Abstract
To avoid host immune surveillance, human cytomegalovirus (HCMV) encoded endoplasmic reticulum (ER)-membrane glycoprotein US2, which interferes with antigen presenting mechanism of major histocompatibility complex (MHC) class Ia and class II molecules. However, not many attempts have been made to study the effect of HCMV US2 on the expression of MHC class Ib molecules. In this study, we examined the effect of HCMV US2 on the expression and function of human CD1d (hCD1d), which presents glycolipid antigens to invariant NKT (iNKT) cells. Our results clearly showed that the physiological interaction between ER lumenal domain of HCMV US2 and α3 domain of hCD1d was observed within ER. Compared with mature form of hCD1d, immature form of hCD1d is more susceptible to ubiquitin-dependent proteasomal degradation mediated by HCMV US2. Moreover, the ectopic expression of HCMV US2 leads to the down-modulation of iNKT cell activity without significant change of hCD1d expression. These results will advance our understanding of the function of HCMV US2 in immune evasive mechanisms against anti-viral immunity of iNKT cells.
Keywords: antigen presentation, HCMV US2 protein, human CD1d, human cytomegalovirus, invariant NKT cell
INTRODUCTION
Several viruses have developed multiple strategies to escape from host immune surveillance mechanisms during specific viral infection. Particularly, human cytomegalovirus (HCMV) encodes unique short glycoproteins (US2, US3, US6, US11) expressed during the certain stages of the HCMV infection to avoid immune responses (Pinto and Hill, 2005; Ploegh, 1998; Seriger et al., 2006). These unique short glycoproteins inhibit the antigen presenting ability of major histocompatibility complex (MHC) class Ia and class II which leads to the down-regulation of T cell activity (Tomazin et al., 1999; Wiertz et al., 1996).
Among several glycoproteins derived from HCMV, US2 can make direct interactions with MHC class Ia or MHC class II molecules (HLA-DM and HLA-DR) and this interaction induces the translocation of MHC class Ia heavy chain or MHC class II α chain from endoplasmic reticulum (ER) to cytoplasm (Tomazin et al., 1999; Wiertz et al., 1996). Consequently, dislocated MHC molecules from ER to cytoplasm are degraded by ubiqui-tin-dependent proteasome complex (Tomazin et al., 1999; Wiertz et al., 1996). The degradation of MHC class Ia or class II molecules leads to inhibit antigen presenting mechanism. Subsequently, HCMV can escape from immune surveillance by CD8+, as well as CD4+ T cells.
MHC class Ib molecules, structurally similar to MHC class Ia molecules, have antigen binding motifs and can function as antigen presenting molecules (Hansen et al., 2007; Rodgers and Cook, 2005). Each MHC class Ib molecule can recognize unique antigen to present antigenic repertoire to activate certain types of T cells (Hansen et al., 2007; Rodgers and Cook, 2005). Compared with those of MHC class Ia molecules, the antigen binding motifs of MHC class Ib molecules are relatively much less polymorphic (Hansen et al., 2007; Rodgers and Cook, 2005). This evidence indicates that each MHC class Ib molecule is evolved as a specialized antigen presenting molecule, adapted to each unique antigen derived from specific pathogens (Hansen et al., 2007; Rodgers and Cook, 2005).
CD1d is very unique among MHC class Ib molecules because CD1d can present lipid antigens instead of conventional peptide antigens (Cohen et al., 2009; Moodey et al., 2005). The nature of T cell subset received antigenic repertoire from CD1d is also unique, so called “invariant NKT cells (iNKT cells)”, since they express both NK cell receptor and invariant T cell receptor (TCR) (Bendelac et al., 2007).
The iNKT cells reside primarily in the thymus and liver; major subsets of iNKT cells are activated when they receive glycolipid (α-galactosylceramide; KRN 7000) delivered from CD1d molecules (Kawano et al., 1997). Since activated iNKT cells promptly release large quantities of cytokines, such as IL-4 and IFN-γ upon activation, iNKT cells have an added immunoregulatory role to bridge between innate and adaptive immunity (Bendelac et al., 2007; Kawano et al., 1997). In support of this idea, several studies have demonstrated that iNKT cells are important to generate the protective immunity against specific parasitic or bacterial infections (Chackerian et al., 2002; Gonzalez-Aseguinolaza et al., 2000). In addition, iNKT cells have an anti-cancer activity (Kawano et al., 1998) and are used as the therapeutics to treat several autoimmune diseases (Wang et al., 2001; Yang et al., 2004). Therefore, the regulation of iNKT cell activity is considered to be a key to the development of immunotherapy against human diseases.
The importance of iNKT cell activity is also emphasized in the protection against several viral diseases. The phenotype of CD1d-deficient mice is much more susceptible to different types of viral infection (De Santo et al., 2008; Grubor-Bauk et al., 2003; Ilyinskii et al., 2006). In addition, activation of iNKT cells by α-galactosylceramide (KRN 7000) through presentation by CD1d enhances the protection of host animals during several tested viral infections (Johnson et al., 2002; Kakimi et al., 2000; van Dommelen et al., 2003). However, not many studies have shown the viral influence on the regulation of iNKT cell activity during infection. In particular, HCMV US2 is well known for modulation of host immunity by down-regulation of MHC class Ia and class II molecules (Tomazin et al., 1999; Wiertz et al., 1996); it is worthwhile to exam the effect of HCMV US2 on the expression or the antigen presenting function of human CD1d (hCD1d) molecule, which present glycolipid antigens to iNKT cells.
In this study, we examined the effect of HCMV US2 on the expression or antigen presenting function of hCD1d molecule. Results clearly indicated that the direct physiological interaction between HCMV US2 and hCD1d leads to the degradation of hCD1d in an ubiquitin-dependent manner. Moreover, HCMV US2 leads to the down-modulation of iNKT cell activity. These results will help our understanding of the mechanism on how specific virus avoids immune surveillance of iNKT cells during specific viral infection.
MATERIALS AND METHODS
Cell culture and antibodies
C1R cell line (human B cell) and Jurkat cell line (human T cell) were obtained from American Type Culture Collection (USA) and DN32.D3 (iNKT hybridoma) cell line was previously described (Park et al., 1998). C1R cells, Jurkat cells, DN32.D3 cells were maintained at 37°C and 5% CO2 in RPMI medium (Invitrogen, USA) supplemented with 10% (v/v) FBS and 1% penicillin/streptomycin (Invitrogen).
The PE conjugated anti-hCD1d antibody (CD1d42 clone, cat # 550255), PE conjugated anti-HLA class Ia (HLA-A, B, C; G46-2.6 clone, cat # 555553) antibody, and PE conjugated isotype control (mouse IgG1κ, cat # 5501617) were purchased from BD Biosciences (USA). The purified mouse anti-hCD1d antibody (C3D5 clone, cat # sc-19632), purified mouse anti-Myc antibody (cat # sc-40), and purified anti-ubiquitin antibody (cat # sc-9133) were purchased from Santa Cruz Biotechnology, Inc. (USA). The purified anti-hCD1d antibody (NOR3.2 clone, cat # MCA982G) was purchased from AbD serotec (UK) and the purified anti-hCD1d antibody (51.1 clone, cat # 14-0016) was purchased from eBioscience, Inc. (USA). The purified anti-β-actin (cat # A5441) antibody was purchased from Sigma (USA). The purified rabbit anti-calreticulin antibody (cat # Ab2907) was purchased from abcam (UK) and Cy3 conjugated goat anti-mouse IgG antibody (cat # 115-165-146) was purchased from Jackson ImmunoResearch laboratories, Inc. (USA). The Alexa 488 conjugated goat anti-mouse IgG antibody (cat # A11001), Alexa Fluor 488 conjugated donkey anti-rabbit IgG antibody (cat # A21206), and Alexa Fluor 594 conjugated donkey anti-rabbit IgG antibody (cat # A21442) were purchased from Invitrogen.
Subcloning of deletion mutants of hCD1d and HCMV US2
Three deletion mutants (Met1-Gln202, Val203-Trp295, Gly296-Leu335) of hCD1d were isolated by PCR using the combination of the following primers (hCD1d-F1, 5′-ACGCGTCGACATGGGGT GCCTGCTG-3′; hCD1d-R1, 5′-ACGCGTCGACTTGCTTCTTC AGTTC-3′; hCD1d-F2, 5′-ACGCGTCGACGTGAAGCCCAAG GCC-3′; hCD1d-R2, 5′-ACGCGTCGACCCAGTAGAGGACG AT-3′; hCD1d-F3, 5′-ACGCGTCGACGGTGGGAGCTACACC-3′; hCD1d-R3, 5′-ACGCGTCGACGGAGGTAAAGCCCAC-3′). PCR products spanning each fragment were cloned into the EcoRI and SalI restriction enzyme sites of the pGilda. Three deletion mutants (Met1-Leu21, Pro22-Thr160, His161-Cys199) of HCMV US2 were isolated by PCR using the combination of the following primers (US2-F1, 5′-CGGGAATTCATGAACAATCTC TGGAAA-3′; US2-R1, 5′-ATTCTCGAGTCACAGGCGGATCA AGGG-3′; US2-F2, 5′-CGGGAATTCCCCGATGGAATCACT AAA-3′; US2-R2, 5′-ATTCTCGAGTCACGTGTATGACTTCCG-3′; US2-F3, 5′-CGGGAATTCCATGTGGCCTGGACAATA-3′; US2-R3, 5′-ATTCTCGAGTCAGCACACGAAAAACCG-3′). PCR products spanning each fragment were cloned into the EcoRI and XhoI restriction enzyme sites of the pJG4-5. Each constructed plasmid was introduced into yeast EGY48 expressing either hCD1d or HCMV US2 hybrid protein.
Quantitation of interaction between hCD1d and HCMV US2
The activity of the interaction between hCD1d and HCMV US2 was verified via measurements of the relative levels of β-galactosidase expression. The β-galactosidase assay was conducted in accordance with the previously described protocols with a slight modification (Lee et al., 2008). In brief, yeast cells (EGY48 strain) containing each construct were cultured in yeast synthetic media (Ura−, His−, Trp−) with 2% (w/v) glucose until they achieved mid-growth phase. Then, the cells were transferred to a yeast medium (Ura−, His−, Trp−) containing 2% (w/v) galactose and 0.2% dimethylsulfoxide (Me2SO). After transformation, equivalent numbers of cells were lysed in 0.7 ml of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 50 mM β-mercaptoethanol, pH 7.0) containing 50 μl of 0.1% SDS and 50 μl of chloroform for 30 s at 30°C. β-Galactosidase activity was then measured via the addition of 140 μl of 4 mg/ml σ-nitrophenyl β-D-galactopyranoside (σNPG). The reaction was conducted at 30°C until a yellow color formed, and then, it was quenched via the addition of 0.4 ml of 1 M Na2CO3. Then, the samples were briefly centrifuged to remove any remaining cell debris, and the absorbance was measured at wavelengths of 420 nm and 550 nm. β-galactosidase activity was calculated using the formula units = [1000 × (A420 - 1.75 × A550)]/(time × volume × A600).
Ectopic expression of hCD1d and HCMV US2 proteins
A cDNA segment corresponding to the hCD1d (GenBank access NM_001766) was subcloned into the pLNCX2 retroviral vector (Invitrogen) through NotI restriction enzyme sites. The cDNA encoding hCD1d in the pLNCX2 was introduced into the 293GPG retrovirus packaging cell line via transfection with Lipofectamine (Invitrogen). After three days, the supernatants were harvested and infected with C1R cells using polybrene (1 μg/ml). After retroviral transduction, hCD1d introduced C1R cells (C1R.hCD1d) were selected with 1.5 mg/ml of neomycin to establish a stable cell line (Invitrogen).
A cDNA segment corresponding to the HCMV US2 was kindly provided by Dr. Kwangseog Ahn (Department of Biological Sciences, Seoul National University, Korea) and the pEGFBsd-IRES3-CL retroviral vector was kindly provided by Dr. Chang-Hwan Park (Graduate School of Biomedical and Engineering, Hanyang University, Korea). For expressing HCMV US2 in C1R.hCD1d cells, a cDNA segment corresponding to the HCMV US2 was subcloned through BamHI restriction enzyme sites into pEGFBsd-IRES3-CL retroviral vector to make cDNA encoding an EGFP fusion HCMV US2 protein (EGFP-US2). The EGFP-US2 cDNA was sequenced by the dideoxy-mediated chain termination method using a 373 automatic sequencer (PerkinElmer, USA). The full-length cDNA encoding EGFP-US2 was found to be identical to the open reading frame of egfp cDNA (GenBank access NM_U55763.1) and HCMV strain AD169 US2 cDNA (GenBank access X17403.1). The cDNA encoding EGFP-US2 in the pEGFBsd-IRES3-CL vector was introduced into the 293GPG retrovirus packaging cell line via transfection with Lipofectamine (Invitrogen). After three days, the supernatants were harvested and infected with C1R.hCD1d cells and Jurkat cells using polybrene (1 μg/ml). As a control, retrovirus generated with an empty pEGFBsd-IRES3-CL retro-viral vector (Mock) was also infected with C1R.hCD1d cells and Jurkat cells. After retroviral transduction, EGFP-US2 or empty vector introduced C1R.hCD1d cells and Jurkat cells were selected with 1 μg/ml of brasticidine (Invitrogen).
Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses
For the RT-PCR analyses, total RNAs from either empty vector or EGFP-US2 introduced C1R.hCD1d cells (1 × 107 cells) and Jurkat cells (1 × 107 cells) were isolated using a Trizol reagent (Invitrogen) according to the manufacturer’s instructions and reverse transcribed into cDNA using the First-Strand cDNA Synthesis Kit with random hexamer and SuperScript RT (Invitrogen). After cDNA synthesis, PCR was conducted using a PTC-100 Thermal Cycler (MJ Research, Inc., USA) for 25 cycles of 1 min at 94°C, 30 s at 65°C, and 30 s at 72°C, followed by a 10 min final extension step at 72°C. Primers for HCMV US2 were designed based on the cDNA sequence of HCMV strain AD169 US2 cDNA (GenBank access X17403.1). The forward primer used was 5′-ATGAACAATCTCTGGAA AGCCTGG-3′, and the reverse primer used was 5′-TCAGCAC ACGAAAAACCGCATCCA-3′. The RT-PCR product was 600 bp in length. Primers for Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) used as an internal control were designed based on the cDNA sequence of human Gapdh (GenBank access NM_002046). The primer sequences for mouse Gapdh were as follows: 5′-ATGACCACAGTCCATGCCATC-3′ (sense), 5′-CCTGCTTCACCACCTTCTTG-3′ (anti-sense), resulting in a 271 bp RT-PCR product. The resulting PCR products were loaded onto a 1.5% agarose gel containing ethidium bromide, which were visualized using ultraviolet light.
Flow cytometry analyses
Flow cytometry analyses were performed to determine expressions of EGFP, HLA class Ia, and hCD1d of either empty vector or EGFP-US2 introduced C1R.hCD1d cells and Jurkat cells. Briefly, cells were harvested and washed three times with cold PBS and incubated with each relevant antibody on ice in Hank’s balanced salt solution (Invitrogen) containing 2% FBS and 0.1% sodium azide (Sigma). For intracellular staining, cells were fixed using 4% paraformaldehyde and permeabilized with 0.15% saponin in PBS containing 3% BSA before staining with each relevant antibody. Antibodies used in flow cytometry analyses were PE conjugated anti-hCD1d antibody (CD1d42 clone, mouse IgG1κ), PE conjugated anti-HLA class Ia (HLA-A, B, C; G46-2.6 clone, mouse IgG1κ) antibody, and PE conjugated isotype control (mouse IgG1κ). All stained cells were analyzed via flow cytometry using a FACSCalibur™ system with Cell-Quest™ software (BD Biosciences).
Confocal microscopy
To observe the EGFP expression of EGFP-US2 or empty vector introduced C1R.hCD1d cells, confocal microscopy was performed according to previous method with slight modification (Cho and Whang, 2011). Briefly, cells were washed three times with PBS and fixed with 4% paraformaldehyde in PBS at room temperature for 10 min. After washing, GFP expressions of cells were imaged by confocal microscopy on a LSM 5 EXCITER (Carl-Zeiss, Germany).
To study co-localization between hCD1d and HCMV US2 using confocal microscopy, a cDNA segment corresponding to the HCMV US2 was subcloned into the pcDNA3.1 myc-His A (Invitrogen) vector through XhoI and HindIII restriction enzyme sites. The cDNA encoding HCMV US2 in the pcDNA3.1 myc-His A (Myc-US2) was introduced into the C1R.hCD1d cells via transfection with Lipofectamine (Invitrogen). After transfection, Myc-US2 introduced C1R.hCD1d cells were permeabilized in 0.2% Triton X-100 in PBS for 5 min and washed three times with PBS. Then, cells were incubated with a blocking solution (3% bovine serum albumin in PBS). Subsequently, cells were washed and incubated with the following antibodies. For staining of hCD1d, the purified mouse anti-hCD1d antibody (C3D5 clone) combined with Cy3 conjugated goat anti-mouse IgG antibody was used. For staining of HCMV US2, the purified mouse anti-Myc antibody combined with Alexa 488 conjugated goat anti-mouse IgG antibody was used. For staining of calreticulin, the purified rabbit anti-calreticulin antibody combined with either Alexa fluor 488 conjugated donkey anti-rabbit IgG or Alexa fluor 594 conjugated donkey anti-rabbit IgG were used. For visualization of the nucleus, DAPI staining was performed according to the manufacturer’s protocol (Vector laboratories, USA). After staining, cells were imaged by confocal microscopy on a LSM 5 EXCITER (Carl-Zeiss).
Measurement of hCD1d protein stability
Stable EGFP-US2 or empty vector introduced C1R.hCD1d cells and Jurkat cells were treated with cycloheximide for 0 h, 2 h, 4 h, and 8 h. After treatment, cells were harvested, washed in PBS, centrifuged, and resuspended in a cell lysis solution (50 mM Tris, pH 7.2, 150 mM NaCl, 1% Triton X-100) supplemented with 200 μg/ml phenylmethylsulfonyl fluoride, phosphatase inhibitor cocktail (Sigma) and protease inhibitor cocktail (EMD Chemicals, Inc., USA). The cell lysates were subsequenttly resolved by 10% SDS-polyacrylamide gel electrophoresis, transferred onto Immobilon P membranes (Millipore Corporation, USA), and immunoblotted with an anti-hCD1d (C3D5 clone) antibody. Immunoreactive bands were visualized using an ECL system (GE Healthcare, UK). Anti-β-actin anti-body was utilized as an internal control.
Ubiquitination assays
Stable EGFP-US2 or empty vector introduced C1R.hCD1d cells were treated with proteasome inhibitor, MG132 (EMD Chemicals, Inc.) for 4 h in culture media. After treatment, cells were washed in PBS, centrifuged, and resuspended in a cell lysis solution (50 mM Tris HCl, pH 8.0, 150 mM NaCl, 1% NP-40) containing protease inhibitor cocktail (EMD Chemicals, Inc.). Cell lysates were incubated with each relevant anti-hCD1d antibody (51.1 clone or NOR3.2 clone) and precipitated with protein G sepharose beads (GE Healthcare, UK). After washing, the immunoprecipitants were resolved by 15% SDS polyacrylamide gel electrophoresis, transferred onto Immobilon P membranes and immunoblotted with the anti-ubiquitin antibody. The anti-hCD1d antibody (C3D5 clone) and anti-β-actin antibody were employed as internal controls.
Measurement of iNKT cell activity
Stable EGFP-US2 or empty vector introduced C1R.hCD1d cells and Jurkat cells were treated with various concentration of the synthetic KRN7000 (Funakoshi Co., Japan) for 6 h. After treatment, cells were inactivated by the treatment of mitomycin C for 4 h. The iNKT cells (DN32.D3) were co-cultured with KRN7000 loaded EGFP-US2 or empty vector introduced C1R.hCD1d cells and Jurkat cells. Three days after incubation, culture media were assayed for IL-2 or IFN-γ concentrations by sandwich ELISA using a mouse IL-2 or IFN-γ ELISA kit (Invitrogen) according to the manufacturer’s protocol.
Statistical analyses
Mean values were compared using a Student’s t-test for independent variables. Significant differences at a confidence level of 99% are shown by an asterisk (*) on each graph.
RESULTS
The endoplasmic reticulum (ER) lumenal domain of HCMV US2 interacts with the α3 domain of hCD1d in the ER
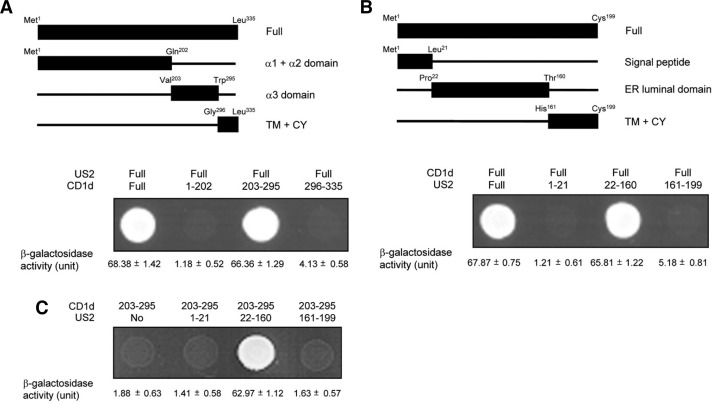
To study effect of HCMV US2 on the antigen presentation of hCD1d and the activity of iNKT cells, we first examine whether HCMV US2 physiologically associates with hCD1d using yeast two-hybrid system. Results clearly indicated that HCMV US2 interacts directly with hCD1d (data not shown). To identify the HCMV US2 binding region of hCD1d, cDNA constructs containing three hCD1d deletion mutants were designed as shown in Fig. 1A. These truncated regions were predicted to contain α1 and α2 domain, α3 domain, transmembrane and cytoplasmic tail (TM + CY), respectively (upper panel in Fig. 1A). In the yeast two-hybrid system, the full-length HCMV US2 cDNA and a plasmid containing either a full-length hCD1d cDNA (upper panel in Fig. 1A, Full) or plasmids containing three truncated mutant forms (upper panel in Fig. 1A, Met1-Gln202, Val203-Trp295, Gly296-Leu335) of cDNAs were co-transformed into EGY48 yeast cells. Cells containing full-length hCD1d cDNA and one deletion mutant (Val203-Trp295) grew on the Ura-, His-, Trp-, and Leu-deficient plates. Yeast cells transformed with the other deletion mutants (Met1-Gln202 and Gly296-Leu335) failed to grow (lower panel in Fig. 1A). To confirm this result, we determined the binding activity of these constructs by measuring the relative expression level of β-galactosidase. As shown in lower panel in Fig. 1A, β-galactosidase assay results confirmed that neither of these mutants (Met1-Gln202 and Gly296-Leu335) can bind to HCMV US2.
Fig. 1.
Mapping of the critical interaction region between hCD1d and HCMV US2 using the yeast two-hybrid system. The cDNA constructs were co-transformed into EGY48 yeast cells to test a protein-protein interaction within the yeast two-hybrid system. The results are representative of three separate experiments. Data are shown as means ± SEM. (A) Upper panel shows the schematic representation of cDNA constructs for each hCD1d (CD1d) deletion mutant and full-length HCMV US2 (US2) fusion proteins in the yeast two-hybrid system. Lower panel shows the result of protein-protein interaction determined in the yeast two-hybrid system. The values of β-galactosidase activity (Unit) measured by σNPG assays are indicated below the corresponding lanes. (B) Upper panel shows the schematic representation of cDNA constructs for each HCMV US2 (US2) deletion mutant and full-length hCD1d (CD1d) fusion proteins in the yeast two-hybrid system. Lower panel shows the result of protein-protein interaction determined in the yeast two-hybrid system. The values of β-galactosidase activity (Unit) measured by σNPG assays are indicated below the corresponding lanes. (C) Interaction between cDNA constructs for an hCD1d (CD1d) deletion mutant (Val203-Trp295) and three HCMV US2 (US2) deletion (Met1-Leu21, Pro22-Thr160 and His161-Cys199) fusion proteins in the two-hybrid system. “No” means empty vector only.
Subsequently, cDNA constructs containing three HCMV US2 truncation mutants were designed to localize the hCD1d binding region of HCMV US2 (Fig. 1B). These truncated regions were predicted to be containing signal peptide, ER lumenal domain, transmembrane and cytoplasmic tail (TM + CY), respectively (upper panel in Fig. 1B). In the yeast two-hybrid system, full-length hCD1d cDNA and either a full-length hCD1d cDNA (upper panel in Fig. 1B, Full) or plasmids containing three truncation mutant forms (upper panel in Fig. 1B, Met1-Leu21, Pro22-Thr160, His161-Cys199) of cDNAs were co-transformed into EGY48 yeast cells. Cells containing full length HCMV US2 cDNA and the one deletion mutant (Pro22-Thr160) grew on the Ura-, His-, Trp-, and Leu-deficient plates. Yeast cells transformed with the other deletion mutants (Met1-Leu21, His161-Cys199) failed to grow (lower panel in Fig. 1B). In addition, we quantitated the binding activity of these constructs by measuring the relative expression level of β-galactosidase. Results from the β-galactosidase assay indicated that the critical HCMV US2 region for binding hCD1d resided within Pro22-Thr160 (lower panel in Fig. 1B).
To confirm these results, one deletion mutant of hCD1d (Val203-Trp295) and either vector cDNA (No) or three truncation mutants of HCMV US2 (Met1-Leu21, Pro22-Thr160, His161-Cys199) were co-transformed into EGY48 yeast cells (Fig. 1C). Consistent with results from Figs. 1A and 1B, cells containing hCD1d deletion mutant (Val203-Trp295) with one HCMV US2 deletion mutant (Pro22-Thr160) only grew on the Ura-, His-, Trp-, and Leu-deficient plates, whereas the other deletion mutants (Met1-Leu21, His161-Cys199) failed to grow. Subsequent results on β-galactosidase assay agreed with these results (Fig. 1C). These results clearly indicated that the ER lumenal domain of HCMV US2 interacts with the α3 domain of hCD1d.
HCMV US2 and hCD1d interact in the ER
Since the ER lumenal domain of HCMV US2 physiologically interacts with the α3 domain of hCD1d, it is likely that HCMV US2 influences on the antigen presentation of hCD1d. To study the functional role of HCMV US2 during the antigen presentation of hCD1d, we attempted to overexpress HCMV US2 in C1R.hCD1d cells and Jurkat cells through retroviral transduction. C1R cells are a good tool to study the effect of HCMV US2 on hCD1d molecule, because C1R cells only express HLA-C (HLA-Cw4 allele) which is less affected by HCMV US2 among HLA class Ia molecules (Schust et al., 1998; Storkus et al., 1987). Jurkat cells express both HCMV US2 susceptible HLA class Ia alleles (HLA-A3 and HLA-B35) and hCD1d molecules (Körner and Burgert, 1994; data not shown). Therefore, we used Jurkat cells to compare the effect of HCMV US2 on hCD1d molecule and HCMV US2 susceptible HLA class Ia molecule.
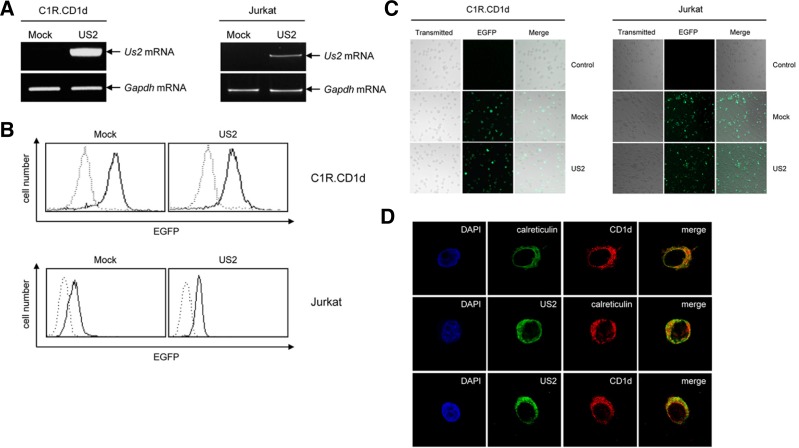
After transduction of HCMV US2 in C1R.hCD1d cells and Jurkat cells, the level of HCMV US2 expression and the efficiency of retroviral transduction were monitored by co-overexpressing EGFP using the IRES-EGFP expression system (Kim et al., 2011). As a result, the mRNA level of HCMV US2 in the EGFP-US2 introduced C1R.hCD1d cells (US2) and Jurkat cells (US2) were significantly higher than those of empty vector transfected C1R.hCD1d cells (Mock) and Jurkat cells (Mock) (Fig. 2A). Then, flow cytometry and confocal microscopy analyses were conducted to identify the level of HCMV US2 expression and the efficiency of retroviral transduction. As shown in Figs. 2B and 2C, EGFP expressions between empty vector transfectants (Mock) and EGFP-US2 transfectants (US2) are equally distributed with the transfection efficiency of approximately 80%.
Fig. 2.
HCMV US2 and hCD1d interact in the ER. Overexpression of the HCMV US2 in C1R.CD1d cells and Jurkat cells (A, B, and C). (A) Detection of HCMV US2 (US2) mRNA transcripts in C1R.CD1d cells and Jurkat cells by RT-PCR. The Gapdh mRNA transcripts were used as a loading control. Mock means empty vector transfectants and US2 means HCMV US2 transfectants. (B) Flow cytometry analyses of the EGFP fusion HCMV US2 protein (EGFP-US2) expression in C1R.CD1d cells and Jurkat cells. Mock means empty EGFP vector transfectants and US2 means EGFP-US2 transfectants. Dotted line, isotype control; solid line, EGFP expression. (C) To measure transfection efficiency, cells were visualized under confocal microscopy after transfection. Control means untransfectants, Mock means empty EGFP vector transfectants and US2 means EGFP-US2 transfectants. The results are representative of three independent experiments. (D) Confocal microscopy analyses shows co-localization of HCMV US2 and hCD1d in the ER. The top panel shows hCD1d (CD1d) is expressed in intracellular compartment and cell surface, and a portion of hCD1d molecules is merged with calreticulin, ER specific marker. The middle panel shows HCMV US2 (US2) is existed in ER. The bottom panel indicates HCMV US2 (US2) and hCD1d (CD1d) proteins are partially co-localized in the ER. The results are representative of three independent experiments.
Next, we investigated co-localization of HCMV US2 and hCD1d in C1R.hCD1d cells using confocal microscopy. Results indicated that hCD1d molecules were localized on the cell surface and intracellular compartment and some of hCD1d partially co-localized with the ER marker calreticulin (upper panel in Fig. 2D). The ER resident-HCMV US2 co-localized with the calreticulin (ER) (middle panel in Fig. 2D). In addition, the ER resident-HCMV US2 co-localized with a portion of hCD1d proteins, when merged (lower panel in Fig. 2D). These results demonstrated that HCMV US2 and hCD1d interact in the ER.
HCMV US2 promotes the ubiquitin-dependent proteasomal degradation of immature form of hCD1d
In case of MHC class Ia or class II, HCMV US2 physically interacts and with heavy chain of MHC molecules and destabilize them (Tomazin et al., 1999; Wiertz et al., 1996). To investigate whether HCMV US2 also destabilizes hCD1d, we tested the stability of hCD1d protein in HCMV US2 overexpressing CD1d.C1R cells (US2), compared with that of empty vector transfectants (Mock) following treatment with cycloheximide. Before treatment with cycloheximide (0 h), the protein expression level of hCD1d in HCMV US2 overexpressing CD1d.C1R cells (US2) was lower than that of hCD1d protein expressed in empty vector transfectants (Mock) (Fig. 3A). Also, the hCD1d protein in HCMV US2 overexpressing CD1d.C1R cells (US2) were degraded much faster than that of empty vector transfectants (Mock) (Fig. 3A). In addition, we tested the stability of hCD1d protein in HCMV US2 overexpressing Jurkat cells (US2), compared with that of empty vector transfectants (Mock). Similar to the observation from CD1d.C1R cells, results were shown that the destability of hCD1d were increased in HCMV US2 overexpressing Jurkat cells (US2), compared with that of empty vector transfectants (Mock) (Fig. 3B). These results indicated that HCMV US2 also destabilizes hCD1d.
Fig. 3.
Destabilization of hCD1d expression by HCMV US2. Analyses on the stability hCD1d proteins in empty vector transfectants (Mock) and HCMV US2 transfectants (US2) (A, B). (A) hCD1d protein level in CD1d. C1R cells at 0 h, 2 h, 4 h, and 8 h after cycloheximide treatment were determined by immunoblotting. Anti-β actin was used as an internal control. Mock means empty vector transfectants and US2 means HCMV US2 transfectants. The results are representative of three independent experiments. (B) hCD1d protein level in Jurkat cells at 0 h, 2 h, 4 h, and 8 h after cycloheximide treatment were determined by immunoblotting. Anti-β actin was used as an internal control. Mock means empty vector transfectants and US2 means HCMV US2 transfectants. The results are representative of three independent experiments. (C) Ubiquitination assays of hCD1d proteins. Immunoblot analysis of ubiquitin conjugation level of CD1d.C1R cells with or without HCMV US2. Cells were treated with MG-132, proteasome inhibitor, harvested, and resuspended with NP-40 lysis buffer. Upper panel shows the immunoprecipitation (IP) of cell lysates using 51.1 antibody (51.1) and Western blot analyses (WB) using anti-ubiquitin antibody. The 51.1 antibody (51.1) detects only mature form of hCD1d. Lower panel shows the immunoprecipitation (IP) of cell lysates using NOR3.2 antibody and Western blot analyses (WB) using anti-ubiquitin antibody. The NOR3.2 antibody (NOR3.2) detects both immature and mature form of hCD1d. The equal amounts of immunoprecipitated hCD1d proteins are confirmed by Western blot analyses using C3D5 antibody and the β-actin was used as the loading control. The results are representative of three independent experiments.
Next, we performed ubiquitination assays to determine whether immature form of hCD1d or mature form of hCD1d is susceptible for HCMV US2 mediated proteasomal degradation. Prior to immunoprecipitation, we treated MG132, the proteasome inhibitor, to detect more ubiquitin-conjugated proteins otherwise undergo ubiquitin-proteasome degradation pathway (data not shown). For immunoprecipitation of hCD1d in CD1d.C1R cells, we used two different anti-hCD1d antibodies to detect hCD1d protein. One anti-hCD1d antibody (51.1 clone) detects only glycosylated mature form of hCD1d (Balk et al., 1994), whereas another anti-hCD1d antibody (NOR3.2 clone) detects both immature and mature forms of hCD1d (Kawana et al., 2007). After immunoprecipitation, anti-ubiquitin antibody was used to perform Western blot analyses to detect the degree of ubiquitination of each different form of hCD1d (immature form vs. mature form) from each transfectant. As a result, the ubiquitination level of mature hCD1d (immunoprecipitated using 51.1 clone) was comparable in both HCMV US2 overexpressing cells (US2) and empty vector transfectants (Mock) (Fig. 3C). However, the ubiquitin conjugation level of all types of hCD1ds including immature and mature form of hCD1d (immunoprecipitated using NOR3.2 clone) was significantly increased in HCMV US2 overexpressing cells (US2) compared with that of empty vector transfectants (Mock) (Fig. 3C). This result indicated the major target of HCMV US2 mediated proteasomal degradation is the immature form of hCD1d rather than the mature form of hCD1d. The equal amount of hCD1d is used for immunoprecipitation, proved by Western blot analyses using other anti-hCD1d antibody (D5 clone) which detects all forms of hCD1d (Fig. 3C) (Kim et al., 1999). Also, the amount of total proteins from whole cell lysates was equal, proved by Western blot analyses using β-actin antibody (Fig. 3C).
HCMV US2 down-regulates iNKT cell activity without significant change of hCD1d expression
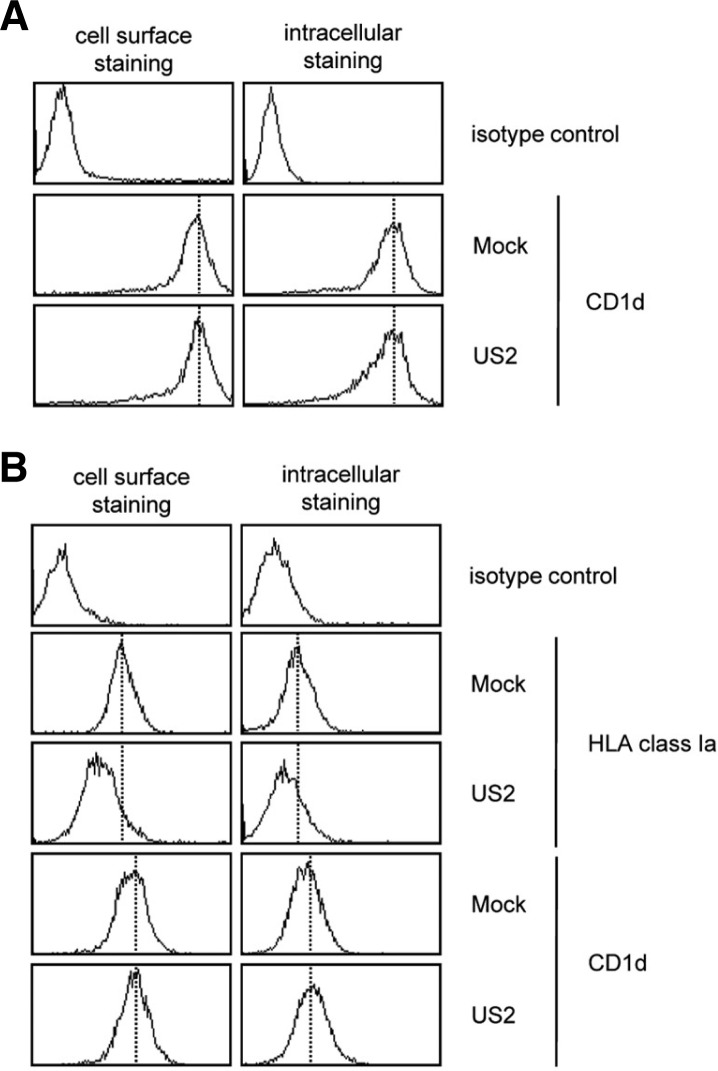
Since HCMV US2 promotes the ubiquitin-dependent proteasomal degradation of immature form of hCD1d, the expression pattern of hCD1d of EGFP-US2 introduced C1R.hCD1d cells and Jurkat cells (US2) were compared with those of empty vector transfected C1R.hCD1d cells and Jurkat cells (Mock) by flow cytometry using anti-hCD1d antibody (CD1d42 clone). When we compared the expression of hCD1d, we observed much higher expression of hCD1d in C1R.hCD1d cells (Fig. 4A), than that of Jurkat cells (Fig. 4B). As shown in Figs. 4A and 4B, hCD1d expressions in HCMV US2 overexpressing C1R.hCD1d cells and Jurkat cells (US2) were not significantly decreased in both intracellular and cell surface level, compared with those of empty vector transfectants (Mock). However, the expression of HLA class Ia in HCMV US2 overexpressing Jurkat cells was significantly reduced in both intracellular and cell surface level (Fig. 4B).
Fig. 4.
The ectopic expression of HCMV US2 does not significantly change the expression of hCD1d. (A) Flow cytometry analyses on the empty vector transfected C1R.CD1d cells (Mock) and HCMV US2 transfected C1R.CD1d cells (US2). Cells were harvested and stained with PE conjugated anti-hCD1d antibody (CD1d42 clone, mouse IgG1κ) and PE conjugated isotype control (mouse IgG1κ). (B) Flow cytometry analyses on the empty vector transfected Jurkat cells (Mock) and HCMV US2 transfected jurkat cells (US2). Cells were harvested and stained with PE conjugated anti-hCD1d antibody (CD1d42 clone, mouse IgG1κ), PE conjugated anti-HLA class Ia (HLA-A, B, C; G46-2.6 clone, mouse IgG1κ) antibody and PE conjugated isotype control (mouse IgG1κ).
Next, we measured the iNKT cell activity to examine whether HCMV US2 affects on iNKT cell activity. The iNKT cells (DN32.D3) were stimulated with various amounts (0.1 μg/ml, 0.5 μg/ml, and 1.0 μg/ml) of glycolipid (KRN7000) loaded empty vector transfectants (Mock) or HCMV US2 overexpressing Jurkat cells (US2). Three days after incubation, culture media were assayed for IL-2 secretion. As shown in Fig. 5A, the IL-2 secretion of DN32.D3 cells stimulated with 0.1 μg/ml of KRN7000 loaded HCMV US2 overexpressing Jurkat cells (US2) was not significantly different than that of DN32.D3 cells stimulated by empty vector transfectants (Mock). However, the IL-2 secretion of DN32.D3 cells stimulated with 0.5 μg/ml and 1.0 μg/ml of KRN7000 loaded HCMV US2 overexpressing Jurkat cells (US2) were decreased significantly compared with those of DN32.D3 cells stimulated by empty vector transfectants (Mock) (Fig. 5A). To confirm these results, we stimulated DN32.D3 cells with 0.5 μg/ml of KRN7000 loaded empty vector transfectants (Mock) or HCMV US2 overexpressing C1R.hCD1d cells (US2) and measured IL-2 and IFN-γ secretion by DN32.D3 cells. Results clearly showed that the IL-2 and IFN-γ secretion of DN32.D3 cells stimulated with KRN7000 loaded HCMV US2 overexpressing C1R.hCD1d cells (US2) were decreased significantly compared with those of DN32.D3 cells stimulated by empty vector transfectants (Mock) (Fig. 5B). These results clearly indicated that HCMV US2 can down-regulate the activity of iNKT cells without significant change of hCD1d expression.
Fig. 5.
Down-regulation of iNKT cell activity by HCMV US2. (A) The empty vector transfected Jurkat cells (Mock) and US2 transfected Jurkat cells (US2) were treated with various concentration of the synthetic KRN7000 and inactivated. Then, inactivated cells were co-cultured with the iNKT cells (DN32.D3). After three days, IL-2 concentrations in culture supernatants were determined by ELISA. Data are shown are means ± SEM. (B) The empty vector transfected C1R.CD1d cells (Mock) and US2 transfected C1R.CD1d cells (US2) were treated with 0.5 μg/ml of the synthetic KRN7000 and inactivated. Then, inactivated cells were co-cultured with the iNKT cells (DN32.D3). After three days, IL-2 and IFN-γ concentrations in culture supernatants were determined by ELISA. Data are shown are means ± SEM.
DISCUSSION
Several previous studies have demonstrated that the deficiency of iNKT cells leads to the greater susceptibility to certain viral infections (De Santo et al., 2008; Grubor-Bauk et al., 2003; Ilyinskii et al., 2006). These results were supported by the fact that animals with the stimulation of iNKT cells through CD1d pulsed with antigen (KRN 7000) were more resistance to specific viral infections (Johnson et al., 2002; Kakimi et al., 2000; van Dommelen et al., 2003). Combined result indicated that iNKT cells are also critical player for supporting host immunity during the viral infections.
Therefore, it is interesting to examine the pattern of expression of CD1d, which gives antigenic repertoire to iNKT cells during the viral infections. Indeed, several previous studies indicated that the antigen presentation of CD1d is abnormal during viral infections (Lin et al., 2005; Sanchez et al., 2005). However, there is no study to explain the mechanism of CD1d down regulation during viral infection.
In this study, we demonstrated that how specific viral product down-modulates the antigen presentation of hCD1d and iNKT cell activity. First, we showed the physiological interaction between HCMV US2 and hCD1d, similar to previous observation (Cho and Jun, 2011). Further analyses indicated that ER lumenal domain of HCMV US2 is required for physiologically interaction with the α3 domain of hCD1d. Previous observation also demonstrated that ER lumenal domain of HCMV US2 is the major binding site for both MHC class Ia and class II molecules (Chevalier et al., 2002; Gewurz et al., 2001). Although hCD1d, heavy chain of MHC class Ia (HLA-A2) and MHC class II molecules (HLA-DM, HLA-DR) share only 25 to 30% of amino acid identity, ER lumenal domain of HCMV US2 still can bind to these molecules. Therefore, this finding explains that molecular conservation of pan-MHC binding site of HCMV US2 during the evolution. In addition, we identified the α3 domain of hCD1d as the major binding site of HCMV US2. Previous observation indicated that HCMV US2 binding site of heavy chain of MHC class Ia molecule (HLA-A2) is located mainly in the α3 domain of the heavy chain, which is remote from the peptide binding motif (Gewurz et al., 2001). Thus, hCD1d and MHC class Ia molecule also share the region that interacts with HCMV US2.
After interaction of MHC molecules, HCMV US2 translocates these to cytoplasm. The translocated MHC molecules are degraded in an ubiquitin-mediated proteasome-dependent manner (Tomazin et al., 1999; Wiertz et al., 1996). Since we found the interaction between HCMV US2 and hCD1d, we tested whether HCMV US2 also leads the degradation of hCD1d by the same manner as those of MHC molecules. Results clearly indicated that binding between HCMV US2 and hCD1d in the ER leads the decreased stability of hCD1d. In addition, we showed that the immature form of hCD1d molecule is more susceptible to ubiquitin conjugation. These results may be evidence supporting that immature hCD1d undergoes ubiquitin mediated degradation. Previous report showed that the expression of group 1 CD1 molecules (hCD1a, hCD1b, hCD1c) is down-regulated after HCMV infection (Raftery et al., 2008). Interestingly, these down-regulation of group 1 CD1 molecules is not due to HCMV encoded short glycoproteins (US2, US3, US6, and US11), but the transcriptional inhibition of group 1 CD1 molecules through HCMV IL-10 homologue (Raftery et al., 2008). However, our results indicated that HCMV US2 interacts with hCD1d through direct protein-protein interaction and leads the proteasomal degradation of hCD1d in an ubiquitin-dependent manner. Thus, our results with previous observation may indicate that hCD1d molecule is the only target molecule of HCMV US2 among a family of hCD1 molecules.
It was surprising to see no difference in cell surface level or the intracellular level of hCD1d expression between HCMV US2 transfectants and empty vector transfectants (Mock) by flow cytometry analyses, since we observed the decreased stability of hCD1d molecule in HCMV US2 transfectants compared with empty vector transfectants (Mock). These results may indicate that hCD1d is more resistant to HCMV US2 mediated proteasomal degradation than HLA-A and HLA-B, because we can see the decreased expression of MHC class Ia molecules in HCMV US2 introducing Jurkat cells, compared with empty vector transfectants (Mock). Indeed, the effect of HCMV US2 is varied in its dependence on HLA class Ia and HLA class Ib alleles. In particular, the stability of HLA-C, one of HLA class Ia alleles, and two HLA class Ib alleles, HLA-E and HLA-G, are less affected by HCMV US2, compared with HLA-A and HLA-B (Furman et al., 2000; Schust et al., 1998). Similar to our observation, a recent report showed that the cell surface expression of hCD1d does not change between HCMV US2 transfectants and empty vector transfectants (Mock) based on flow cytometry analyses (Cho and Jun, 2011).
To test whether HCMV US2 can modulate iNKT cell activity by affecting CD1d antigen presentation, we stimulated iNKT cell (DN32.D3) with glycolipid (KRN7000) loaded, empty vector transfectants (Mock) or HCMV US2 overexpressing Jurkat cells. Results on IL-2 ELISA assays indicated that iNKT cell activity was not affected by HCMV US2 expression on Jurkat cells on lower dose (0.1 μg/ml) of KRN7000, but affected by HCMV US2 expression on Jurkat cells on higher dose (0.5 μg/ml and 1.0 μg/ml) of KRN7000. Confirming these results, DN32.D3 cells pulsed with 0.5 μg/ml of KRN7000 in HCMV US2 overexpressing C1R.CD1d cells secreted less amount of IL-2 and IFN-γ compared with that of DN32.D3 cells stimulated with empty vector transfectants pulsed with glycolipid (Mock).
The fact that HCMV US2 down-regulates iNKT cell activity without significant change of hCD1d expression comes up with several possibilities. First, it is possible that anti-hCD1d antibody (CD1d42 clone) used for flow cytometry may not detect the alteration of conformational change of hCD1d by HCMV US2 in the ER. A second possibility is that HCMV US2 may affect on one of several molecular chaperones involves in the antigen presentation of hCD1d molecule in the ER. In the same manner as the heavy chain of MHC class Ia molecules, the heavy chain of CD1d molecule interacts with calnexin, calreticulin, ERp57, and associates with β2-microglobulin before it exits from the ER (Kang and Cresswell, 2002). Furthermore, the heavy chain of CD1d molecules can associate with an invariant chain in the ER (Jayawardena-Wolf et al., 2001), similar to MHC class II molecules. Although the exact mechanism of lipid antigen loading to CD1d is not defined, the microsomal triglyceride transfer protein (MTP) can mediate the loading of lipid antigen to CD1 molecules in the ER (Dougan et al., 2005). Supporting this idea, chemical inhibitors of MTP can down-regulate iNKT cell activity stimulated with KRN7000 pulsed CD1d molecule (Dougan et al., 2005). However, it is unlikely that HCMV US2 influences on β2-microglobulin, invariant chain, and MTP molecules associated with hCD1d because the inhibition of β2-microglobulin and MTP causes the decreased expression of CD1d on the cell surface (data not shown; Dougan et al., 2005) and the lack of expression of invariant chain causes the increased expression of CD1d on the cell surface (Jayawardena-Wolf et al., 2001). A third possibility is that HCMV US2 may affect the loading of lipid antigen to CD1 molecules in the endosomal compartment, not in the ER. However, this possibility is unlikely because the expression of HCMV US2 molecule is strictly restricted in the ER (Gewurz et al., 2002).
In summary, we have shown that the ER lumenal domain of HCMV US2 interacts with α3 domain of hCD1d in the ER. Our observation has indicated that HCMV US2 facilitate the proteasomal degradation of immature form of hCD1d on ubiquitin-dependent manner. Although, there is no significant change of hCD1d expression detected by flow cytometry analyses, the ectopic expression of HCMV US2 leads to the decreased activity of iNKT cell. These results may show additional evidence that HCMV developed strategies to escape from host immune surveillance mechanisms.
Acknowledgments
This work was supported by Next-Generation BioGreen 21 program (No. PJ008089), Rural Development Administration, Republic of Korea, and a Korea University Grant (2012).
REFERENCES
- Balk SP, Burke S, Polischuk JE, Frantz ME, Yang L, Porcelli S, Colgan SP, Blumberg RS. β2-microglo-bulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994;265:259–262. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects Mice from tuberculosis. Infect Immun. 2002;70:6302–6309. doi: 10.1128/IAI.70.11.6302-6309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier MS, Daniels GM, Johnson DC. Binding of human cytomegalovirus US2 to major histocompatibility complex class I and II proteins is not sufficient for their degradation. J Virol. 2002;76:8265–8275. doi: 10.1128/JVI.76.16.8265-8275.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Jun Y. Human CD1d molecules are resistant to human cytomegalovirus US2- and US11-mediated degradation. Biochem Biophys Res Commun. 2011;413:616–622. doi: 10.1016/j.bbrc.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Cho S, Hwang ES. Status of mTOR activity may phenotypically differentiate senescence and quiescence. Mol. Cells. 2012;33:597–604. doi: 10.1007/s10059-012-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, Khurana A, Kronenberg M, Johnson C, Exley M, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman MH, Ploegh HL, Schust DJ. Can viruses help us to understand and classify the MHC class I molecules at the maternal-fetal interface? Hum Immunol. 2000;61:1169–1176. doi: 10.1016/s0198-8859(00)00203-2. [DOI] [PubMed] [Google Scholar]
- Gewurz BE, Gaudet R, Tortorella D, Wang EW, Ploegh HL, Wiley DC. Antigen presentation subverted: structure of the human cytomegalovirus protein US2 bound to the class I molecule HLA-A2. Proc. Natl. Acad. Sci. USA. 2001;98:6794–6799. doi: 10.1073/pnas.121172898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz BE, Ploegh HL, Tortorella D. US2, a human cytomegalovirus-encoded type I membrane protein, contrains a non-cleavable amino-terminal signal peptide. J Biol Chem. 2002;277:11306–11313. doi: 10.1074/jbc.M107904200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, Taniguchi M, Bendelac A, Van Kaer L, Koezuka Y, et al. alpha-galactosylcera-mide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc. Natl. Acad. Sci. USA. 2000;97:8461–8466. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impared clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant Vα14-Jα281 TCR. J Immunol. 2003;170:1430–1434. doi: 10.4049/jimmunol.170.3.1430. [DOI] [PubMed] [Google Scholar]
- Hansen TH, Huang S, Arnold PL, Fermont DH. Patterns of nonclassical MHC antigen presentation. Nat Immunol. 2007;8:563–568. doi: 10.1038/ni1475. [DOI] [PubMed] [Google Scholar]
- Ilyinskii PO, Wang R, Balk SP, Exley MA. CD1d mediates T-cell-Dependent resistance to secondary infection with encephalomyocarditis virus (EMCV) in vitro and immune response to EMCV infection in vivo. J Virol. 2006;80:7146–7158. doi: 10.1128/JVI.02745-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena-Wolf J, Benlagha K, Chiu YH, Mehr R, Bendelac A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15:897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS. NKT cells contribute to expansion of CD8+ T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002;76:4294–4303. doi: 10.1128/JVI.76.9.4294-4303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Cresswell P. Calnexin, calreticulin, and ERp57 cooperate in disulfide bond formation in human CD1d heavy chain. J Biol Chem. 2002;277:44838–44844. doi: 10.1074/jbc.M207831200. [DOI] [PubMed] [Google Scholar]
- Kawana K, Quayle AJ, Ficarra M, Ibana JA, Shen L, Kawana Y, Yang H, Marrero L, Yavagal S, Greene SJ, et al. CD1d degradation in Chlamydia trachomatis-infected epithelial cells is the result of both cellular and chlamydial proteasomal activity. J Biol Chem. 2007;282:7368–7375. doi: 10.1074/jbc.M610754200. [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Garcia J, Exley M, Johnson KW, Balk SP, Blumberg RS. Biochemical characterization of CD1d expression in the absence of beta2-microglobulin. J Biol Chem. 1999;274:9289–9295. doi: 10.1074/jbc.274.14.9289. [DOI] [PubMed] [Google Scholar]
- Kim EM, Lee HH, Kim SH, Son YO, Lee SJ, Han J, Bae J, Kim SJ, Park CG, Park Y, et al. The mouse small ubiquitin-like modifier-2 (SUMO-2) inhibits interleukin-12 (IL-12) production in mature dendritic cells by blocking the translocation of the p65 subunit of NFκB into the nucleus. Mol Immunol. 2011;48:2189–2197. doi: 10.1016/j.molimm.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Körner H, Burgert HG. Down-regulation of HLA antigens by the adenovirus type 2 E3/19K protein in a T-lymphoma cell line. J Virol. 1994;68:1442–1448. doi: 10.1128/jvi.68.3.1442-1448.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Rho SB, Park SY, Chun T. Interaction between fortilin and transforming growth factor-beta stimulated clone-22 (TSC-22) prevents apoptosis via the destabilization of TSC-22. FEBS Lett. 2008;582:1210–1218. doi: 10.1016/j.febslet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Lin Y, Roberts TJ, Spence PM, Brutkiewicz RR. Reduction in CD1d expression on dendritic cells and macrophages by an acute virus infection. J Leukoc Biol. 2005;77:151–158. doi: 10.1189/jlb.0704399. [DOI] [PubMed] [Google Scholar]
- Moody DB, Zajonc DM, Wilson IA. Anatomy of CD1-lipid antigen complexes. Nat Rev Immunol. 2005;5:387–399. doi: 10.1038/nri1605. [DOI] [PubMed] [Google Scholar]
- Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J Immunol. 1998;160:3128–3134. [PubMed] [Google Scholar]
- Pinto AK, Hill AB. Viral interference with antigen presentation to CD8+ T cells lessons from cytomegalovirus. Viral Immunol. 2005;18:434–444. doi: 10.1089/vim.2005.18.434. [DOI] [PubMed] [Google Scholar]
- Ploegh HL. Viral strategies of immune evasion. Science. 1998;280:249–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- Raftery MJ, Hitzler M, Winau F, Giese T, Plachter B, Kaufmann SH, Schonrich G. Inhibition of CD1 antigen presentation by human cytomegalovirus. J Virol. 2008;82:4308–4319. doi: 10.1128/JVI.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest. 2005;115:1369–1378. doi: 10.1172/JCI24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schust DJ, Tortorella D, Seebach J, Phan C, Ploegh HL. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J Exp Med. 1998;188:497–503. doi: 10.1084/jem.188.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seriger B, Ritz U, Ferrone S. Molecular mechanisms of HLA class I antigen abnormalities following viral infection and transformation. Int. J. Cancer. 2006;118:129–138. doi: 10.1002/ijc.21312. [DOI] [PubMed] [Google Scholar]
- Storkus WJ, Howell DN, Salter RD, Dawson JR, Cresswell P. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987;138:1657–1659. [PubMed] [Google Scholar]
- Tomazin R, Boname J, Hegde NR, Lewinsohn DM, Altschuler Y, Jones TR, Cresswell P, Nelson JA, Riddell SR, Johnson DC. Cytomegalovirus US2 destroys two components of the MHC class II pathways, preventing recognition by CD4+ T cells. Nat Med. 1999;5:1039–1043. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- van Dommelen SL, Tabarias HA, Smyth MJ, Degli-Esposti MA. Activation of NKT cells during murine cytomegalovirus infection enhances the antiviral response mediated by NK cells. J Virol. 2003;77:1877–1894. doi: 10.1128/JVI.77.3.1877-1884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Geng YB, Wang CR. CD1-restricted NKT cells protect nonobese Diabetic mice from developing diabetes. J Exp Med. 2001;194:313–320. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- Yang JQ, Chun T, Liu H, Hong S, Bui H, Van Kaer L, Wang CR, Singh RR. CD1d deficiency exacerbates inflammatory dermatitis in MRL-lpr/lpr mice. Eur J Immunol. 2004;34:1723–1732. doi: 10.1002/eji.200324099. [DOI] [PMC free article] [PubMed] [Google Scholar]