Abstract
Recent reports have proposed a novel function for the N-methyl-d-aspartate (NMDA) receptor (NMDAR), a well-known excitatory, ionotropic receptor. A series of observations employing pharmacological techniques has proposed that upon ligand binding, this ionotropic receptor can actually function via signaling cascades independent of traditional ionotropic action. Moreover, the “metabotropic” action of NMDARs is suggested to mediate a form of synaptic plasticity, namely long-term synaptic depression (LTD), which shares cellular mechanisms with the synaptic deficits observed in Alzheimer’s disease. Given that a growing body of clinical and preclinical evidence strongly recommends NMDAR antagonists for their therapeutic potentials and advantages in a variety of diseases, further investigation into their molecular and cellular mechanisms is required to better understand the “metabotropic” action of NMDARs.
Keywords: Alzheimer’s disease, glutamate receptors, ionotropic, long term depression, metabotropic, synaptic plasticity
INTRODUCTION
The N-methyl-d-aspartate (NMDA) receptor is probably one of the most extensively studied ionotropic glutamate receptors. Recent studies have proposed that the NMDA receptor (NMDAR) actually exerts long-lasting changes in synaptic transmission via initiation of intracellular signaling pathways, even in the absence of ion flux. In other words, NMDAR can also function as a metabotropic receptor (Kessels et al., 2013; Nabavi et al., 2013). This proposal is quite striking, and urges researchers to take a fresh look at the working principles of the central synapse as well as at the accumulated observations from the past decades.
TRADITIONAL UNDERSTANDING OF NMDA RECEPTORS
It is well known that glutamate exerts its excitatory action upon binding to ionotropic or metabotropic partners in the postsynaptic density (PSD) of the central nervous system. These receptors have been studied extensively for decades. Once released, glutamate activates its receptors that, in turn, either causes immediate ion influx (“ionotropic”) or initiates signaling cascades upon ligand binding (“metabotropic”). It has been well documented that ionotropic receptors for glutamate include the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-receptor (AMPAR), NMDAR, and kainate receptor (Collingridge and Lester, 1989). Metabotropic receptors include 8 isoforms of G-protein coupled receptors, namely mGluR1-8 (Conn and Pin, 1997). Ionotropic receptors mediate fast, immediate responses, whereas metabotropic receptors mediate relatively slower synaptic responses (Traynelis et al., 2010).
In general, ionotropic glutamate receptors are tetrameric, ligand-gated cation channels. Among these, NMDAR has been the center of attention predominantly due to its unique features: 1) it allows Ca2+ influx upon activation, in addition to other cation-mediated currents, and 2) it requires additional conditions for activation (specifically, the Mg2+ block has to be released for opening) (MacDermott et al., 1986; McBain and Mayer, 1994; Regehr and Tank, 1990). The combination of 2 duplicated subunits (generally, GluN1 subunits with co-activator, glycine binding sites, and regulatory GluN2 subunits with glutamate binding sites) consist of NMDARs, and the subunit composition (particularly among GluN2 isoforms) is known to determine ion conductance through the receptor and be developmentally regulated (Al-Hallaq et al., 2007; Collingridge et al., 2009; Monyer et al., 1994; Rauner and Kohr, 2011; Sheng et al., 1994; Stocca and Vicini, 1998). The mature human brain is known to express mainly GluN1 and GluN2A (Petralia et al., 1994). Each NMDAR subunit exhibits an extracellular N-terminal domain for tetramerization, a ligand-binding domain for glutamate or co-activator glycine, a transmembrane domain for pore-formation, and a C-terminal domain for receptor localization and modulation (Traynelis et al., 2010). The size of the C-terminal domain differs depending on the subunits. A number of postsynaptic proteins are known to interact with NMDARs through the C-terminal domain, including postsynaptic density protein 95 (PSD-95), synaptic scaffolding molecule (S-SCAM), Homer, and collapsing response mediator protein 2 (CRMP2) (Al-Hallaq et al., 2007; Kim and Sheng, 2004).
Because the opening of NMDARs requires both glutamate binding to its GluN2 subunits and depolarization to eliminate the voltage-dependent Mg2+ block, conventional fast synaptic transmission has been depicted as follows: AMPAR will be opened immediately by the released glutamate, causing depolarization of postsynaptic neurons, which in combination with the released glutamate, activates NMDARs. The opening of NMDARs allows cation influx with additional Ca2+ influx. This series of events has been extensively investigated (McBain and Mayer, 1994; Traynelis et al., 2010).
ROLE OF NMDARS IN SYNAPTIC PLASTICITY
NMDAR is often referred as the “coincidence detector” due to its concurrent requirement for ligand binding and depolarization induced by AMPARs for opening. Together with its ability to allow Ca2+ influx, its dual opening requirement suggests that NMDARs might play an essential role in basal transmission as well as in synaptic plasticity (Malenka and Nicoll, 1993). Synaptic plasticity, a well-established cellular model of learning and memory, occurs in 2 forms, depending on the electrical stimulation patterns: long-term potentiation (LTP; long-lasting enhancement of synaptic efficacy after certain patterns of stimulation) and long-term depression (LTD; long-lasting depression of synaptic transmission after a pattern of stimulation) (Bliss and Collingridge, 1993; Kandel, 2001; Malenka and Bear, 2004; Morris et al., 2003). Currently, textbooks explain that the level of Ca2+ influx resulting from a given pattern of stimulation determines whether to produce LTP or LTD by inserting or removing AMPARs from the surface, respectively (Kessels and Malinow, 2009; Neveu and Zucker, 1996). Multiple forms of LTP and LTD have been characterized in various brain structures. LTP and LTD occur in an NMDAR-dependent manner in the hippocampus, a structure deeply implicated for learning and memory. The electrical stimulation pattern, such as brief, high frequency stimulation (HFS; for example, 100 Hz for 0.2–2 s) or theta burst stimulation (TBS; for example, 4–5 pulses at 100 Hz, repeated 5–10 times at 5 Hz), induces an immediate but brief increase in Ca2+ levels, thus resulting in LTP (Bashir et al., 1991; Berretta et al., 1991; Lee et al., 2000). However, low frequency stimulation (LFS; for example, 1 Hz for 5–15 min in the hippocampus) induces a slower and lower Ca2+ rise, which contributes to AMPAR internalization and thus to LTD induction (Gean and Lin, 1993; Man et al., 2000; Montgomery and Madison, 2002; Montgomery et al., 2005). Ca2+ influx is thought to activate a group of kinases and/or phosphatases, including Ca2+/calmodulin-dependent protein kinase II (CaMKII), protein kinase C (PKC), protein kinase A (PKA), the tyrosine kinase Src, and the protein phosphatases PP1/PP2A, within the cell to eventually modulate the number of surface AMPARs as well as the number of spines, thus leading to LTP or LTD (Berridge et al., 2003; Harnett et al., 2009; Lee et al., 2000; Li et al., 2011; Liu and Zukin, 2007; Morishita et al., 2005). Therefore, NMDARs have been proposed to be hallmarks of synaptic plasticity due to their instrumental role in long-lasting changes in synaptic efficacy (Malenka and Bear, 2004; Malenka and Nicoll, 1993).
The role of Ca2+ through NMDARs has been extensively investigated in long-term synaptic potentiation. Classical studies using NMDAR antagonists failed to induce LTP (Harris et al., 1984; Morris et al., 1986), as did experiments with Ca2+ chelators to block Ca2+ elevation within the cell (Lynch et al., 1983). Another line of evidence came from additional electrical stimulation patterns developed to induce LTP. In addition to HFS, the pairing moderate stimulation (1–2 Hz) with depolarization (holding at 0 mV) induces robust potentiation in synaptic responses, suggesting that release of the Mg2+ block during moderate activity is sufficient to activate NMDARs, thereby causing LTP (Chen et al., 1999; Malinow and Tsien, 1990). Similar observations have been obtained for LTD. Treatment with Ca2+ chelators or NMDAR antagonists successfully blocked LTD induction in given synapses (Cummings et al., 1996; Dudek and Bear, 1992), supporting the idea that elevated Ca2+, presumably through NMDAR together with voltage-gated Ca2+ channels, is instrumental for synaptic plasticity, and for learning and memory.
EVIDENCE FOR THE IONOTROPIC GLUTAMATE RECEPTOR AS A METABOTROPIC RECEPTOR
The provocative idea that NMDAR, the most traditional ionotropic glutamate receptor, may work as a metabotropic receptor arises from observations on synaptic depression induced by LFS, which employed a series of NMDAR antagonists. LFS-induced synaptic depression has been well characterized in acute hippocampal slice preparations and is referred to as NMDAR-dependent LTD (Thiels et al., 1996). Blockade of NMDAR function by using APV [D-(-)-2-amino-5-phosphonopentanoic acid], a widely used antagonist, completely blocked LTD induction in the hippocampus, consistent with previous observations. Striking differences were observed upon application of other antagonists, namely, MK-801 and 7CK (7-chlorokynurenate). Unlike APV, these NMDAR antagonists failed to block NMDAR-dependent LTD in given synapses (Nabavi et al., 2013).
Pharmacologically inconsistent action of antagonists sometimes reveals a novel function of given receptors or uncovers the involvement of unknown pathways, including unexpected receptors. For example, in a previous study on long-term synaptic depression of excitatory synapses onto interneurons in the hippocampus, the possible involvement of endocannabinoid receptors (CB1R) was investigated using two different antagonists (Gibson et al., 2008). Blockade of CB1R using its antagonist SR141716A abolished interneuron LTD, whereas blockade by another antagonist AM251 failed to do so. As AM251 exhibited no deficits as the CB1R antagonist when applied with a CB1R agonist, unknown receptors were sought that were possibly blocked by SR141716A but not by AM251. It was previously known that this type of interneuron LTD is independent of NMDAR activation so the authors examined the possible involvement of unknown receptors. Upon careful examination, it was determined that SR141716A also inhibits the function of transient receptor potential V1 (TRPV1) receptors in addition to CB1Rs (De Petrocellis et al., 2001). It turns out that extrasynaptic TRPV1 contributes to the interneuron LTD in the hippocampus. This view is now widely accepted in the field and led to further studies (for review, see Kullmann et al., 2012). Similarly, in the NMDAR-dependent LTD study discussed above, all three antagonists effectively blocked NMDAR-mediated currents. Instead, the pharmacological sites of action of these antagonists differ. MK-801 blocks NMDAR by clogging the pore for ion flux, and so does 7CK. 7CK competitively binds to a co-activator (such as glycine) binding site. However, APV binds to glutamate binding sites on GluN2 subunits, while leaving the pore open (Traynelis et al., 2010). Therefore, the glutamate binding itself appears to be important for successful induction of NMDAR-dependent synaptic depression, thereby dissociating NMDAR function into ionotropic and metabotropic (Nabavi et al., 2013) (Fig. 1).
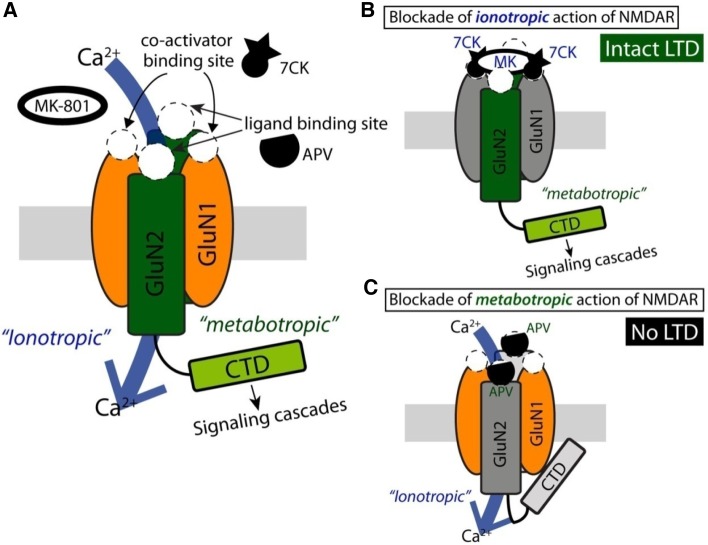
Fig. 1.
Schematic diagram of a new model that depicts ionotropic and metabotropic actions of NMDARs. (A) NMDARs are tetramers, composed of 2 GluN1 subunits and 2 GluN2 subunits. The GluN1 subunit contains glycine-binding sites that can be antagonized by 7CK. The GluN2 subunit contains glutamate-binding sites that can be antagonized by APV and R-CPP. MK-801 is a pore blocker that eliminates ion flux through NMDARs in a use-dependent manner as ketamine. The new model proposes that upon glutamate binding and release of the Mg2+ block, NMDARs allow ion flux and initiate signaling cascades, presumably via the C-terminal domain (CTD). (B) When ionotropic action is selectively inhibited by antagonists such as 7CK, MK-801, and ketamine, NMDAR-dependent LTD in the hippocampus is expected to be intact. (C) When the metabotropic action of NMDAR is selectively inhibited using competitive antagonists such as APV and R-CPP, NMDAR-dependent LTD is anticipated to be eliminated due to blockade of the necessary signaling cascades.
More striking observations come from experiments where LTD was induced with HFS (a stimulation pattern generally used to induce LTP) in the presence of MK-801, a pore blocker of NMDARs. This result suggests that as long as no Ca2+ enters the neuron, the metabotropic activation of NMDARs seems to favor depression of given synapses (Nabavi et al., 2013). Is there any signaling pathway turned on by NMDAR activation upon ligand binding, then? Indeed, binding of glutamate to NMDAR increased the level of activated p38 MAPK in cultured neurons and Ca2+ influx was found to play no role in p38 MAPK phosphorylation (Nabavi et al., 2013), which lends further support for this new model of NMDAR action.
Therefore, now what determines whether to induce LTP or LTD does not seem to be the level of Ca2+ increase or the pattern of electrical stimulation, but rather the activation modes of NMDARs. The ionotropic activation of NMDARs (i.e., massive Ca2+ influx) induces LTP, while the metabotropic activation of NMDARs (i.e., conformational change of NMDAR, initiating p38 MAPK signaling cascades) induces LTD. Note that Ca2+ increase would still induce LTP; however, as long as the Ca2+ levels remain at the basal intracellular level, metabotropic NMDAR activation will lead to synaptic depression (Fig. 1).
In fact, it has been previously documented that NMDAR could function independent of ion flux. In the hippocampus, phosphorylation of the NMDAR subunit, which is responsible for receptor trafficking was shown to be regulated in an activity-dependent manner regardless of ion influx through the pore (Vissel et al., 2001). In addition, it has been reported that manipulations of the C-terminal domain of NMDARs, without altering synaptic transmission per se, could modulate synaptic plasticity (Kohr et al., 2003; Ryan et al., 2013). In hippocampal Schaffer collateral synapses, truncation of the C-terminal domain of an isoform of the GluN2 subunit was shown to have a minimal effect on the amount of Ca2+ influx (Kohr et al., 2003). Another recent study took advantage of a line of genetically engineered animals that have swapped C-terminal domains between the GluN2A and 2B subunits and examined the effect of this domain at the cellular as well as behavioral level (Ryan et al., 2013). Synapses prepared from these animals showed no alterations in basal synaptic transmission, which was evaluated by measuring NMDAR-mediated evoked excitatory postsynaptic current (eEPSC) and AMPAR-mediated eEPSC ratio, paired pulse facilitation, and the kinetics of NMDAR-eEPSC. These data indicate that synaptic transmission, including ion influx, is minimally disrupted. However, swapping C-terminal domains of the GluN2 subunits caused serious defects in various learning or emotional behaviors (Ryan et al., 2013), implying that a distinct mechanism beyond altered synaptic transmission drives synaptic as well as behavioral plasticity, possibly via metabotropic signaling cascades induced by the C-terminal domain of GluN2.
CLINICAL IMPLICATIONS OF “METABOTROPIC” NMDARS
The most common progressive neurodegenerative disease, Alzheimer’s disease (AD), exhibits neurofibrillary tangles as a distinct pathophysiological hallmark. The tangles are an accumulation of overproduced beta amyloid (Aβ) proteins and hyperphosphorylated tau protein (Selkoe and Schenk, 2003). One of the currently accepted explanations regarding the pathophysiology of AD is the “amyloid hypothesis”, which proposes that the accumulated hydrophobic Aβ peptides are the main pathological consequence observed in the brain of patients with AD. Aβ is generated from amyloid precursor protein (APP) via sequential processing by a series of secretases (Kamenetz et al., 2003; Selkoe and Schenk, 2003).
Aβ is known to exert detrimental effects at the synaptic level before the onset of behavioral symptoms such as memory loss and cognition deficits (Venkitaramani et al., 2007). It has been reported that preparations obtained from a number of animal models of AD exhibit impaired LTP, thereby recapitulating cognitive dysfunction observed in patients with AD (Shankar et al., 2008; Snyder et al., 2005; Walsh et al., 2002). These studies reported that the type of LTP disrupted in AD model synapses is NMDAR-dependent (Shankar et al., 2007; 2008; Snyder et al., 2005). In addition, it has been well documented that Aβ-mediated synaptic depression shares signaling pathways with NMDAR-dependent LTD, including major players such as calcineurin, a calcium-activated phosphatase, and caspase-3 (Hsieh et al., 2006; Venkitaramani et al., 2007). Moreover, some reports suggest that blockade of NMDAR alleviates Aβ-mediated synaptic depression, suggesting NMDAR as a potential drug target (Shankar et al., 2007). However, the detailed action of Aβ on synaptic depression remains uncertain because the pathological outcomes of Aβ used in experimental settings differ depending on oligomerization status of Aβ and/or its location (Gasparini and Dityatev, 2008).
It seems clear that functional NMDAR mediates the deleterious effects of Aβ on synaptic transmission because blockade of NMDARs using APV and R-CPP [3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid], both of which act on GluN2 subunits, protects synapses from the Aβ-induced synaptic depression (Kessels et al., 2013; Shankar et al., 2007; Snyder et al., 2005). Organotypic slice preparations sparsely infected with Aβ precursor proteins were used to allow direct comparison of the synaptic efficacy between control and Aβ-overexpressed neurons. As suggested previously (Hu et al., 2009), GluN2B-containing NMDARs appear to mediate the deleterious effect of Aβ on synaptic transmission because the GluN2B-specific antagonists Ro 25-6981 and ifenprodil minimized Aβ-mediated synaptic deficits (Kessels et al., 2013). Additionally, blockade of GluN1 subunits exhibits no such protective effect, and Aβ seems to promote the switch between GluN2A and GluN2B, implying that Aβ has precise target sites on synapses. Differential interaction with various postsynaptic proteins (such as neuronal nitric oxide synthase, Homer, β-catenin, and CRMP2) depending on the subunit composition may help to explain the specificity of the deleterious effects of Aβ (Al-Hallaq et al., 2007). Given the similarity in cellular mechanisms shared between NMDAR-dependent LTD and Aβ-mediated synaptic depression, whether Aβ-mediated synaptic dysfunction also requires activation of metabotropic NMDARs has been examined (Nabavi et al., 2013). Pore blockers of NMDARs, such as ketamine and MK-801, are also unable to abolish Aβ-induced synaptic depression, strongly suggesting that synaptic defects shown in AD are mediated by “ion flux-independent” actions of NMDARs (Kessels et al., 2013).
CONCLUSION
The new model for NMDAR working principles presents dual action upon activation (ionotropic and metabotropic) proposing the distinct involvement of each action of one receptor under different physiological as well as pathophysiological conditions. Evidence suggesting that pathological Aβ accumulation may exert its effects through selective disruption in metabotropic action of NMDARs provides more convincing arguments for this new model, although further examination and careful verification of this model needs to be preceded.
Blockade of NMDAR appears to be one of the favorite target sites for clinical treatment of a number of diseases. For example, a non-competitive NMDAR antagonist ketamine is currently under clinical evaluation for its therapeutic action in depressive disorders (for review, see Chung, 2012; Kavalali and Monteggia, 2012). Ketamine, however, is shown to bind to the allosteric site and blocks Ca2+ influx (Anis et al., 1983), thus implying the role of ionotropic action of NMDARs in the pathophysiology of depressive disorders. However, this does not suggest that the metabotropic action of NMDARs is not involved in depressive disorders because administration of R-CPP, a competitive antagonist for glutamate binding to GluN2 subunits, has been reported to have antidepressant-like effects (Autry et al., 2011). Likewise, the possibility that the ionotropic action of NMDARs also contributes to AD symptoms cannot be ruled out because a previous study using Ca2+ imaging approaches reported that Aβ oligomers reduce Ca2+ influx into the spine head (Shankar et al., 2007). In addition, memantine, an effective AD medication, is a fast, voltage-sensitive pore blocker of NMDARs, which hampers Ca2+ influx through its pore (Danysz and Parsons, 2003; Frankiewicz et al., 1996; Misztal et al., 1996).
The observations obtained thus far are limited to samples prepared in vitro. Therefore, evidence of such metabotropic functions of NMDARs in vivo is expected to provide further support for this new member of the metabotropic glutamate receptors. Furthermore, the detailed signaling cascades initiated by NMDARs need to be identified. Thorough investigation of the functional distinction between the ionotropic and metabotropic actions of NMDARs is anticipated to provide an opportunity to revise our knowledge of the working principles of NMDARs, as well as to contribute for developing possibly more powerful clinical interventions for neurodegenerative diseases.
Acknowledgments
Research in my laboratory is supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (2011-A010-0043 and 2012-A010-0030), and from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (C.C. Grant Number: 2012-A419-0160).
REFERENCES
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. (2007). NMDA diheteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 27, 8334–8343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis NA, Berry SC, Burton NR, Lodge D. (1983). The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 79, 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. (1991). Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature 349, 156–158 [DOI] [PubMed] [Google Scholar]
- Berretta N, Berton F, Bianchi R, Brunelli M, Capogna M, Francesconi W. (1991). Long-term potentiation of NMDA receptor-mediated EPSP in Guinea-pig Hippocampal Slices. Eur J Neurosci. 3, 850–854 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. (2003). Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 [DOI] [PubMed] [Google Scholar]
- Chen HX, Otmakhov N, Lisman J. (1999). Requirements for LTP induction by pairing in hippocampal CA1 pyramidal cells. J Neurophysiol. 82, 526–532 [DOI] [PubMed] [Google Scholar]
- Chung C. (2012). New perspectives on glutamate receptor antagonists as antidepressants. Arch Pharm Res. 35, 573–577 [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Lester RA. (1989). Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 41, 143–210 [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. (2009). A nomenclature for ligand-gated ion channels. Neuropharmacology 56, 2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. (1997). Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 37, 205–237 [DOI] [PubMed] [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. (1996). Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron 16, 825–833 [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. (2003). The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: preclinical evidence. Int J Geriatr Psychiatry 18, S23–32 [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. (2001). The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem. 276, 12856–12863 [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. (1992). Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA 89, 4363–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankiewicz T, Potier B, Bashir ZI, Collingridge GL, Parsons CG. (1996). Effects of memantine and MK-801 on NMDA-induced currents in cultured neurones and on synaptic transmission and LTP in area CA1 of rat hippocampal slices. Br J Pharmacol. 117, 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini L, Dityatev A. (2008). Beta-amyloid and glutamate receptors. Exp Neurol. 212, 1–4 [DOI] [PubMed] [Google Scholar]
- Gean PW, Lin JH. (1993). D-2-amino-5-phosphonovaleate blocks induction of long-term depression of the NMDA receptor-mediated synaptic component in rat hippocampus. Neurosci Lett. 158, 170–172 [DOI] [PubMed] [Google Scholar]
- Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. (2008). TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57, 746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett MT, Bernier BE, Ahn KC, Morikawa H. (2009). Burst-timing-dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron 62, 826–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EW, Ganong AH, Cotman CW. (1984). Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 323, 132–137 [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. (2006). AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron 52, 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu NW, Klyubin I, Anwyl R, Rowan MJ. (2009). GluN2B subunit-containing NMDA receptor antagonists prevent Abeta-mediated synaptic plasticity disruption in vivo. Proc Natl Acad Sci USA 106, 20504–20509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. (2003). APP processing and synaptic function. Neuron 37, 925–937 [DOI] [PubMed] [Google Scholar]
- Kandel ER. (2001). Neuroscience - the molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. (2012). Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry 169, 1150–1156 [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. (2009). Synaptic AMPA receptor plasticity and behavior. Neuron 61, 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Nabavi S, Malinow R. (2013). Metabotropic NMDA receptor function is required for beta-amyloid-induced synaptic depression. Proc Natl Acad Sci USA 110, 4033–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. (2004). PDZ domain proteins of synapses. Nat Rev Neurosci. 5, 771–781 [DOI] [PubMed] [Google Scholar]
- Kohr G, Jensen V, Koester HJ, Mihaljevic AL, Utvik JK, Kvello A, Ottersen OP, Seeburg PH, Sprengel R, Hvalby O. (2003). Intracellular domains of NMDA receptor subtypes are determinants for long-term potentiation induction. J Neurosci. 23, 10791–10799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. (2012). Plasticity of inhibition. Neuron 75, 951–962 [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. (2000). Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955–959 [DOI] [PubMed] [Google Scholar]
- Li HB, Jackson MF, Yang K, Trepanier C, Salter MW, Orser BA, Macdonald JF. (2011). Plasticity of synaptic GluN receptors is required for the Src-dependent induction of long-term potentiation at CA3-CA1 synapses. Hippocampus 21, 1053–1061 [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. (2007). Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 30, 126–134 [DOI] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. (1983). Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305, 719–721 [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. (1986). NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 321, 519–522 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. (2004). LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. (1993). NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 16, 521–527 [DOI] [PubMed] [Google Scholar]
- Malinow R, Tsien RW. (1990). Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature 346, 177–180 [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. (2000). Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron 25, 649–662 [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. (1994). N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 74, 723–760 [DOI] [PubMed] [Google Scholar]
- Misztal M, Frankiewicz T, Parsons CG, Danysz W. (1996). Learning deficits induced by chronic intraventricular infusion of quinolinic acid--protection by MK-801 and memantine. Eur J Pharmacol. 296, 1–8 [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Madison DV. (2002). State-dependent heterogeneity in synaptic depression between pyramidal cell pairs. Neuron 33, 765–777 [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Selcher JC, Hanson JE, Madison DV. (2005). Dynamin-dependent NMDAR endocytosis during LTD and its dependence on synaptic state. BMC Neurosci. 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540 [DOI] [PubMed] [Google Scholar]
- Morishita W, Marie H, Malenka RC. (2005). Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat Neurosci. 8, 1043–1050 [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. (1986). Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319, 774–776 [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O’Carroll C. (2003). Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 358, 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, Malinow R. (2013). Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc Natl Acad Sci USA 110, 4027–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu D, Zucker RS. (1996). Postsynaptic levels of [Ca2+]i needed to trigger LTD and LTP. Neuron 16, 619–629 [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. (1994). The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci. 14, 6102–6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner C, Kohr G. (2011). Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 286, 7558–7566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Tank DW. (1990). Postsynaptic NMDA receptor-mediated calcium accumulation in hippocampal CA1 pyramidal cell dendrites. Nature 345, 807–810 [DOI] [PubMed] [Google Scholar]
- Ryan TJ, Kopanitsa MV, Indersmitten T, Nithianantharajah J, Afinowi NO, Pettit C, Stanford LE, Sprengel R, Saksida LM, Bussey TJ, et al. (2013). Evolution of GluN2A/B cytoplasmic domains diversified vertebrate synaptic plasticity and behavior. Nat Neurosci. 16, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D. (2003). Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 43, 545–584 [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. (2007). Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 27, 2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. (2008). Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 14, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. (1994). Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368, 144–147 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, et al. (2005). Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 8, 1051–1058 [DOI] [PubMed] [Google Scholar]
- Stocca G, Vicini S. (1998). Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 507, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Xie X, Yeckel MF, Barrionuevo G, Berger TW. (1996). NMDA receptor-dependent LTD in different subfielffds of hippocampus in vivo and in vitro. Hippocampus 6, 43–51 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 62, 405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Chin J, Netzer WJ, Gouras GK, Lesne S, Malinow R, Lombroso PJ. (2007). Beta-amyloid modulation of synaptic transmission and plasticity. J Neurosci. 27, 11832–11837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. (2001). A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci. 4, 587–596 [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. (2002). Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]