Kit 387-A (Sigma, USA) in the dark at 37°C. After rinsing the cells, TRAP-positive multi-nucleated cells were visualized by phase-contrast light microscopy (Olympus Optical, Japan). TRAP-positive multinucleated cells with more than five nuclei were considered to be osteoclasts. Using the same conditions, the fixed and permeabilized cells were treated with 100 μl of citrate buffer (100 mM, pH 5) containing 50 mM sodium tartrate and 3 mM p-nitrophenyl-phosphate. After 1 h incubation, the enzyme reaction mixtures were transferred to new plates and mixed with an equal volume of 0.1 N NaOH. The absorbance was measured using a Wallac EnVision microplate reader (PerkinElmer, Finland). All experiments were performed in triplicate.
Quantitative real-time RT-PCR (qRT-PCR) analysis
Total RNA was isolated from BMMs or C2C12 cells using TRI-zol reagent (Life Technologies, USA), and cDNA was synthesized from 1 μg of total RNA using the Omniscript Reverse Transcriptase Kit (Qiagen, USA) according to the manufacturer’s instructions. Quantitative PCR was performed using the Brilliant SYBR Green Master Mix (Stratagene, USA) and the Mx3000P Real-Time PCR system (Stratagene, USA) as described in a previous study (Kim and Kim, 2010). The primer sequences used in this study are shown in Table 1. All reactions were performed in triplicate, and the data were analyzed using the 2 −ΔΔCT method. GAPDH was used as an internal standard. Statistical significance was determined with the Student’s t-test using GAPDH-normalized 2 −ΔΔCT values (Livak and Schmittgen, 2001).
Table 1.
Sequences of the primers used in this study
| Target gene | Forward (5′ – 3′) | Reverse (5′ – 3′) |
|---|---|---|
| c-Fos | CCAGTCAAGAGCATCAGCAA | AAGTAGTGCAGCCCGGAGTA |
| NFATc1 | GGGTCAGTGTGACCGAAGAT | GGAAGTCAGAAGTGGGTGGA |
| TRAP | GATGACTTTGCCAGTCAGCA | ACATAGCCCACACCGTTCTC |
| ALP | ATGGGCGTCTCCACAGTAAC | TCACCCGAGTGGTAGTCACA |
| BMP-4 | CCTGGTAACCGAATGCTGAT | AGCCGGTAAAGATCCCTCAT |
| GAPDH | AACTTTGGCATTGTGGAAGG | ACACATTGGGGGTAGGAACA |
Western blot analysis
The cytoplasmic fractions and nuclear fractions of cell lysates were prepared using the NucBuster Protein Extraction kit (Novagen, Germany). After the protein concentration was quantified, the cytoplasmic or nuclear proteins were loaded and separated on 8–15% polyacrylamide gels, and then transferred to PVDF membranes. After membrane blocking with 5% skim milk, the expression of specific proteins was detected using primary antibodies. After incubation with HRP-conjugated secondary antibodies, the signals were developed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, USA) and analyzed with the LAS-3000 luminescent image analyzer (Fuji Photo Film Co., Ltd., Japan).
Cell viability assay
C2C12 cells (4 × 103 cells/well) were seeded in a 96-well plate and incubated for 24 h. The cells were treated with BMP-2 (25 ng/ml) or its combination with CX-4945 in media containing 5% FBS for 6 days. Cell viability was measured using the Cell Counting Kit-8 (Dojindo Molecular Technologies, USA) according to the manufacturer’s instruction. The absorbance was measured using the Wallace EnVision microplate reader (PerkinElmer, Finland). All experiments were performed in triplicate.
Alkaline phosphatase (ALP) staining
C2C12 cells (4 × 103 cells/well) were seeded in a 96-well plate and incubated for 24 h. Cells were replenished with BMP-2 (25 ng/ml) or its combination with CX-4945 in media containing 5% FBS every 3 days. After 6 days, cells were fixed with 3.7% formaldehyde, rinsed with PBS, and stained with ALP staining kit (Sigma, USA). ALP-positive cells were visualized by phase-contrast light microscopy (Olympus Optical, Japan).
RESULTS
CX-4945 inhibits RANKL-induced osteoclast differentiation
To determine the concentration of CX-4945 to use for investigating its possible effect on osteoclast differentiation, we assessed its cytotoxicity in cell culture by monitoring LDH production, and found that no cell death was observed at the concentrations used in this study (Fig. 1B). We next determined whether CX-4945 affects the RANKL-induced osteoclast differentiation. As shown in Figs. 1C and 1D, RANKL strongly stimulated the differentiation of BMMs into osteoclasts, but CX-4945 significantly inhibited the formation of RANKL-induced TRAP-positive multinucleated cells and TRAP activity in a dose-dependent manner. The anti-osteoclastogenic activity of CX-4945 was confirmed by qRT-PCR analysis. As shown in the left panel of Fig. 2A, the relative mRNA level of TRAP was induced by RANKL in time-dependent manner, but its induction was attenuated by the addition of CX-4945.
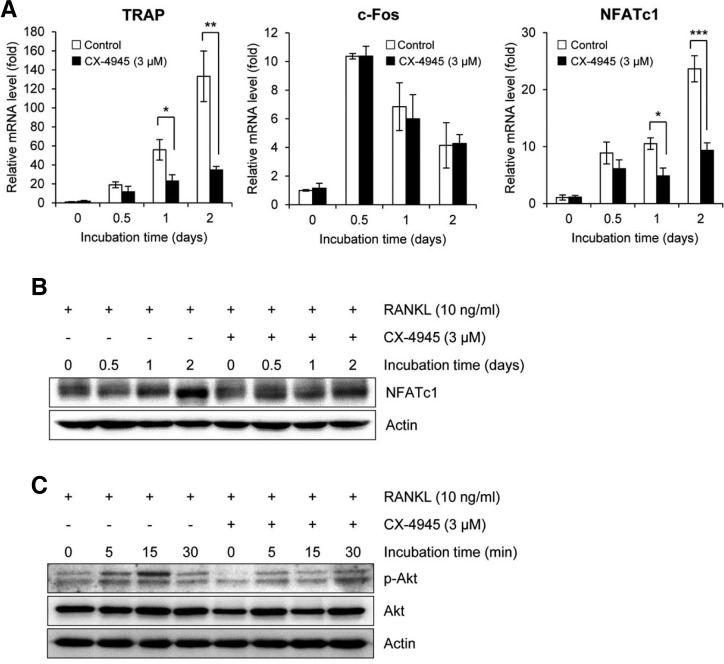
Fig. 2.
CX-4945 inhibits the expression of osteoclast differentiation markers and Akt phosphorylation. (A) The effect of CX-4945 on the RANKL-induced expression of molecules related to osteoclast differentiation was evaluated by qRT-PCR. The effects of CX-4945 on the protein expression of NFATc1 (B) and the phosphorylation of Akt (C) were evaluated by Western blot analysis. CX-4945 was used at 3 μM. Actin was used as a loading control in Western blot analysis. All data were obtained in at least 3 independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001 (versus cells treated with RANKL alone).
CX-4945 inhibits the expression of osteoclast differentiation markers and phosphorylation of Akt
During the RANKL-induced osteoclast differentiation, the signal transduction is mediated by the induction and autoamplification of NFATc1 that is the most distal transcription factor to be controlled by c-Fos (Grigoriadis et al., 1994; Kim et al., 2012; Matsuo et al., 2004; Takayanagi et al., 2002). Transcriptional induction of molecules related to the differentiation and bone resorption of osteoclasts such as TRAP, cathepsin K and OSCAR has been shown to be regulated by NFATc1 (Asagiri and Takayanagi, 2007; Kim et al., 2012). As shown in Fig. 2A, RANKL strongly induced the mRNA expression of c-Fos and NFATc1, but CX-4945 significantly inhibited the RANKL-mediated induction of NFATc1, but not that of c-Fos. The inhibitory effect of CX-4945 on the expression of NFATc1 was confirmed by Western blot analysis (Fig. 2B).
Since CK2 affects Akt activation (Di Maira et al., 2005) and its signaling cascade regulates NFATc1 expression during osteoclast differentiation (Moon et al., 2012), the effect of CX-4945 on the Akt activation was further evaluated. Akt phosphorylation was induced by RANKL within 15 min, but its induction was attenuated by the addition of CX-4945 (Fig. 2C).
CX-4945 stimulates BMP-2-induced osteoblast differentiation via Smad signaling
CK2 is a negative regulator of BMP signaling; the inhibition of its interaction between BMP receptor type Ia or a dominant negative CK2α led to an increase in BMP signaling and mineralization in C2C12 cells (Bragdon et al., 2010). Accordingly, we evaluated the effect of CX-4945 on the BMP-2-induced osteoblast differentiation using C2C12 cells. Since CX-4945 exhibited the cytotoxicity at 30 μM, non-cytotoxic dosages of CX-4945 (upto 10 μM) were used in the following experiments (Fig. 3A). In C2C12 cells, BMP-2 induced the expression of alkaline phosphatase (ALP) that is a representative marker for osteoblast differentiation (Franceschi and Iyer, 1992), and its induction was enhanced by CX-4945 in a dose-dependent manner (Fig. 3B). The enhancing effect of CX-4945 on the induction of ALP was confirmed by qRT-PCR analysis (Fig. 3C), and moreover, the BMP-2-induced mRNA expression of osteogenic BMP-4 was also significantly enhanced by CX-4945 at 10 μM (Bessa et al., 2009).
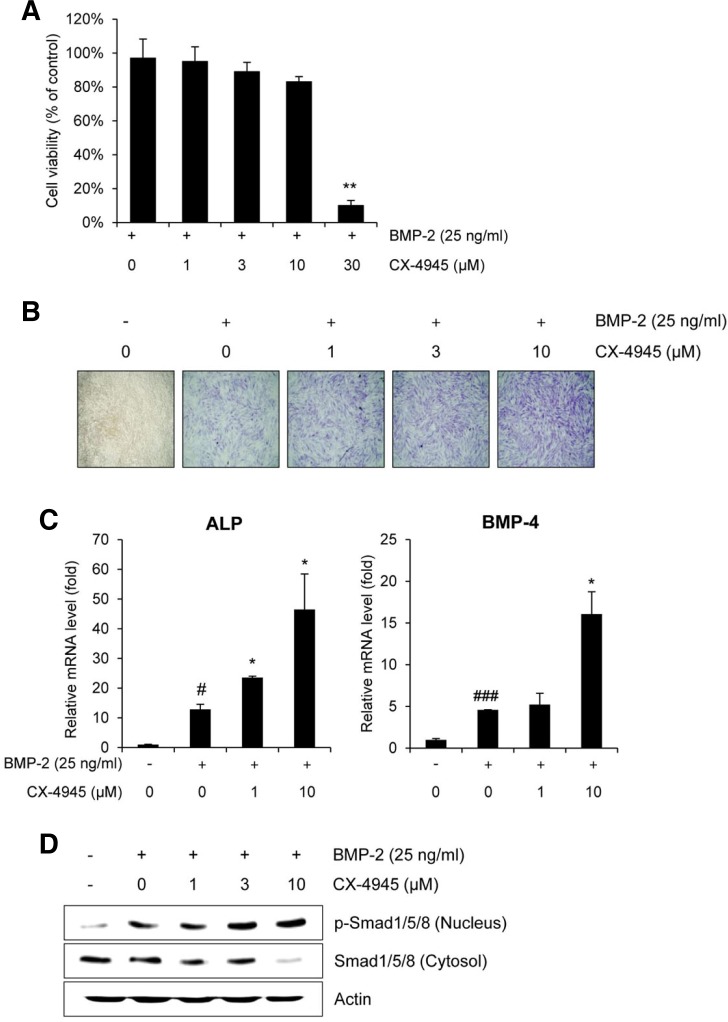
Fig. 3.
CX-4945 stimulates osteoblast differentiation via Smad signaling. (A) The effect of CX-4945 on the viability of C2C12 cells were evaluated using the CCK-8 assay. (B) The effect of CX-4945 on the BMP-2-induced osteoblast differentiation was evaluated by visualized the induction of ALP in C2C12 cells. DMSO was used as a vehicle control. (C) The transcriptional induction of ALP and BMP-2 in C2C12 cells was evaluated. Cells were replenished with BMP-2 (25 ng/ml) or its combination with CX-4945 in media containing 5% FBS every 3 days. After 6 days, the mRNA expression of osteoblast differentiation markers was detected by qRT-PCR. (D) The effect of CX-4945 on the nuclear translocation of Smad1/5/8 was evaluated. C2C12 cells were treated with BMP-2 (25 ng/ml) and CX-4945 (1, 3, or 10 μM) for 6 days. Cells were replenished with BMP-2 (25 ng/ml) or its combination with CX-4945 in media containing 5% FBS every 3 days. After 6 days, the cells were lysed and fractionated, and the phosphorylation levels of nuclear Smad1/5/8 and cytosolic Smad 1/5/8 were determined by Western blot analysis. #p < 0.05; ###p < 0.001 (versus the control); *p < 0.05; **p < 0.01 (versus cells treated with BMP-2 alone).
In addition, the effect of CX-4945 on BMP-2-induced phosphorylated Smad1/5/8 was evaluated by Western blot analysis (Fig. 3D). CX-4945 enhanced the BMP-2-mediated nuclear accumulation of phosphorylated Smad1/5/8 in a dose-dependent manner.
CX-4945 stimulates BMP-2-induced osteoblast differentiation via ERK signaling pathway
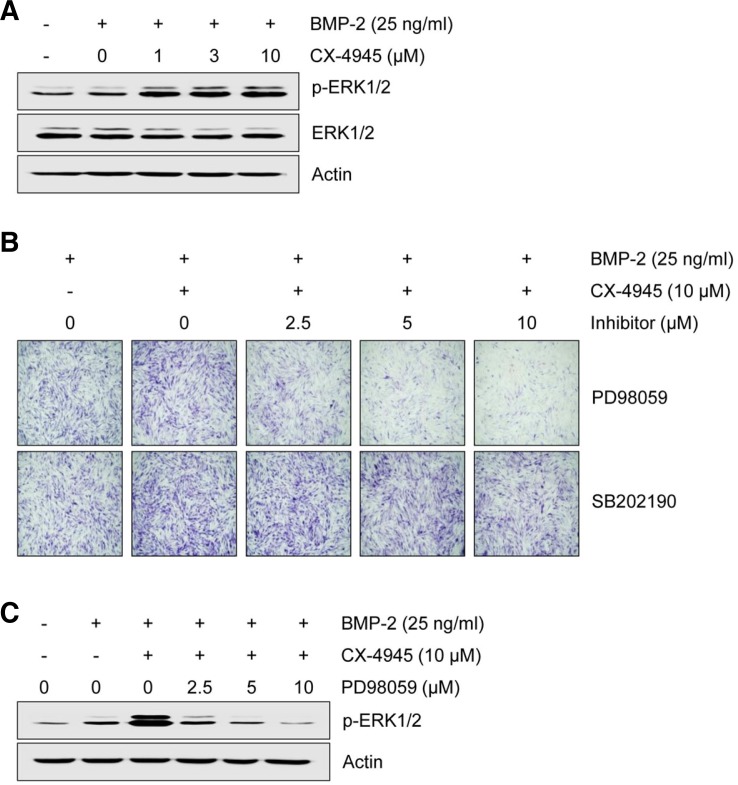
Since CK2 is required for activating extracellular signal-regulated kinase (ERK) signaling when it interacts with partner molecules (Ritt et al., 2007) and BMP-2 activates mitogen-activated protein kinases (MAPKs), including ERK, p38 and c-Jun NH2 terminal kinase (JNK), we investigated the effect of CX-4945 on BMP-2-induced activation of MAPK signaling. As shown in Fig. 4A, CX-4945 enhanced BMP-2-induced phosphorylation of ERK1/2, but phosphorylation of p38 and JNK was not changed (data not shown). The involvement of ERK1/2 signaling in the CX-4945-mediated enhancement of BMP-2-induced osteoblast differentiation was further confirmed by pharmacologic inhibition study; CX-4945-mediated enhancement of BMP-2-induced ALP was attenuated by PD98059, an inhibitor of MEK that is an upstream molecule of ERK, but not by SB202190, a p38 inhibitor (Fig. 4B). CX-4945-mediated enhancement of BMP-2-induced activation of ERK1/2 was also attenuated by PD98059 in a dose-dependent manner (Fig. 4C).
Fig. 4.
CX-4945 activates BMP-2-induced osteoblast differentiation via MAPK ERK1/2 signaling. (A) The effect of CX-4945 on the activation of ERK1/2 was evaluated. C2C12 cells were treated with BMP-2 (25 ng/ml) and with CX-4945 (1, 3 or 10 μM as indicated) for 6 days. After 6 days, ERK1/2 phosphorylation was measured by Western blot analysis. ERK1/2 involvement was confirmed by pharmacologic inhibition study. C2C12 cells were treated with the indicated concentrations of PD98059 or SB 202190 in the presence of BMP-2 (25 ng/ml) and CX-4945 (10 μM) for 6 days. After 6 days, ALP was detected using an ALP staining assay (B) and the phosphorylation of ERK1/2 was assessed by Western blot analysis (C). Actin was used as a loading control.
DISCUSSION
During the past decade, R&D expenditures by the pharmaceutical industry have grown by over 10% each year, but the approval of new drugs has remained sluggish (Booth and Zemmel, 2004). To increase the success rate in drug development, several strategies has been applied. Of these cost-cutting strategies for drug discovery, the most fruitful strategy could be ‘drug repositioning’ that is to identify new indication for existing drugs (Ashburn and Thor, 2004).
Protein kinase CK2, a highly conserved, constitutively expressed enzyme, that has recently emerged as a specific target for cancer therapy (Gyenis et al., 2013). However, since a number of CK2 substrates have been existed, many CK2 inhibitors have been developed and widely utilized (Pagano et al., 2004; 2008; Prudent et al., 2009; Sarno et al., 2001; Zien et al., 2005), the validation of ‘druggability’ of kinase inhibitors is very important in a various CK2-mediated regulatory mechanism. Previous studies identified CX-4945 as a CK2 inhibitor that regulates human cancer cell survival and angiogenesis (Kim and Kim, 2012; Pierre et al., 2011a; 2011b; Siddiqui-Jain et al., 2010), and our recent study showed that CX-4945 has favorable pharmacokinetic characteristics (Son et al., 2013). Since several studies have reported the possible involvement of CK2 and its effect Akt in bone metabolism (Bragdon et al., 2010; Moon et al., 2012), here we evaluated and found the anti-osteoclastogenic and anabolic activity of CX-4945; CX-4945 inhibited the RANKL-induced osteoclast differentiation accompanied with suppression of Akt/NFATc1 signaling axis, but it enhanced the BMP-2-induced osteoblast differentiation via the ERK1/2-Smad signaling axis. Since BMP-2 enhances the expression of other BMP genes during osteoblast differentiation (Chen et al., 1997; Kawai et al., 2006), these reports demonstrate that CX-4945 could enhance the BMP-2-induced increases in ALP and BMP-4 mRNA levels (Fig. 3C). Recent report represents that mutation of the CK2 phosphorylation site or blocking CK2 from binding to BMP receptor type Ia (BMPRIa) led specifically to osteogenesis through the MEK/ERK signaling pathway (Moseychuk et al., 2013). This report demonstrates that mutations of BMPRIa at CK2 binding domain induced osteogenesis and this osteogenic differentiation was mediated through MEK-ERK signaling pathway. Additionally, the BMP-2-induced osteoblast differentiation was inhibited by the MEK inhibitor PD98059, not p38 inhibitor SB203580. This report supports our result (Fig. 4) that blocked CK2 enhances BMP-2-induced osteoblast differentiation through MEK-ERK signaling pathway and osteoblast differentiation is independent to p38-mediated signaling pathway. Although much more information is needed before pre-clinical or clinical trials can be conducted, CX-4945 or other CK2 inhibitor might be developed for treating bone-related disorder including osteoporosis.
Acknowledgments
We thank Dr. Seong Hwan Kim (Laboratory of Translational Therapeutics, Pharmacology Research Center, Bio-Organic Science Division, Korea Research Institute of Chemical Technology, Republic of Korea) for critical discussion. This work was supported by a Korea Research Institute of Chemical Technology project grant, SI-1304, which was funded by the Korea Ministry of Knowledge Economy.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Ahmed K. Nuclear matrix and protein kinase CK2 signaling. Crit Rev Eukaryot Gene Expr. 1999;9:329–336. doi: 10.1615/critreveukargeneexpr.v9.i3-4.170. [DOI] [PubMed] [Google Scholar]
- Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: An emerging role for protein kinase CK2. Trends Cell Biol. 2002;12:226–230. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- Allende JE, Allende CC. Protein kinase CK2-an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses or existing drugs. Nat Rev Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Bessa PC, Cerqueira MT, Rada T, Gomes ME, Neves NM, Nobre A, Reis RL, Casal M. Expression, purification and osteogenic bioactivity of recombinant human BMP-4, -9, -10, -11 and -14. Protein Expr Purif. 2009;63:89–94. doi: 10.1016/j.pep.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Booth B, Zemmel R. Prospects for productivity. Nat Rev Drug Discov. 2004;3:451–456. doi: 10.1038/nrd1384. [DOI] [PubMed] [Google Scholar]
- Bragdon B, Thinakaran S, Moseychuk O, King D, Young K, Litchfield DW, Petersen NO, Nohe A. Casein kinase 2 beta-subunit is a regulator of bone morphogenetic protein 2 signaling. Biophys J. 2010;99:897–904. doi: 10.1016/j.bpj.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Harris MA, Rossini G, Dunstan CR, Dallas SL, Feng JQ, Mundy GR, Harris SE. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60:283–290. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- Choi SW, Son YJ, Yun JM, Kim SH. Fisetin inhibits osteoclast differentiation via downregulation of p38 and c-Fos-NFATc1 signaling pathways. Evid Based Complement Alternat Med. 2012;2012:810563. doi: 10.1155/2012/810563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozza G, Pinna LA, Moro S. Protein kinase CK2 inhibitors: a patent review. Expert Opin Ther Pat. 2012;22:1081–1097. doi: 10.1517/13543776.2012.717615. [DOI] [PubMed] [Google Scholar]
- Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Guerra B, Issinger OG. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis. 1999;20:391–408. doi: 10.1002/(SICI)1522-2683(19990201)20:2<391::AID-ELPS391>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gyenis L, Turowec JP, Bretner M, Litchfield DW. Chemical proteomics and functional proteomics strategies for protein kinase inhibitor validation and protein kinase substrate identification: applications to protein kinase CK2. Biochim. Biophys. Acta. 2013;1834:1352–1358. doi: 10.1016/j.bbapap.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Haltmaier HK. A Potenz aus dem Labor: Die Erektionspille VIAGRA (‘Potency from the Lab: The Erection Pill Viagra’) Reinbek bei Hamburg, Germany: Rowohlt Taschenbuch Verlag GmbH; 1998. [Google Scholar]
- Kawai M, Bessho K, Maruyama H, Miyazaki J, Yamamoto T. Simultaneous gene transfer of bone morphogenetic protein (BMP)-2 and BMP-7 by in vivo electroporation induces rapid bone formation and BMP-4 expression. BMC Musculoskelet Disord. 2006;7:62. doi: 10.1186/1471-2474-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim SH. Tanshinone IIA enhances BMP-2-stimulated commitment of C2C12 cells into osteoblasts via p38 activation. Amino Acids. 2010;39:1217–1226. doi: 10.1007/s00726-010-0557-8. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim SH. Druggability of the CK2 inhibitor CX-4945 as an anticancer drug and beyond. Arch Pharm Res. 2012;35:1293–1296. doi: 10.1007/s12272-012-0800-9. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim JH, Moon JB, Lee J, Kwak HB, Park YW, Kim N. The transmembrane adaptor protein, linker for activation of T cells (LAT), regulates RANKL-induced osteoclast differentiation. Mol. Cells. 2012;33:401–406. doi: 10.1007/s10059-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longman R. Pharmaceutical strategies: jumpstart to products. In Vivo. 2004;22:17. [Google Scholar]
- Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279:26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee SY, Kim N. Akt induces osteoclast differentiation through regulating the GSK3β/NFATc1 signaling cascade. J Immunol. 2012;188:163–169. doi: 10.4049/jimmunol.1101254. [DOI] [PubMed] [Google Scholar]
- Moseychuk O, Akkiraju H, Dutta J, D'Angelo A, Bragdon B, Duncan RL, Nohe A. Inhibition of CK2 binding to BMPRIa induces C2C12 differentiation into osteoblasts and adipocytes. J Cell Commun Signal. 2013 doi: 10.1007/s12079-013-0199-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano MA, Meggio F, Ruzzene M, Andrzejewska M, Kazimierczuk Z, Pinna LA. 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: a novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun. 2004;321:1040–1044. doi: 10.1016/j.bbrc.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Pagano MA, Bain J, Kazimierczuk Z, Sarno S, Ruzzene M, Maira Di G, Elliott M, Orzeszko A, Cozza G, Meggiom F, et al. The selectivity of inhibitors of protein kinase CK2: an update. Biochem J. 2008;415:353–365. doi: 10.1042/BJ20080309. [DOI] [PubMed] [Google Scholar]
- Parvanta C, Roth Y, Keller H. Crowdsourcing 101: a few basics to make you the leader of the pack. Health Promot Pract. 2013;14:163–167. doi: 10.1177/1524839912470654. [DOI] [PubMed] [Google Scholar]
- Pierre F, Chua PC, O’Brien SE, Siddiqui-Jain A, Bourbon P, Haddach M, Michaux J, Nagasawa J, Schwaebe MK, Stefan E, et al. Pre-clinical characterization of CX-4945, a potent and selective small molecule inhibitor of CK2 for the treatment of cancer. Mol Cell Biochem. 2011a;356:37–43. doi: 10.1007/s11010-011-0956-5. [DOI] [PubMed] [Google Scholar]
- Pierre F, Chua PC, O’Brien SE, Siddiqui-Jain A, Bourbon P, Haddach M, Michaux J, Nagasawa J, Schwaebe MK, Stefan E, et al. Discovery and SAR of 5-(3-chlorophenyl-amino)benzo[c][2,6]naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer. J Med Chem. 2011b;54:635–654. doi: 10.1021/jm101251q. [DOI] [PubMed] [Google Scholar]
- Prudent R, Cochet C. New protein kinase CK2 inhibitors: jumping out of the catalytic box. Chem Biol. 2009;16:112–120. doi: 10.1016/j.chembiol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Ritt DA, Zhou M, Conrads TP, Veenstra TD, Copeland TD, Morrison DK. CK2 Is a component of the KSR1 scaffold complex that contributes to Raf kinase activation. Curr Biol. 2007;17:179–184. doi: 10.1016/j.cub.2006.11.061. [DOI] [PubMed] [Google Scholar]
- Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’) FEBS Lett. 2001;296:44–48. doi: 10.1016/s0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- Sarno S, Papinutto E, Franchin C, Bain J, Elliott M, Meggio F, Kazimierczuk Z, Orzeszko A, Zanotti G, Battistutta R, et al. ATP site-directed inhibitors of protein kinase CK2: an update. Curr Top Med Chem. 2011;11:1340–1351. doi: 10.2174/156802611795589638. [DOI] [PubMed] [Google Scholar]
- Siddiqui-Jain A, Drygin D, Streiner N, Chua P, Pierre F, O’Brien SE, Bliesath J, Omori M, Huser N, Ho C, et al. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010;70:10288–10298. doi: 10.1158/0008-5472.CAN-10-1893. [DOI] [PubMed] [Google Scholar]
- Son YH, Song JS, Kim SH, Kim J. Pharmacokinetic characterization of CK2 inhibitor CX-4945. Arch Pharm Res. 2013;36:840–845. doi: 10.1007/s12272-013-0103-9. [DOI] [PubMed] [Google Scholar]
- Stuart M. Rediscovering existing drugs. Start-Up. 2004;9:23–30. [Google Scholar]
- Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- Zien P, Duncan JS, Skierski J, Bretner M, Litchfield DW, Shugar D. Tetrabromobenzotriazole (TBBt) and tetra-bromobenzimidazole (TBBz) as selective inhibitors of protein kinase CK2: evaluation of their effects on cells and different molecular forms of human CK2. Biochim. Biophys. Acta. 2005;1754:271–280. doi: 10.1016/j.bbapap.2005.07.039. [DOI] [PubMed] [Google Scholar]