Abstract
Tumor-associated macrophages (TAMs) accumulate in various cancers and promote tumor angiogenesis and metastasis, and thus may be ideal targets for the clinical diagnosis of tumor metastasis with high specificity. However, there are few specific markers to distinguish between TAMs and normal or inflammatory macrophages. Here, we show that TAMs localize in green fluorescent protein-labeled tumors of metastatic lymph nodes (MLNs) from B16F1 melanoma cells but not in necrotic tumor regions, suggesting that TAMs may promote the growth of tumor cells and the progression of tumor metastasis. Furthermore, we isolated pure populations of TAMs from MLNs and characterized their gene expression signatures compared to peritoneal macrophages (PMs), and found that TAMs significantly overexpress immunosuppressive cytokines such as IL-4, IL-10, and TGF-β as well as proangiogenic factors such as VEGF, TIE2, and CD31. Notably, immunological analysis revealed that TIE2+/CD31+ macrophages constitute the predominant population of TAMs that infiltrate MLNs, distinct from tissue or inflammatory macrophages. Importantly, these TIE2+/CD31+ macrophages also heavily infiltrated MLNs from human breast cancer biopsies but not reactive hyperplastic LNs. Thus, TIE2+/CD31+ macrophages may be a unique histopathological biomarker for detecting metastasis in clinical diagnosis, and a novel and promising target for TAM-specific cancer therapy.
Keywords: breast cancer, melanoma, metastasis, proangiogenic macrophages, tumor-associated macrophages
INTRODUCTION
Tumor-associated macrophages (TAMs) are the most abundant cancer stromal cells in the tumor microenvironment (Mantovani et al., 2002; 2008; Pollard, 2004). Recent clinical and experimental studies have suggested that TAMs play pivotal roles in tumor immunosuppression and provide a suitable microenvironment for cancer development and progression (Mantovani and Sica, 2010; Pollard, 2008; Qian and Pollard, 2010). Their tumor-promoting role presumably results from their ability to mediate tumor angiogenesis, lymphangiogenesis, increase the breakdown of extracellular matrix, promote tumor cell invasion and migration, and suppress the antitumor immune response (Mantovani and Sica, 2010; Pollard, 2009; Qian and Pollard, 2010; Yang et al., 2011). Notably, high TAM infiltration is well correlated with worse clinical outcome in most malignant tumors such as breast, cervical, ovarian, prostate, and thyroid cancers, Hodgkin’s lymphoma, hepatocellular carcinoma, lung carcinoma, and cutaneous melanoma (Fei et al., 2008; Groblewska et al., 2007; Huang et al., 1996; Lan et al., 2013; Mantovani and Sica, 2010; Ryder et al., 2008; Qian and Pollard, 2010; Schoppmann et al., 2006; Steidl et al., 2010; Xu et al., 2013). In contrast, high TAM infiltration may also be associated with increased survival in cervical, pancreatic, and lung cancers (Kim et al., 2008; White et al., 2003; Zijlmans et al., 2006). These discrepancies among various cancers may result from relatively nonspecific markers for the detection of macrophages (e.g., CD68 and CD163), which do not distinguish between TAMs and inflammatory macrophages (Quatromoni and Eruslanov, 2012). Moreover, CD68 and CD163 antibodies can detect inflammatory macrophages as well as TAMs, with overlapping features of pro- and anti-tumor subsets (Heusinkveld and van der Burg, 2011). To address the seemingly conflicting evidence regarding the roles of TAMs in the tumor microenvironment, it would be useful to identify unique markers that can detect distinct subsets of TAMs.
In the present study, we focused on proximal lymph nodes (LNs) as a target organ of early metastasis because malignant tumors such as melanoma and breast cancers preferentially metastasize via LNs. To identify a TAM-specific marker that is distinct from tissue and inflammatory macrophages, we isolated TAMs from the metastatic LNs (MLNs) of B16F1 melanoma mice using fluorescence-activated cell sorting (FACS) and performed comparative gene expression analysis of macrophages. Immunohistological analyses suggested that proangiogenic TIE2+/CD31+ macrophages are the predominant macrophage subset that infiltrates MLNs. Our findings suggest that macrophages with high levels of TIE2 and CD31 may play an important role in tumor angiogenesis and might be useful as a specific marker for TAMs. TIE2+/CD31+ TAMs can potentially be used as a prognostic marker for clinical diagnosis and may represent potential targets for cancer therapeutics.
MATERIALS AND METHODS
Animal care and use
Animal studies were approved by the Center for Animal Care and Use and performed according to institutional ethics and safety guidelines at Gachon University of Medicine and Science, Lee Gil Ya Cancer and Diabetes Institute, Incheon, Korea.
Human breast cancer specimens
The study protocol was approved by the Medical Ethics Committee of Seoul National University Hospital (Korea). The study cohort consisted of 41 patients who received treatment at SNUH between 2007 and 2010. Multiple sections of resected MLNs (n = 36) from patients with breast cancer and reactive hyperplastic LNs (n = 5) from patients with benign cancer were stained with hematoxylin and eosin (H&E). Images of the stained slides were captured with the Mirax Desk scanner (Carl Zeiss, Germany).
Inoculation of B16F1 melanoma mice
We inoculated B16F1 or B16F1-GFP melanoma cells (4 × 105 per mouse) to 8-week-old C57BL/6 male mice into the left front footpad (Hill et al., 1984). The tumor was allowed to grow to approximately 1.0 cm in diameter, at which point we sacrificed the mice and harvested the primary tumors, regional LNs, and non-regional LNs.
Isolation and activation of thioglycollate-elicited peritoneal macrophages (PMs)
We obtained thioglycollate-elicited PMs from C57BL/6 mice. Briefly, we injected mice intraperitoneally with 2 ml 3% Brewer thioglycollate medium (Difco, USA). After 3 days, cells were harvested by washing the peritoneal cavity with cold PBS. Resident macrophages were obtained by peritoneal lavage of naïve mice. The cells were centrifuged (1,000 rpm, 5 min). The cell pellets were washed three times with PBS, resuspended in DMEM medium supplemented with 10% FBS (Gibco), and cultured on chambered coverglasses (Nunc, Fisher Scientific) at a density of 1 × 105 cells ml−1. Cells were allowed to adhere for 3 h, and then the slides were washed to remove non-adherent cells. Next, adherent PMs were incubated with or without 100 ng ml−1 LPS for 3 h. Following incubation, the cells were harvested.
Histochemistry, immunohistochemistry (IHC) and immunofluorescence
MLNs that contained B16F1 melanoma cells were fixed with 10% neutral-buffered formalin and embedded in paraffin wax. To confirm the presence of LN metastases, we obtained GFP images from 2.5-μm-thick sections of MLNs that contained B16F1-GFP melanoma cells using the Zeiss Axio Imager Z1 microscope (Carl Zeiss). H&E staining was performed using a standard protocol and stained sections were imaged with the Zeiss Axio Imager Z1 microscope. For IHC, antigen retrieval was performed in Tris/EDTA (pH 9.0) for 20–30 min. Then we incubated sections with the following primary antibodies at 25°C for 2 h: monoclonal F4/80 (BM8; eBioscience, USA), goat polyclonal CD31 (M-20; Santa Cruz), and rabbit polyclonal TIE2 (H-176, Santa Cruz) for mouse LNs and MLNs, and polyclonal TIE2 (C-19; Santa Cruz), monoclonal CD31 (JC70A; Dako, Denmark), goat polyclonal CD31 (M-20; Santa Cruz), and mo-noclonal CD163 (10D6; Novocastra, England) for human hyperplastic LNs and MLNs. For IHC, detection of horseradish peroxidase-conjugated secondary antibodies with 3,3′-diami-nobenzidine tetrahydrochloride substrate (Dako) was followed by counterstaining with hematoxylin (Dako). For immunofluo-rescence staining, fluorescently conjugated secondary antibodies were added and the slides were incubated at room temperature for 30 min in the dark. The sections were counters-tained with DAPI to visualize the nuclei. After mounting, the sections were imaged with a Zeiss LSM 710 laser-scanning confocal microscope (Carl Zeiss).
Flow cytometry
For further phenotypic analysis of FACS-sorted macrophages, we obtained MLNs from 24 B16F1 melanoma mice (tumor size, 1 cm diameter). Harvested cells were filtered through cell strainers (pore diameter, 70 μm; BD Falcon, USA) and washed in FACS buffer (0.2% FBS, 2 mM EDTA in PBS). Single-cell suspensions were stained on ice for 30 min with a PE-conjugated anti-CD11b antibody (M1/70, BD Biosciences) and an APC-conjugated anti-CD206 antibody (eBioscience). Stained samples were sorted with a FACSAria II cell sorter (BD Biosciences), and the data were collected and analyzed using the FACSDiva (BD Biosciences) and FlowJo (Tree Star, USA) software packages.
qRT-PCR analysis
Total RNA was treated with RQ1 RNase-Free DNase (Prome-ga). Reverse transcription was performed with the PrimeScript First-Strand cDNA Synthesis kit (TaKaRa Bio, Japan). qRT-PCR was performed with the SYBR Green PCR Master Mix (TaKaRa Bio) on an ABI PRISM 7900 Sequence detection system with gene-specific primers (Supplementary Table S1).
Statistical analysis
All of the graphs show the mean ± SD of at least three independent experiments. Student’s t-tests were used to identify statistically significant differences between groups, using P < 0.05 as the cut-off. To determine the significance of the differences in expression of TIE2 and CD31 between groups, Fisher’s exact test was used to analyze the differences in the expression of TIE2 and CD31 between groups. P < 0.05 was considered to be significant.
RESULTS
Macrophage infiltration of MLNs in the orthotropic B16F1 melanoma model
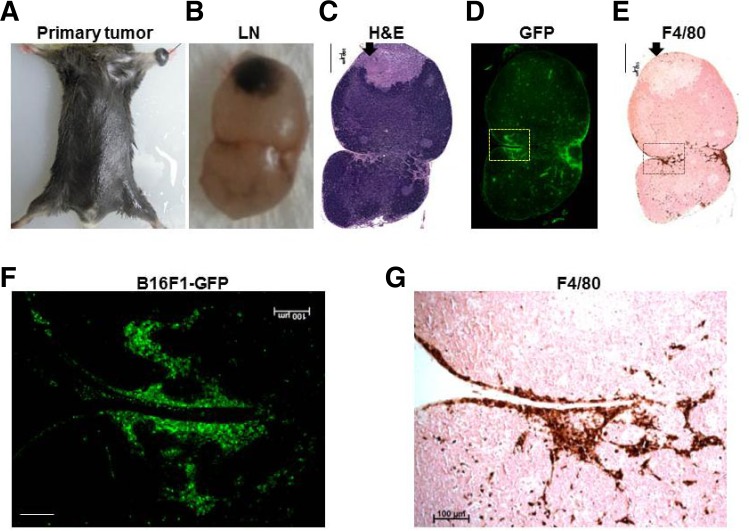
To investigate the significant role of macrophages in MLNs, we initially used the orthotropic B16F1 melanoma mouse model, which is useful for studies of metastasis because it consistently results in metastasis in the brachial or axillary node after melanoma implantation (Hill et al., 1984). We inoculated B16F1 melanoma cells harboring GFP (B16F1-GFP) (4 × 105 per mouse) to 8-week-old C57BL/6 male mice into the left front footpad. Tumors were allowed to grow to approximately 1.0 cm in diameter, until about 14 days after implantation (Fig. 1A), at which point we obtained axillary MLNs from B16F1 melanoma mice (Fig. 1B) and histopathologically confirmed the presence of LN metastasis by H&E staining (Fig. 1C) and GFP imaging (Fig. 1D).
Fig. 1.
Histopathological analysis of a metastatic lymph nodes from a B16F1-GFP melanoma mouse. (A) Representative B16F1-GFP melanoma mouse with a primary tumor. (B-E) Histological analyses of a representative metastatic lymph node section: gross photograph (B), H&E staining (C), GFP fluorescence (D), and IHC staining with the F4/80 antibody (E). Scale bars, 500 μm. The arrow indicates necrotic regions of the tumor. (F-G) Magnified views of fluorescence images of the rectangular regions (F) and IHC staining of the same magnified rectangular regions (G). Scale bars, 100 μm.
To confirm whether macrophages are recruited to MLNs, immunohistochemical staining was performed using an anti-F4/80 (a macrophage-specific marker) antibody. MLN sections with the anti-F4/80 antibody correlated well with the presence of tumor cells, as verified by the GFP images (Figs. 1D and 1F). Most F4/80+ macrophages were located along the invasive front of the MLNs (Figs. 1E and 1G), rather than the necrotic regions of the tumor mass (Figs. 1C and 1E, arrow). Consistent with previous results (Qian and Pollard, 2010), our data suggest that the preferential recruitment of macrophages to GFP+ tumor cells may stimulate tumor cell migration or invasion.
Gene expression profiles of TAMs are distinct from those of tissue or inflammatory macrophages
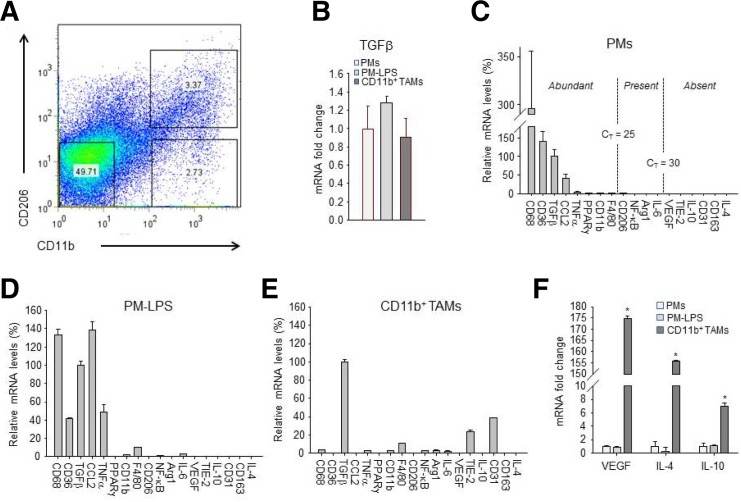
To identify TAM-specific markers that are distinct from tissue or inflammatory macrophages, we isolated a pure population of CD11b+ macrophages as primary TAMs from MLNs by FACS (Fig. 2A). To screen a panel of cytokines and cell surface markers associated with the different phenotypes of macrophages under various conditions (Mantovani and Sica, 2010; Pollard, 2009), we performed qRT-PCR to compare the gene expression profiles of TAMs with those of either PMs or LPS-stimulated PMs.
Fig. 2.
Isolation of CD11b+ TAMs from metastatic lymph nodes and comparative expression profiles of 18 genes associated with the macrophage phenotype for PMs, LPS-stimulated PMs (PM-LPS), and CD11b+ TAMs. (A) TAMs were isolated from metastatic lymph nodes of 24 B16F1 melanoma mice each time, and stained with anti-CD11b and anti-CD206 antibodies before sorting by FACS. (B) mRNA expression of TGF-β in PMs, PM-LPS, and FACS-sorted CD11b+ TAMs from metastatic lymph nodes. Data are presented as means ± SE for each group (n = 3). *P < 0.05, compared to PMs. (C-E) qRT-PCR analysis of gene expression in PMs (C), PM-LPS (D), and FACS-sorted CD11b+ TAMs (E) from metastatic lymph nodes (n = 24). All values are expressed relative to cyclophilin A and adjusted arithmetically to depict the TGF-β expression for each macro-Phage as 100 units. Values represent the means and ± SE of three independent samples for each macrophage. Arbitrary cut-offs were set at CT < 25 (abundant), 25 < CT < 30 (present), and CT > 30 (absent), as shown by broken lines in PM. For VEGF, IL-4, and IL-10, all values are expressed as mean fold-change relative to PMs. (F) mRNA expression in PMs, PM-LPS, and FACS-sorted CD11b+ TAMs from metastatic lymph nodes. Data are presented as means ± SE for each group (n = 3). *P < 0.05, compared to PMs.
This allowed for rank order determination of mRNA levels for 18 genes within a given cell type. We found that these macrophages exhibited relatively high levels of TGF-β expression, but there were no significant differences between the types of macrophages (Fig. 2B). When the values were adjusted arithmetically to depict the TGF-β expression for each macrophage as 100 units, the relative expression profiles clearly showed that CD68, CD36, TGF-β, and CCL2 were the most abundantly expressed genes in PMs (Fig. 2C). In response to LPS, the LPS-stimulated PMs highly overexpressed pro-inflammatory cytokines such as TNF-α and IL-6 through NF-κB (p65) activation (Fig. 2D). CD11b+ TAMs showed markedly decreased expression levels of mRNAs for CCL2, CD36, and CD68 but the expression levels of mRNAs for CD31 and TIE2 were significantly upregulated in TAMs compared with PMs or LPS-stimulated PMs (Figs. 2D and 2E). Interestingly, the most abundant protein in CD11b+ TAMs was TGF-β, whose mRNA levels were equivalent to the housekeeping gene cyclophilin A (CT ∼ 18.5). In addition, mRNA levels of VEGF, IL-4, and IL-10 were increased 174.8-, 154.7-, and 7.0-fold, respectively, in CD11b+ TAMs compared with PMs, or LPS-stimulated PMs (Fig. 2F). Overall, the expression profiles of TAMs from MLNs show that TAMs significantly overexpress immunosuppressive cytokines (IL-4, IL-10, and TGFβ), as well as proangiogenic factors (VEGF, TIE2, and CD31) compared with PMs or LPS-stimulated PMs. This suggests that TAMs may result from the tumor microenvironment (Jenkins et al., 2011), rather than classical inflammation, and may provide an immunosuppressive microenvironment for tumor growth (Qian and Pollard, 2010).
TIE2+/CD31+ macrophages are the predominant population of TAMs that infiltrate MLNs from B16F1 melanoma cells
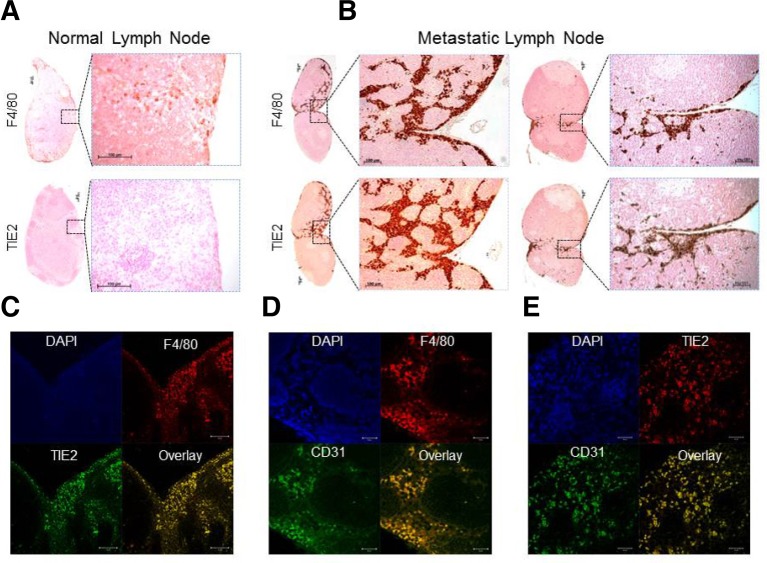
To confirm that the gene expression profiles were correlated with protein levels, we stained both healthy LNs and MLNs for anti-TIE2 or anti-F4/80 by IHC. Normal LN-resident macrophages expressed F4/80+ but not TIE2 (angiopoietin receptor 2) protein (Fig. 3A). However, MLNs strongly expressed TIE2 protein, which correlated well with F4/80 staining, indicating that TIE2+ macrophages were predominantly localized within MLNs. Furthermore, the majority of F4/80+/TIE2+ macrophages were located along the invasive front of MLNs, not in necrotic regions of tumors (Fig. 3B). To extend the expression profiling of TIE2+ macrophages to different tissues, we performed IHC analysis of TIE2 and anti-F4/80 in a range of normal mouse tissues. In most of the tissue macrophages tested (e.g., those from the kidney, liver, and spleen), TIE2 was not detectable, although intense expression of F4/80 protein was detectable in normal tissue macrophages (Supplementary Fig. S1), indicating that TIE2+ macrophages are only found in MLNs. Thus, TIE2 expression is strongly upregulated in TAMs within metastatic tumor regions but not in normal tissue macrophages, suggesting that it could be a specific marker for TAMs.
Fig. 3.
Immunological assessment of TIE2 and CD31 protein as TAM-specific markers of micro- or macro-metastasis. (A) IHC staining of serial sections from normal lymph nodes using anti-F4/80 and anti-TIE2 antibodies. Boxed regions (left) are magnified views of the right panel. Scale bars, 100 μm. (B) IHC staining of micro-meta-static lymph nodes (left) and macro-metastatic lymph nodes (right) using anti-F4/80 and anti-TIE2 antibodies. Boxed regions are shown in magnified views in the right panel. Scale bars, 100 μm. (C-E) Double immunofluorescence staining of a representative metastatic LN section using anti-TIE2 and anti-F4/80 antibodies (C), anti-F4/80 and anti-CD31 antibodies (D), and anti-TIE2 and anti-CD31 antibodies (E). Scale bars (left to right), 50, 20, and 20 μm.
To confirm whether TIE2 or CD31 are expressed on the surface of macrophages, we performed co-immunostaining with F4/80. Consistent with the IHC data, our studies clearly show that TIE2+ macrophages had an approximate 96% overlap with F4/80+ macrophages (Supplementary Fig. S2), suggesting that TIE2+ macrophages constitute the major population of TAMs that infiltrate MLNs. This contradicts previous reports that TIE2-expressing monocytes/macrophages (TEMs) can be classified into F4/80+/TIE2+ and F4/80+/TIE2−subsets (Welford et al., 2011). Furthermore, immunofluorescence analysis showed that TIE2 or CD31 are highly overexpressed on the surface of the macrophages, confirming that TIE2 or CD31-expressing cells are morphologically normal macrophages that infiltrate MLNs (Figs. 3C and 3D). Moreover, TIE2+ macrophages were co-localized with CD31+ macrophages in MLNs (Fig. 3E). Our findings therefore strongly suggest that TIE2+/CD31+ macrophages are a TAM-specific hallmark of tumor metastasis that allows discrimination between TAMs and normal tissue macrophages, providing more reliable and accurate diagnosis of tumor metastasis in sentinel LN biopsies from human patients.
Exclusive expression of proangiogenic TIE2+/CD31+ macrophages in MLNs from human breast tumors
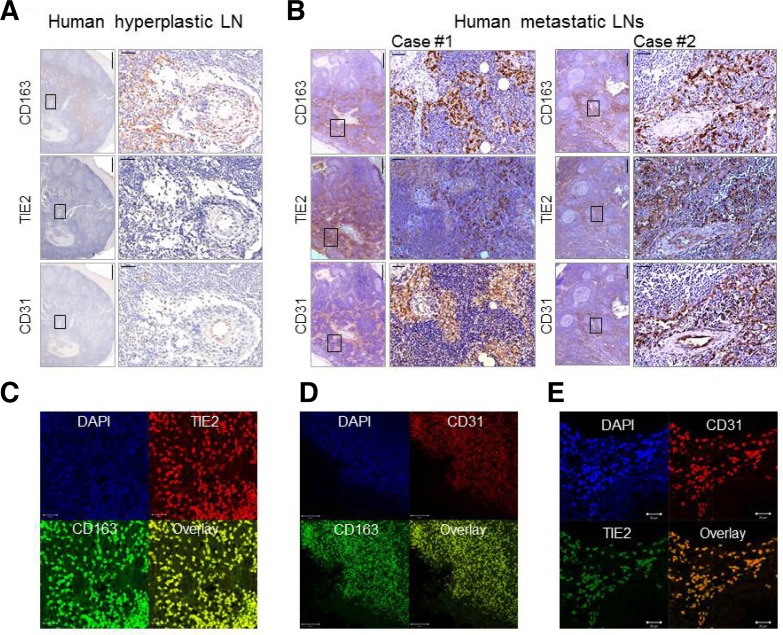
To determine whether our findings are relevant for human samples, we obtained sentinel LNs from human breast cancer patients. We stained both benign hyperplastic LNs and MLNs by IHC with anti-CD163, anti-TIE2, and anti-CD31 antibodies. Because CD163 is expressed exclusively on monocytes and macrophages (Heusinkveld and van der Burg, 2011), we selected it as a macrophage marker for the human samples. CD163+ macrophages were highly accumulated in both hyperplastic LNs (n = 5) and MLNs (n = 36), suggesting that CD163 does not discriminate between TAMs and inflammatory macrophages. However, TIE2 or CD31 immunoreactivity was significantly different between hyperplastic LNs and MLNs (p < 0.0001). Expression was not detected in hyperplastic LNs (0/5, 0%), while TIE2+ or CD31+ macrophages infiltrated the MLNs in all cases of human breast cancer (36/36, 100%). TIE2 and CD31 proteins were also detectable in the endothelial cells of blood vessels both in hyperplastic LNs (Fig. 4A), and MLNs (Fig. 4B). However, TIE2+ and CD31+ macrophages are normal macrophages that morphologically differ significantly from endothelial cells (Fig. 4B), suggesting that TIE2+ and CD31+ macrophages are unique populations of TAMs within MLNs in human breast cancer patients.
Fig. 4.
Assessment of CD163, TIE2, and CD31 expression in reactive hyperplastic lymph nodes and metastatic lymph nodes from healthy and breast tumor-bearing human subjects. (A) Immunohistochemical images for anti-CD163, anti-TIE2, and anti-CD31 antibodies are shown for representative reactive hyperplastic lymph nodes from human subjects. Boxed regions are shown as magnified views in the right panel. Scale bars, 500 μm (left) and 50 μm (right). (B) Immunohisto-chemical images for anti-CD163, anti-TIE2, and anti-CD31 antibodies are shown for metastatic lymph nodes from breast tumor-bearing human subjects. Boxed regions are shown as magnified views in the right panel. Scale bars, 500 μm (left) and 50 μm (right). (C-E) Double immunofluorescence staining of two representative metastatic lymph node sections from a breast tumor-bearing human subject using anti-TIE2 and anti-CD163 antibodies (C), anti-CD31 and anti-CD163 antibodies (D), and anti-CD31 and anti-TIE2 antibodies (E), Scale bars (left to right), 20, 50, and 20 μm.
Next, we investigated whether TIE2+ and CD31+ cells in MLNs are macrophage subsets. Immunofluorescence analysis clearly showed that TIE2+ and CD31+ cells were highly co-localized with CD163, a macrophage marker (Figs. 4C and 4D), confirming that these cells are morphologically normal macrophages. Immunofluorescence staining with anti-TIE2 and anti-CD31 antibodies further showed that TIE2 expression overlapped with CD31 expression within endothelial layers of blood vessels in MLNs, but the fluorescence intensities of TIE2 and CD31 were dramatically lower than those in macrophages (Supplementary Fig. 3S). Consistent with the gene expression profiles of TAMs, these results further confirmed that the expression levels of TIE2 and CD31 in TAMs were significantly upregulated compared to endothelial cells. Furthermore, immunofluorescence analysis of invasive regions of MLNs clearly revealed that TIE2+ macrophages had ∼90% overlap with CD31+ macrophage staining (Fig. 4E), confirming that TIE2+/CD31+ macrophages represent the predominant population of TAMs that infiltrate MLNs from human breast cancer patients. These findings further suggest that TIE2+/CD31+ macrophages may be a good diagnostic indicator of tumor metastasis, in that they clearly differentiate between metastasis and inflammatory responses, such as reactive hyperplasia, which often contribute to misdiagnoses.
DISCUSSION
In this study, we clearly showed that TAMs highly overexpress immunosuppressive cytokines (e.g., IL-4, IL-10, and TGF-β) as well as proangiogenic factors (e.g., VEGF, TIE2, and CD31). Immunological analysis further demonstrated that proangiogenic TIE2+/CD31+ macrophages are unique and predominant populations of TAMs in MLNs from both an experimental model of melanoma and human breast cancer patients, but not normal LNs or reactive hyperplastic LNs. Thus, our studies demonstrated that proangiogenic TIE2+/CD31+ macrophages are TAM- specific markers that can be used to distinguish between tissue and inflammatory macrophages, suggesting that our data may be of high clinical relevance for the diagnosis of tumor metastasis and may provide potential targets for TAM-specific cancer therapy.
Most clinical studies have used CD68 or CD163 as a marker for the clinical identification of TAMs. However, these surface markers recognize most macrophages, including proinflammatory and tissue macrophages as well as TAMs (Heusinkveld and van der Burg, 2011; Quatromoni and Eruslanov, 2012). This lack of unique surface markers for TAMs has made it difficult to pinpoint the significance of TAMs in tumorigenesis. To address this, we demonstrated using the experimental B16F1 melanoma model that TIE2+/CD31+ macrophages are found exclusively within tumor regions and constitute predominant populations of TAMs that infiltrate MLNs. Neither TIE2 nor CD31 protein was expressed in inflammatory macrophages or other tissue macrophages. These findings strongly suggest that TIE2+/ CD31+ macrophages might be useful as TAM-specific marker for clinical diagnosis of MLNs.
Accumulating evidence indicates that TAMs play an important role in regulating angiogenesis. In previous studies, depleting TAMs with clodronate (Zeisberger et al., 2006) or CD11b-neutralizing antibody (Ahn et al., 2010) inhibited tumor angiogenesis and reduced blood vessel density in tumor tissues, suggesting that TAMs promote angiogenesis in the tumor microenvironment. However, the mechanisms by which TAMs promote angiogenesis are incompletely understood. Our studies may provide evidences that TAMs may play an important role in tumor angiogenesis because they highly overexpress various proangiogenic markers such as VEGF, TIE2, and CD31. Importantly, TIE2+ or CD31+ macrophages in solid tumors have been distinguished from endothelial cells and inflammatory macrophages (McKenney et al., 2001; Pucci et al., 2009). The genetic depletion of TIE2+ macrophages inhibits tumor angiogenesis in various subcutaneous tumor models (Mazzieri et al., 2011). Moreover, proangiogenic roles of CD31+ or TIE2+ macrophages have been highlighted by experimental models of parabiosis (Kim et al., 2009) and murine tumor models (Mazzieri et al., 2011). These data provide important evidence in support of our hypothesis that CD31+ macrophages as well as TIE2+ macrophages have pivotal roles in tumor angiogenesis. These findings, together with our results, strongly suggest that TAMs express proangiogenic TIE2 and/or CD31 protein within tumor regions.
Moreover, co-localization analysis further demonstrated that TAMs in MLNs expressed both TIE2 and CD31, which correlated well with F4/80, a macrophage marker, in the experimental B16F1 melanoma mouse model. Clinically, these findings are highly relevant for MLNs from human breast cancer patients, as we found that TIE2 and CD31 were also co-expressed in TAMs in MLNs from human breast cancer patients, and that this expression correlated strongly with the macrophage marker CD163. Notably, these CD31+/TIE2+ macrophages were morphologically normal macrophages that are distinguishable from endothelial cells and inflammatory macrophages. Although TIE2+ or CD31+ macrophage infiltration has been independently highlighted by experimental tumor models (Garcia et al., 2012; Giatromanolaki et al., 1998; Kim et al., 2009; Mazzieri et al., 2011; McKenney et al., 2001; Pucci et al., 2009) and TIE2+ macrophages can be classified into distinct subsets of TAMs (Welford et al., 2011), these important studies did not find that both TIE2 and CD31 are co-localized in the same macrophages infiltrating solid tumors or MLNs. Until recently, there had been a lack of direct evidence that these TIE2+/CD31+ macrophages represent a notable proportion of TAMs in MLNs from both an experimental mouse model and human cancer patients. However, for the first time our studies demonstrate that TIE2+/CD31+ macrophages form the major population of TAMs that comprise TIE2+ macrophages in human MLNs that are distinct from tissue or inflammatory macrophages. Importantly, our study provides strong evidences that these TAMs may play an important role in tumor angiogenesis because they highly overexpress various proangiogenic markers such as VEGF, TIE2, and CD31.
In conclusion, our studies suggest that proangiogenic TIE2+/ CD31+ macrophages are a TAM-specific hallmark of tumor metastasis for clinical diagnosis as well as a potential therapeutic target for the treatment of metastasis. Pharmacological inhibition of proangiogenic TIE2+/CD31+ macrophages may be a novel and promising strategy for future cancer therapies.
Acknowledgments
This study was supported by grants from the Basic Science Research Program (2010-0024500) of the National Research Foundation, the Korea Health Technology R&D Project of the Ministry of Health &Welfare (A111345), and the Next-Generation BioGreen 21 Program (no. PJ00954001) of the Rural Development Administration, Republic of Korea.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl. Acad. Sci. USA. 2010;107:8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei YY, Landry JP, Sun YS, Zhu XD, Luo JT, Wang XB, Lam KS. A novel high-throughput scanning microscope for label-free detection of protein and small-molecule chemical microarrays. Rev Sci Instrum. 2008;79:013708. doi: 10.1063/1.2830286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Sandi MJ, Cordelier P, Binetruy B, Pouyssegur J, Iovanna JL, Tournaire R. Tie1 deficiency induces endothelial-mesenchymal transition. EMBO Rep. 2012;13:431–439. doi: 10.1038/embor.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giatromanolaki A, Koukourakis MI, Kakolyris S, Kaklamanis L, Barbatis K, O’Byrne KJ, Theodosssiou D, Harris AL, Gatter KC. Focal expression of thymidine phosphorylase associates with CD31 positive lymphocytic aggregation and local neoangiogenesis in non-small cell lung cancer. Anticancer Res. 1998;18:71–76. [PubMed] [Google Scholar]
- Groblewska M, Mroczko B, Wereszczynska-Siemiatkowska U, Mysliwiec P, Kedra B, Szmitkowski M. Serum levels of granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) in pancreatic cancer patients. Clin Chem Lab Med. 2007;45:30–34. doi: 10.1515/CCLM.2007.025. [DOI] [PubMed] [Google Scholar]
- Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RP, Chambers AF, Ling V, Harris JF. Dynamic heterogeneity: rapid generation of metastatic variants in mouse B16 melanoma cells. Science. 1984;224:998–1001. doi: 10.1126/science.6719130. [DOI] [PubMed] [Google Scholar]
- Huang S, Xie K, Bucana CD, Ullrich SE, Bar-Eli M. Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clin Cancer Res. 1996;2:1969–1979. [PubMed] [Google Scholar]
- Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Min HS, Lee KH, Kim YJ, Oh DY, Jeon YK, Lee SH, Im SA, Chung DH, Kim YT, et al. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br. J. Cancer. 2008;98:1118–1124. doi: 10.1038/sj.bjc.6604256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim JS, Papadopoulos J, Wook Kim S, Maya M, Zhang F, He J, Fan D, Langley R, Fidler IJ. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am J Pathol. 2009;174:1972–1980. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, Lu J, Liu J. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol. Cancer Res. Treat. 2013;12:259–267. doi: 10.7785/tcrt.2012.500312. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- McKenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol. 2001;25:1167–1173. doi: 10.1097/00000478-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, Di Serio C, Naldini L, De Palma M. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376–389. [PMC free article] [PubMed] [Google Scholar]
- Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr. Relat. Cancer. 2008;15:1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139:839–846. doi: 10.1016/j.surg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969–1973. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res. 2003;9:853–860. [PubMed] [Google Scholar]
- Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Kim C, Kim MJ, Schwendener RA, Alitalo K, Heston W, Kim I, Kim WJ, Koh GY. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol. Cancer. 2011;10:36. doi: 10.1186/1476-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br. J. Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers PH, Kenter GG, Gorter A. The absence of CCL2 expression in cervical carcinoma is associated with increased survival and loss of heterozygosity at 17q11.2. J Pathol. 2006;208:507–517. doi: 10.1002/path.1918. [DOI] [PubMed] [Google Scholar]