Abstract
The migration and proliferation of vascular smooth muscle cells (VSMCs) are essential elements during the development of atherosclerosis and restenosis. An increasing number of studies have reported that extracellular matrix (ECM) proteins, including the CCN protein family, play a significant role in VSMC migration and proliferation. CCN4 is a member of the CCN protein family, which controls cell development and survival in multiple systems of the body. Here, we sought to determine whether CCN4 is involved in VSMC migration and proliferation. We examined the effect of CCN4 using rat cultured VSMCs. In cultured VSMCs, CCN4 stimulated the adhesion and migration of VSMCs in a dose-dependent manner, and this effect was blocked by an antibody for integrin α5β1. CCN4 expression was enhanced by the pro-inflammatory cytokine tumor necrosis factor α (TNF-α). Furthermore, knockdown of CCN4 by siRNA significantly inhibited the VSMC proliferation. CCN4 also could up-regulate the expression level of marker proteins of the VSMCs phenotype. Taken together, these results suggest that CCN4 is involved in the migration and proliferation of VSMCs. Inhibition of CCN4 may provide a promising strategy for the prevention of restenosis after vascular interventions.
Keywords: CCN4, marker protein, small interfering RNA, vascular smooth muscle cell
INTRODUCTION
Atherosclerotic stenosis and its ischemic complications necessitate arterial reconstruction. Current therapeutic strategies to restore blood flow in stenotic coronary arteries include percutaneous transluminal angioplasty, intracoronary stents and coronary artery bypass surgery. Although these therapies have shown considerable promise in reducing the incidence of restenosis (Grinius et al., 2007), it remains a considerable medical challenge; the incidence of restenosis is high: up to 40% within 6 months after percutaneous transluminal angioplasty (Schillinger et al., 2003) and up to 20% after bypass surgery (Griffiths et al., 2004). Moreover, the trend towards primary stenting has not significantly improved the patency rate (Choy et al., 2001; Coolong and Kuntz, 2007). Therefore, restenosis remains the Achilles’ heel of coronary intervention, requiring continuous advances in our understanding of its mechanism and new innovative solutions. A dominant cellular event of restenosis is smooth muscle cell (SMC) proliferation and migration. After vascular injury, the SMCs start to proliferate and then migrate into the developing neointima, thus becoming the major cellular substrate of the restenotic tissue (Andrés et al., 2012).
Several studies have demonstrated that the extracellular matrix (ECM) plays a major role in the development of restenosis. CCNs are relatively small among ECM proteins, they regulate a broad spectrum of cellular responses, including cell adhesion and migration, differentiation and proliferation, apoptosis and survival, as well as the generation of reactive oxygen species and alterations of gene expression (Perbal, 2004). CCNs can also regulate the activities of other growth factors and cytokines by modulating their bioavailability and triggering signaling crosstalk (Brigstock, 2003). Therefore, CCNs may function in a context-dependent manner in vivo, as they modulate the activities of other growth factors and cytokines that are co-expressed. Studies in cell culture systems and in animal models have shown that CCNs play critical roles in angiogenesis and cardiovascular development, chondrogenesis and skeletal development, wound healing and tissue repair, and the pathobiology of chronic diseases such as fibrosis and cancer (ChenLau, 2009; Rachfal and Brigstock, 2005; Schutze et al., 2005).
CCN4 is a member of the CCN family that is defined by the first three members of the family, cysteine-rich protein 61, connective tissue growth factor, and Nephroblastoma overexpressed gene (Berschneider and Königshoff, 2011). CCN4 was initially identified as a gene in a mouse mammary epithelial cell line (Pennica et al., 1998), was later determined to modulate gastric tumor growth (Davies et al., 2007), and was regulated by Wnt1, a cysteine-rich glycosylated protein that controls cell development and survival in multiple systems of the body (Chong et al., 2011; He et al., 2005; Maiese et al., 2008). CCN4 also promotes TNF-α-stimulated cardiac fibroblast proliferation, and is up-regulated in myocardial infarction, pulmonary fibrosis (Kramer et al., 2009) and colitis (Wang et al., 2009a). Knockdown of CCN1 by siRNA inhibits neointimal hyperplasia after balloon angioplasty (Matsumae et al., 2008). CCN3 could inhibit neointimal hyperplasia through modulation of SMC growth and migration (Shimoyama et al., 2010). According to their structural similarity, we speculated that CCN4 may play a role in cardiovascular disease.
In this study, to test the hypothesis that CCN4 participates in VSMC migration and proliferation, we characterized the effect of CCN4 on VSMC migration and proliferation, and investigated whether the knockdown of CCN4 could inhibit VSMC proliferation. Furthermore, we examined the effect of CCN4 on VSMCs markers. These results suggested that CCN4 facilitates VSMC migration in cultured VSMCs. The application of small interfering RNAs (siRNAs) targeting CCN4 greatly suppressed VSMC proliferation. Meanwhile, CCN4 could up-regulate the expression level of marker proteins of the VSMCs phenotype.
MATERIALS AND METHODS
Materials
Monoclonal antibodies (mAbs) against integrins such as anti-α5β1, anti-α5, and anti-β1 were purchased from Abcam (USA). The anti-CCN4 antibody, anti-vascular cell adhesion molecule-1 (VCAM-1), anti-elastin, anti-osteopontin and anti β-actin antibodies were purchased from Invitrogen (USA). All other chemicals and reagents were purchased from Sigma (USA).
VSMC culture
Primary cultures of rat aortic SMC were isolated from Sprague-Dawley rats (Chamley-Campbell et al., 1979). In brief, the tunica media was minced and digested in 5 ml of the digestion solution (2 mg/ml collagenase, 0.125 mg/ml elastase, 0.25 mg/ml soybean trypsin inhibitor, 2.0 mg/ml crystallized bovine albumin) for 45 min at 37°C. The cellular digests were filtered through sterile 100-μM nylon mesh, centrifuged at 1,000 rpm for 10 min, and washed twice in DMEM medium containing 10% fetal bovine serum (FBS). All SMC lineage was confirmed by the presence of immune-reactivity for SM α-actin in > 95% of the cells. Experiments were performed with mouse SMCs from 3–6 passages. All studies followed the guideline the Animal Care and Use Committee of Southern Medical University.
Real-time quantitative PCR analysis
Total RNA was extracted using the RNA plus kit (Takara, China). One to five micrograms of total RNA per sample was primed with Oligo dT primer and reverse transcribed using the AMV Reverse Transcription System (Takara, China). The levels of gene mRNA transcripts were analyzed by using the specific primers and SYBR Green I reagent and the RT-PCR kit, according to the manufacturer’s instructions, on Bio-Rad iQ5 Quantitative PCR System (Takara, China). The specific primers for rat CCN4 were sense, 5′-AGAGCCGCCTCTGCAACTT-3′ and antisense, 5′-GGAGAAGCCAAGCCCATCA-3′; for osteopontin were 5′-CCAGCACACAAGCAGACGTT-3′ and anti-sense, 5′-TCAGTCCATAAGCCAAGCTAT-3′; for elastin were sense, 5′-CCCGCAGTTACCTTTCCG-3′, and antisense, 5′-GGCACTTTCCCAGGCTTC-3’; and for β-actin were sense, 5′-GATCATTGCTCCTCCTGAGC-3′ and antisense, 5′-ACTCC TGCTTGCTGATCCAC-3′. Briefly, 20 μl reactions containing 50 ng of total RNA, 10 μl 2× SYBR Green I reagent, 6.25 U Multi-Scribe reverse transcriptase, 10U RNase inhibitor and 0.1 mM primers were subjected to one cycle of 94°C for 10 min and then 40 cycles of 94°C for 30 s, 59°C for 30 and 72°C for 30 s. For relative quantification, the levels of individual gene mRNA transcripts were firstly normalized to the control β-actin. Subsequently, the differential expression of these genes was analyzed by the ΔCt method and expressed as the fold changes.
Western blotting
Total protein extracts were prepared using RIPA lysis buffer (Beyotime, China) according to the operating instructions. The protein concentration in the lysates was evaluated using a BCA protein assay kit (Beyotime, China). For western blotting, the proteins lysates (30 μg/lane) were separated by SDS-PAGE and transferred onto polybinylidene difluoride membranes (Whatman Schleicher & Schuell, UK). After blocking, the target proteins were probed with primary antibody (anti-CCN4, anti-VCAM-1, anti-elastin, anti-osteopontin or β-actin) overnight at 4°C. Then the blots were washed and incubated with horseradish peroxidase-conjugated secondary antibody. After washing, the sites of antibody binding were visualized by chemiluminescence (Boehringer Mannheim, Germany) and the relative levels of each protein to the β-actin were analyzed.
Recombinant lentivirus construction and CCN4 siRNA transfection
Lentivirus-mediated siRNA constructs were designed as previously described (Yoshida et al., 2007). In brief, the annealed oligonucleotides encoding sense and antisense strands linked by the loop sequence were sbu-cloned into a pSINsi-Mu6 vector (Invitrogen). The sequences corresponding to the siRNA of CCN4 were sense 5′-AGGUGGAGUUAACAAGAA-3′, and antisense, 5′-UUCUUGUUAACTCCACCU-3′. The siRNA-producing construct was introduced into a lentivirus vector, pLenti6/V5-D-TOPT (Invitrogen), and the recombinant lentiviruses were propagated in 293T cells. Transfection of VSMCs was incubated in the virus-containing culture medium for 24 h and in serum-free medium for 48 h; the cells were then used for analysis. The transfection efficiency was examined by western blotting, as described above.
VSMCs adhesion and migration assay
A solid-phase binding assay was performed as previously described with some modifications to investigate VSMCs adhesion (Cobbold and Waldmann, 1981). Briefly, microtiter wells were coated with 0.5% BSA and incubated at 4°C overnight. Recombinant CCN4 (1–40 μg/ml) was added to the wells and blocked with 1% BSA for 4 h at 4°C. Non-treated VSMCs or VSMCs treated with function blocking mAbs for 1 h were then incubated at room temperature for 1 h. After washing, adherent cells were fixed with 1% glutaraldehyde, stained with crystal violet, and quantified by dye extraction with the measurement of absorbance at 595nm. VSMCs migration was measured with the transwell migration assay (Furukawa et al., 1999). In brief, VSMCs were added into the upper chamber of a seeded trans-well containing serum-free medium, while the lower chamber was supplemented with serum-free medium containing the indicated recombinant CCN4 or PDGF-BB as a positive control. VSMCs were subsequently allowed to migrate across a poly-carbonate filter. After incubation in a humidified atmosphere of 5% CO2/95% air at 37°C for 12 h, cells on the upper membrane surface were removed by washing with PBS, and those that had migrated on the bottom side of the filter were subsequently fixed in methanol and stained with Harris hematoxylin solution for 5 min. The number of cells per four high power fields was counted under a microscope in order to determine the average number of cells that had migrated. The number of cells represented migration activity.
VSMCs proliferation assay and BrdU uptake
For the BrdU incorporation, the rat VSMCs were plated at a density of 1.0 × 104/well in a 24-well tissue culture plate. Cell proliferation was confirmed by quantifying cell numbers using a cell counter (Kaihong, China), and BrdU incorporation into DNA was quantified by using the Cell Proliferation ELISA BrdU kit (Takara, China). In some cases, cells containing various anti-integrin antibodies were treated with CCN4 (10 μg/ml); other cells were treated with siRNA-CCN4 containing CCN4 (0–10 μg/ml). After 24 h serum starvation, the cells were incubated in the presence of BrdU at 37°C in a humidified incubator containing 5% CO2 for 24 h.
Statistical analysis
All results are reported as means ± SD. Statistical analysis involved use of the Student’s t-test for the comparison of 2 groups or 1-way ANOVA for multiple comparisons. P < 0.05 was considered to be significant.
RESULTS
Effect of CCN4 on VSMC adhesion in vitro
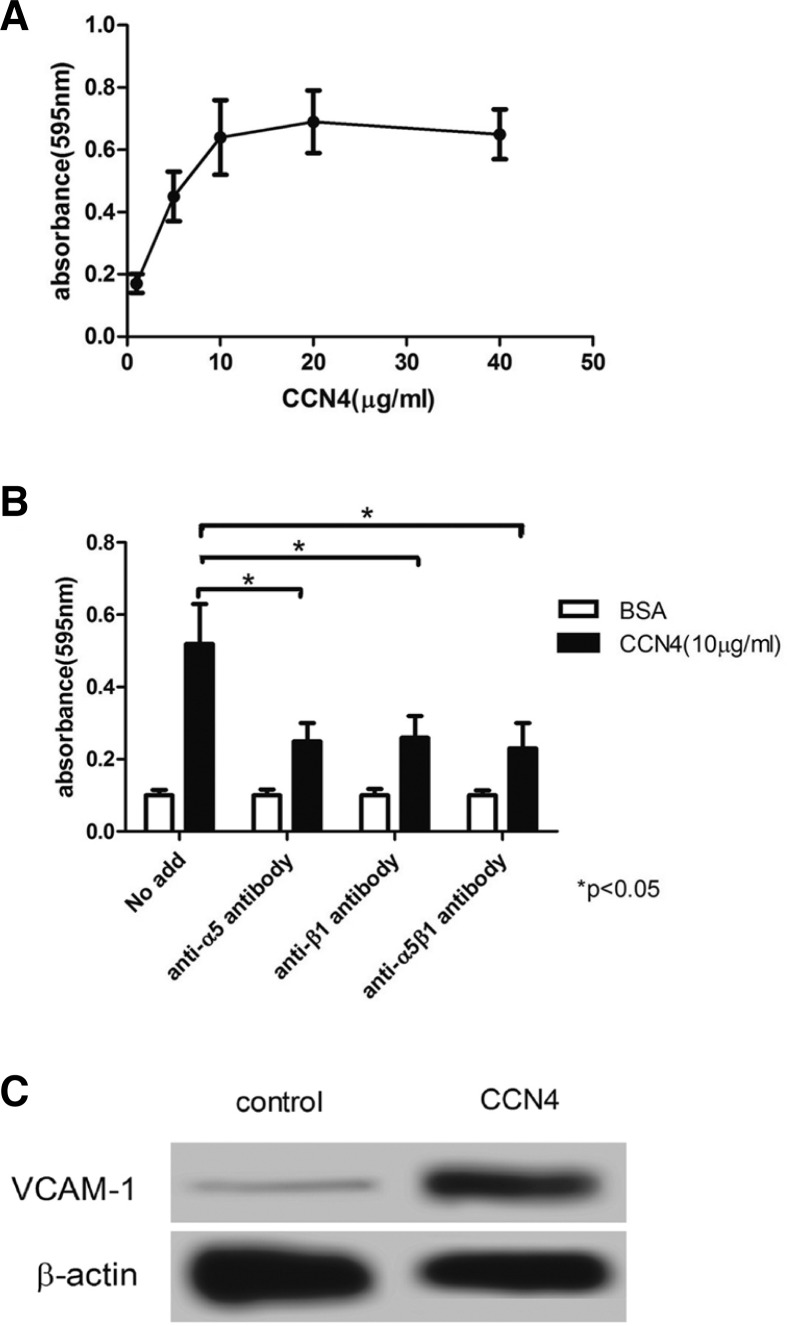
To examine the effect of CCN4 on adhesion of VSMC, we performed a solid-phase binding assay. As shown in Fig. 1A, CCN4 enhanced the VSMC adhesion in a concentration-dependent manner; the absorbance showed that the efficiency of VSMCs adhesion was only 0.17 ± 0.02 when 1 μg/ml was applied to VSMCs, which was markedly increased at the concentration of 5 μg/ml and 10 μg/ml CCN4, to 0.45 ± 0.07 and 0.63 ± 0.09, respectively, reaching a maximum (0.68 ± 0.07) when treated with 20 μg/ml CCN4. In contrast, pretreatment of cells with 40 μg/ml CCN4 slightly decreased the absorbance (0.62 ± 0.06). Furthermore, blocking antibodies against integrins α5, β1 and α5β1 were applied to explore the mechanism of CCN4 on VSMC adhesion; the result indicated that adhesion of VSMCs to CCN4 was significantly suppressed by anti-integrin antibodies, and pretreatment of VSMCs with anti-integrin antibodies declined the absorbance by 50% compared to the control group (*P < 0.05) (Fig. 1B). Then we examined the effect of CCN4 on expression of vascular cell adhesion molecule-1 (VCAM-1), western blot results showed that CCN4 significantly enhanced the expression of VCAM-1 (Fig. 1C). These results suggested that integrin may play a vital role between CCN4 and VSMCs adhesion.
Fig. 1.
The effect of CCN4 on VSMCs adhesion. (A) CCN4 was bound to VSMCs in a dose-dependent manner. (B) Integrin α5β1 regulated VSMCs adhesion to CCN4. (C) CCN4 enhanced the expression of VCAM-1. Data are mean ± SD of six dishes from three separate experiments. *P < 0.05 compared with control group.
Effect of CCN4 on VSMC migration and proliferation through integrin
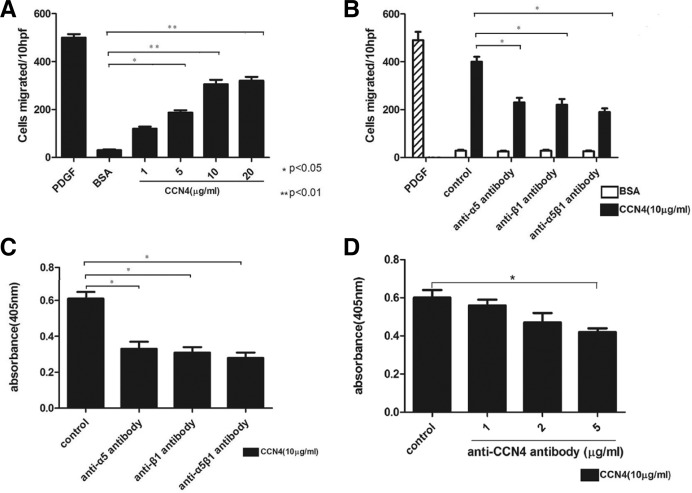
Since CCN4 has an effect on VSMCs adhesion, we asked whether it also has an effect on VSMC migration and proliferation. To explore the effect of CCN4 on VSMC migration, we adopted a transwell migration assay. As shown in Fig. 2A, we found that the enhanced VSMC migration induced by CCN4 was dose-dependent. The number of VSMCs migrating was greatly increased when various concentrations of CCN4 was employed as compared with the control group, beginning at the concentration of 1 μg/ml CCN4 and peaking around at 20 μg/ml (*P < 0.05, **P < 0.01). Meanwhile, in order to determine the mechanism of CCN4 on VSMCs migration, we used a blocking antibody against the integrins α5, β1 and α5β1 to treat VSMCs (Pickering et al., 2000). As indicated in Fig. 2B, anti-integrin antibodies decrease the VSMCs migration by 43%, 49% and 56%, respectively, as compared with the control (*P < 0.05). The result showed that the effect of CCN4 on VSMCs migration was regulated through integrin α5β1. We next performed a BrdU incorporation assay to identify the effect of CCN4 on VSMCs proliferation. As indicated in Fig. 2C, compared with the control, the application of anti-integrin antibodies markedly declined BrdU incorporation by 46%, 49% and 53%, respectively. Meanwhile, anti-CCN4 antibody was used to further confirm the role of CCN4 in VSMCs proliferation, as shown in Fig. 2D, anti-CCN4 antibody declined BrdU incorporation in a concentration-dependent manner (*P < 0.05). These results indicated that integrin α5β1 regulated the effect of CCN4 on VSMCs migration and proliferation.
Fig. 2.
Effect of CCN4 on VSMCs migration and proliferation through integrin. (A) CCN4-induced VSMCs migration in a dose-dependent manner. (B) VSMCs migration was regulated by α5β1. (C) VSMCs proliferation was mediated by α5β1. (D) Anti-CCN4 antibody declined BrdU incorporation in a concentration-dependent manner. (A-D) Data are mean ± SD of six dishes from three separate experiments. *P < 0.05, **P < 0.01 compared with control.
TNF-α induces CCN4 expression in VSMCs
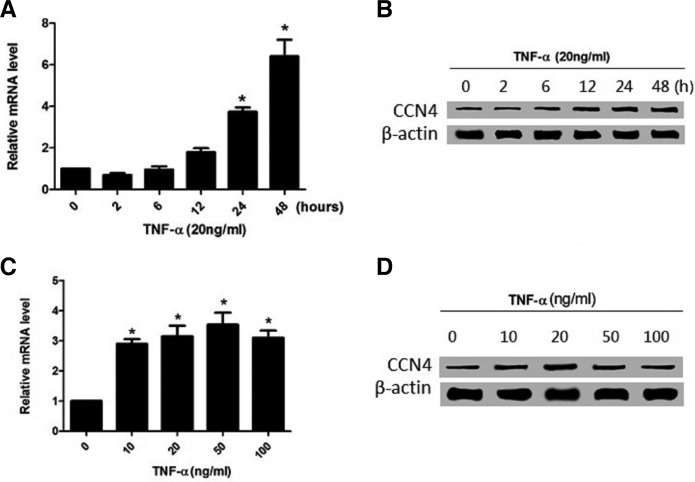
Tumor necrosis factor (TNF)-α is one of the pro-inflammatory cytokines that mediate a wide range of immune and inflammatory responses and have been found to be involved in the development of post-PCI restenosis and atherosclerosis (Bonta et al., 2010; Maddaluno et al., 2012). It has been reported that TNF-α stimulates the proliferation and migration of VSMCs, and is up-regulated at the site of vascular injury and in atherosclerotic plaque specimens (Gupta et al., 2012; Wang et al., 2009b). CCN4 was also involved in VSMCs adhesion, migration, and proliferation. Therefore, we examined the response of CCN4 to TNF-α in cultured VSMCs. As shown in Fig. 3A, after 6 h, TNF-α (20 ng/ml) greatly increased the mRNA level of CCN4, reaching a peak around 48 h. Then, to determine the optimal concentration of TNF-α, we used various concentrations. When 10 ng/ml was applied to CCN4, the level of CCN4 mRNA started to increase; while the level was decreased by treatment with 100 ng/ml TNF-α (Fig. 3C), suggesting that the elevated level of CCN4 mRNA by TNF-α was time- and concentration-dependent. Moreover, consistent with the mRNA level, the level of CCN4 protein was also increased by TNF-α (Figs. 3B and 3D).
Fig. 3.
TNF-α induced CCN4 expression in VSMCs. (A, B) Time-dependent induction of CCN4 mRNA and protein expression. (C, D) concentration-dependent induction of CCN4 by TNF-α. Results are means ± SD from three independent experiments performed in duplicate. Relative expression is expressed in arbitrary units. *P < 0.05 compared with no TNF-α treatment.
CCN4 knockdown by siRNA inhibits VSMC proliferation in vitro
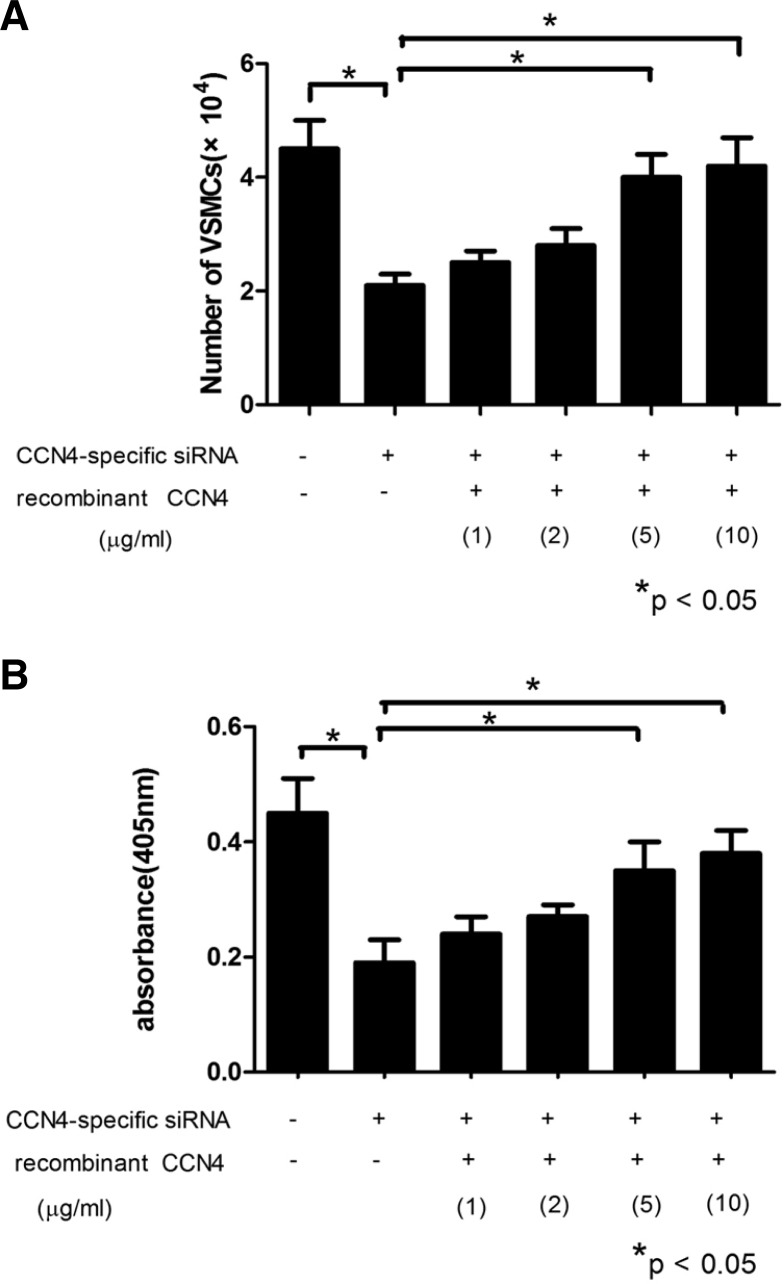
It is possible that a reduction of CCN4 levels could inhibit VSMC proliferation in vitro. Therefore, a lentivirus-mediated siRNA construct was designed, and transfected into the VSMCs in order to investigate the effect of siRNA-CCN4 on VSMCs proliferation. As shown in Fig. 4, proliferation assay showed that the number of VSMCs with siRNA-CCN4 was still almost half of that with control, and the incorporation of BrdU by VSMCs was also significantly decreased in the siRNA-CCN4 group compared with the control (*P < 0.05). Moreover, to corroborate the above analysis, we used the recombinant CCN4 protein to treat the VSMCs, which reversed the effect of CCN4 knockdown on VSMC proliferation and BrdU incorporation, exhibiting a dose-dependent manner (*P < 0.05). Taken together, these results suggested that the knockdown of CCN4 could inhibit VSMC proliferation in vitro.
Fig. 4.
Knockdown of CCN4 by siRNA inhibited the VSMC proliferation in vitro. (A) The number of VSMCs. (B) The incorporation of BrdU by VSMCs. There was a significant reduction in cells numbers and incorporation of BrdU, while the pretreatment of cells with the recombinant CCN4 protein reversed the effect of CCN4 knockdown on VSMC proliferation and BrdU uptake in a dose-dependent manner. *P < 0.05 compared with control.
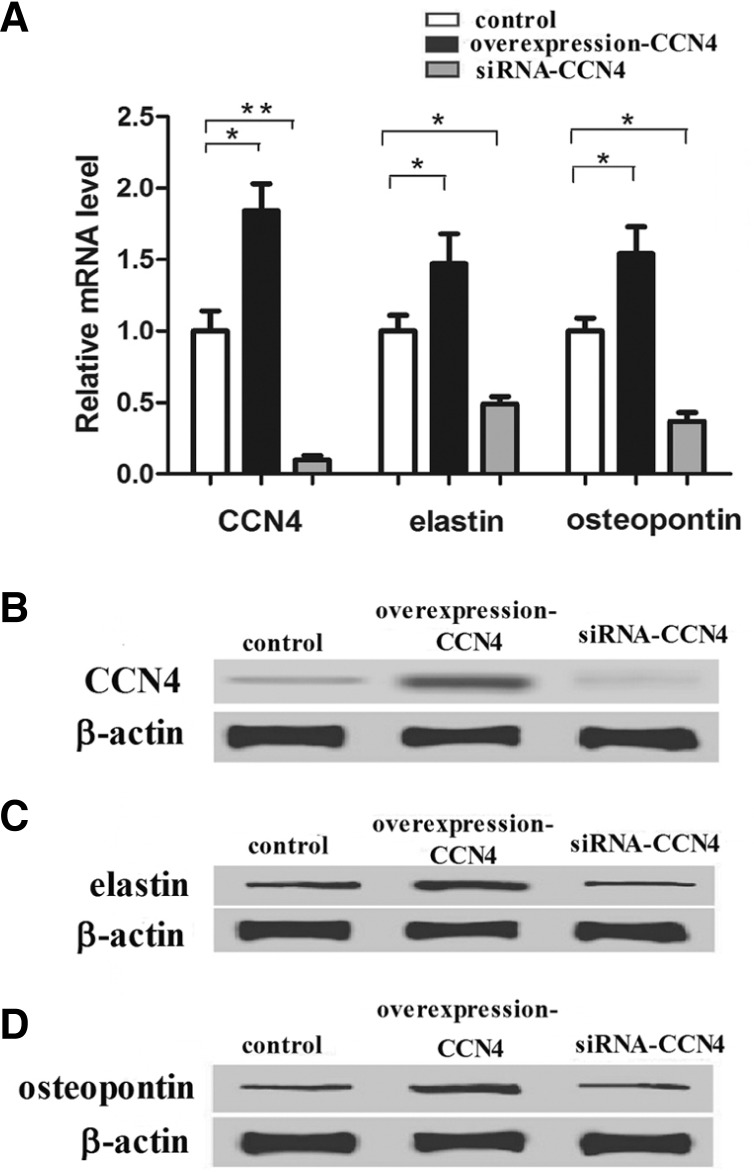
Effect of CCN4 on the expression of VSMCs markers
We found that CCN4 was involved in VSMC proliferation, which was closely connected with the VSMC phenotype. Therefore, elastin and osteopontin, VSMC marker proteins expressed in the synthetic phenotype, were applied to determine the effect of CCN4 on expression of VSMCs markers in this study. CCN4, elastin and osteopontin mRNA levels were significantly decreased by siRNA-CCN4 and increased by CCN4 overexpression (*P < 0.05, **P < 0.01) (Fig. 5A). Consistent with RT-PCR analysis, the protein level was quantified by the western blot in three independent experiments and normalized using the β-actin signal. As indicated in Figs. 5B, 5C, and 5D, down-regulation of CCN4, elastin and osteopontin was also observed after treatment of VSMCs with siRNA-CCN4, while pretreatment of cells with the CCN4 overexpression up-regulated the protein level of CCN4, elastin and osteopontin (*P < 0.05). Taken together, these results demonstrated that CCN4 could up-regulate the expression level of marker proteins of the VSMCs phenotype.
Fig. 5.
Effect of CCN4 on expression of the VSMCs marker proteins. (A) Representative images of relative mRNA level of CCN4 and VSMCs marker proteins treated with CCN4 overexpression and siRNA-CCN4. (B, C, and D) represent Western blots of control, CCN4 overexpression and siRNA-CCN4. The expression levels of proteins were normalized based on the β-actin levels. *P < 0.05, **P < 0.01 compared with control.
DISCUSSION
Restenosis is a maladaptive response of the coronary artery to trauma caused by angioplasty. Inflammation, thrombosis, cellular proliferation, and extracellular matrix production contribute to post-procedural lumen loss (Nikol and Huehns, 1996). Activated medial smooth muscle cells proliferate and migrate to the intima and synthesize the extracellular matrix, resulting in restenosis (Weintraub, 2007). In the present study, we focused on the function of CCN4 in VSMC migration and proliferation. Here we provide the first evidence that CCN4 expression was induced by TNF-α stimulation in VSMCs. Furthermore, VSMCs invasion/migration was accelerated by CCN4 up-regulation. Knockdown of CCN4 by lentiviral delivery of siRNA significantly inhibited the proliferation of VSMCs. Also, CCN4 could up-regulate the expression level of marker proteins of the VSMCs phenotype.
Integrins link the ECM with the actin cytoskeleton within VSMCs. The β1 subunit is the main β subunit in VSMCs both in vivo and in vitro; the major α-integrin subunits expressed in VSMCs in vivo are α1, α3, and α5 (Moiseeva, 2001). Ono et al reported that CCN4 has a positive influence on bone cell differentiation and function and may work by enhancing the effects of bone morphogenetic protein 2 (BMP-2) to increase osteogenesis through a mechanism potentially involving binding to integrin α5β1 (Ono et al., 2011). Hou et al demonstrated that pretreatment of OASFs (osteoarthritis synovial fibroblasts) with αvβ5 but not α5β1 and αvβ3 integrin antibodies reduced CCN4-induced IL-6 (interleukin-6) production (Hou et al., 2013). In this study, to specify the underlying mechanism of the effect of CCN4 on SMC proliferation, blocking antibodies against the integrins α5 or β1 were used to determine whether they regulate the effect of CCN4 on VSMCs. The result showed that CCN4 support VSMC adhesion and migration through the α5β1 integrin, and it is noteworthy that CCN4 also has a function on VSMC proliferation through the α5β1 integrin.
Recently, a research reported that although the pro-inflammatory cytokine TNF-α alone is not cytotoxic, the presence of CCN1, CCN2, or CCN3 can unmask TNF-α cytotoxic effects and induce fibroblast death. These authors show that CCN1 stimulates reactive oxygen species (ROS) generation and suggest that the high and sustained levels of ROS induced by CCN1/TNF-α result in the oxidative inactivation of JNK phosphatases, leading to the persistent activation of JNK and subsequent cell death (Bai et al., 2010). Although the induction of CCN family members by pro-inflammatory cytokines has already been reported, the novel result of this study is that a combination of TNF-α and CCN4 induce VSMCs proliferation; that is to say, CCN4 expression was induced by TNF-α stimulation in primary VSMCs.
Excessive proliferation of SMCs with the deposition of ECM proteins at the arterial anastomotic site is a critical problem during cardiovascular diseases (Janssens et al., 1998; Zahedmanesh and Lally, 2009). In this study, the direct contribution of CCN4 to VSMCs proliferation was examined using RNA interference. RNA interference is a new modality in gene therapy which can elicit down-regulation of gene expression and has enormous potential in the treatment of cardiovascular diseases (Fountaine et al., 2005). Many studies have demonstrated the power of in vivo administration of RNA interference in mammals, and the stability of the siRNA, the route of administration, and the choice of delivery tool are also crucial for success (Behlke, 2006; Rubinson et al., 2003). For example, CCN5 is required for the anti-proliferative effect of heparin in VSMCs by using RNA interference. The knockdown of CCN5 in VSMC causes changes in VSMCs morphology and cytoskeletal organization (Lake and Castellot, 2003). In this study, CCN4 knockdown was achieved by lentiviral transduction of small interfering RNA. The results demonstrated that RNA interference (RNAi)-mediated CCN4 knockdown may suppress VSMCs proliferation.
Early morphological and biochemical studies indicated that VSMCs exhibited two distinct phenotypes and that a change from the contractile to the synthetic phenotype was a prerequisite for progression of vascular disease (Shanahan and Weissberg, 1998). In this study, we adopted elastin and osteopontin as marker proteins of the VSMC synthetic phenotype to determine the effect of CCN4 on VSMCs marker proteins. The results indicated that CCN4 could up-regulate the expression level of marker proteins of the VSMCs phenotype. Matchkov et al reported that different Ca2+ transport proteins not only control averaged intracellular Ca2+ but also through their differences in the character of the Ca2+ signal modulate the activity of transcription factors and thus initiate phenotype switch (Matchkov et al., 2012). Li et al demonstrated that CCN3 physically interacts with the calcium binding protein S100A4 and induces a significant transient increase of intracellular calcium in pathological conditions (Li et al., 2002). However, the precise mechanism of CCN4 on marker proteins remains to be elucidated. Therefore, careful understanding of the cross talk between CCN4 and marker proteins of VSMCs phenotype is very important for more effective therapies.
In conclusion, this report demonstrates that CCN4 is involved in the migration and proliferation of VSMCs. Therefore, CCN4 may play an important role in human cardiovascular disease states such as atherosclerosis and restenosis after angioplasty, and may represent a novel therapeutic target in the prevention of restenosis after vascular interventions.
Acknowledgments
This research was funded by the Natural Science Foundation of Guangdong Province (grant number: 10151051501000058). The authors also would like to thank the lab animal resources of Southern Medical University for taking excellent care of our animals.
REFERENCES
- Andrés V, Fuster JJ, Silvestre-Roig C, Wessely R. (2012). Modulating the proliferative response to treat restenosis after vascular injury. Mol Trans Vas Med. 227–248 [Google Scholar]
- Bai T, Chen CC, Lau LF. (2010). Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 184, 3223–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behlke MA. (2006). Progress towards in vivo use of siRNAs. Mol Ther. 13, 644–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berschneider B, Königshoff M. (2011). WNT1 inducible signaling pathway protein 1 (WISP1): a novel mediator linking development and disease. Int J Biochem Cell B 43, 306–309 [DOI] [PubMed] [Google Scholar]
- Bonta PI, Pols TW, van Tiel CM, Vos M, Arkebout EK, Rohlena J, Koch KT, de Maat MP, Tanck MW, de Winter RJ, et al. (2010). Nuclear receptor Nurr1 is expressed in and is associated with human restenosis and inhibits vascular lesion formation in mice involving inhibition of smooth muscle cell proliferation and inflammation. Circulation 121, 2023–2032 [DOI] [PubMed] [Google Scholar]
- Brigstock D. (2003). The CCN family: a new stimulus package. J Endocrinol. 178, 169–175 [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J, Campbell G, Ross R. (1979). The smooth-muscle cell in culture. Physiol Rev. 59, 1–61 [DOI] [PubMed] [Google Scholar]
- Chen CC, Lau LF. (2009). Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell B 41, 771–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. (2011). EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3β, and β-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 8, 103–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy JC, Granville DJ, Hunt DWC, McManus BM. (2001). Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 33, 1673–1690 [DOI] [PubMed] [Google Scholar]
- Cobbold S, Waldmann H. (1981). A rapid solid-phase enzyme-linked binding assay for screening monoclonal antibodies to cell surface antigens. J Immunol Methods 44, 125–133 [DOI] [PubMed] [Google Scholar]
- Coolong A, Kuntz RE. (2007). Understanding the drug-eluting stent trials. Am J Cardiol. 100, 17–24 [DOI] [PubMed] [Google Scholar]
- Davies SR, Leigh Davies M, Sanders A, Parr C, Torkington J, Jiang WG. (2007). Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol. 36, 1129–1136 [DOI] [PubMed] [Google Scholar]
- Fountaine TM, Wood MJA, Wade-Martins R. (2005). Delivering RNA interference to the mammalian brain. Curr Gene Ther. 5, 399–410 [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, Matsushima K, Sasayama S. (1999). Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 84, 306–314 [DOI] [PubMed] [Google Scholar]
- Griffiths H, Bakhai A, West D, Petrou M, De Souza T, Moat N, Pepper J, Di Mario C. (2004). Feasibility and cost of treatment with drug eluting stents of surgical candidates with multi-vessel coronary disease. Eur J Cardiothorac Surg. 26, 528–534 [DOI] [PubMed] [Google Scholar]
- Grinius V, Navickas R, Unikas R. (2007). Stents in interventional cardiology. Medicina 43, 183–189 [PubMed] [Google Scholar]
- Gupta GK, Agrawal T, Del Core MG, Hunter WJ, Agrawal DK. (2012). Decreased expression of vitamin D receptors in neointimal lesions following coronary artery angioplasty in atherosclerotic swine. PLoS One 7, e42789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. (2005). Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene 24, 3054–3058 [DOI] [PubMed] [Google Scholar]
- Hou CH, Tang CH, Hsu CJ, Hou SM, Liu JF. (2013). CCN4 induces IL-6 production through avb5 receptor, PI3K, Akt, and NF-kB singling pathway in human synovial fibroblasts. Arthrit Res Ther. 15, 2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S, Flaherty D, Nong Z, Varenne O, van Pelt N, Haustermans C, Zoldhelyi P, Gerard R, Collen D. (1998). Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation 97, 1274–1281 [DOI] [PubMed] [Google Scholar]
- Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L. (2009). WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 119, 772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake AC, Castellot JJ., Jr (2003). CCN5 modulates the anti-proliferative effect of heparin and regulates cell motility in vascular smooth muscle cells. Cell Commun Signal. 1, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Martinez V, He B, Lombet A, Perbal B. (2002). A role for CCN3 (NOV) in calcium signalling. Mol Pathol. 55, 250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaluno M, Grassia G, Di Lauro MV, Parisi A, Maione F, Cicala C, De Filippis D, Luvone T, Guglielnotti A, Maffia P, et al. (2012). Bindarit inhibits human coronary artery smooth muscle cell proliferation, migration and phenotypic switching. PLoS One 7, e47464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ, Shang YC. (2008). The Wnt signaling pathway: aging gracefully as a protectionist?. Pharmacol Therapeut. 118, 58–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchkov VV, Kudryavtseva O, Aalkjaer C. (2012). Intra-cellular Ca2+ signalling and phenotype of vascular smooth muscle cells. Basic Clin Pharmacol Toxicol. 110, 42–48 [DOI] [PubMed] [Google Scholar]
- Matsumae H, Yoshinori Y, Ono K, Togi K, Inoue K, Furukawa Y, Nakashima Y, Kojima Y, Nobuyoshi M, Kita T, et al. (2008). CCN1 knockdown suppresses neointimal hyperplasia in a rat artery balloon injury model. Arterioscler Thromb Vasc Biol. 28, 1077–1083 [DOI] [PubMed] [Google Scholar]
- Moiseeva EP. (2001). Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 52, 372–386 [DOI] [PubMed] [Google Scholar]
- Nikol S, Huehns T. (1996). Molecular biology and post-angio-plasty restenosis. Atherosclerosis 123, 17–32 [DOI] [PubMed] [Google Scholar]
- Ono M, Inkson CA, Kilts TM, Young MF. (2011). WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J Bone Miner Res. 26, 193–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M. (1998). WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA 95, 14717–14722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. (2004). CCN proteins: multifunctional signalling regulators. Lancet 363, 62–64 [DOI] [PubMed] [Google Scholar]
- Pickering JG, Chow LH, Li S, Rogers KA, Rocnik EF, Zhong R, Chan BM. (2001). α5β1 integrin expression and luminal edge fibronectin matrix assembly by smooth muscle cells after arterial injury. Am J Pathol. 156, 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. (2005). Structural and functional properties of CCN proteins. Vitam Horm. 70, 69–103 [DOI] [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT. (2003). A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 33, 401–406 [DOI] [PubMed] [Google Scholar]
- Schillinger M, Exner M, Mlekusch W, Haumer M, Sabeti S, Ahmadi R, Schwarzinger I, Wagner O, Minar E. (2003). Restenosis after femoropopliteal PTA and elective stent implantation: predictive value of monocyte counts. J Endovasc Ther. 10, 557–565 [DOI] [PubMed] [Google Scholar]
- Schutze N, Noth U, Schneidereit J, Hendrich C, Jakob F. (2005). Differential expression of CCN-family members in primary human bone marrow-derived mesenchymal stem cells during osteogenic, chondrogenic and adipogenic differentiation. Cell Commun Signal. 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan CM, Weissberg PL. (1998). Smooth muscle cell heterogeneity patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscl Throm Vas. 18, 333–338 [DOI] [PubMed] [Google Scholar]
- Shimoyama T, Hiraoka S, Takemoto M, Koshizaka M, Tokuyama H, Tokuyama T, Watanabe A, Fujimoto M, Kawamura H, Sato S, et al. (2010). CCN3 inhibits neointimal hyperplasia through modulation of smooth muscle cell growth and migration. Arterioscler Thromb Vasc Biol. 30, 675–682 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang R, Wen S, McCafferty DM, Beck PL, MacNaughton WK. (2009a). Nitric oxide increases Wnt-induced secreted protein-1 (WISP-1/CCN4) expression and function in colitis. J Mol Med. 87, 435–445 [DOI] [PubMed] [Google Scholar]
- Wang L, Zheng J, Bai X, Liu B, Liu CJ, Xu Q, Zhu Y, Wang N, Kong W, Wang X. (2009b). ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. 104, 688–698 [DOI] [PubMed] [Google Scholar]
- Weintraub WS. (2007). The pathophysiology and burden of restenosis. Am J Cardiol. 100, 36–46 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Togi K, Matsumae H, Nakashima Y, Kojima Y, Yamamoto H, Ono K, Nakamura T, Kita T, Tanaka M. (2007). CCN1 protects cardiac myocytes from oxidative stress viaβ1 integrin-Akt pathway. Biochem Biophys Res Commun. 355, 611–618 [DOI] [PubMed] [Google Scholar]
- Zahedmanesh H, Lally C. (2009). Determination of the influence of stent strut thickness using the finite element method: implications for vascular injury and in-stent restenosis. Med Biol Eng Comput. 47, 385–393 [DOI] [PubMed] [Google Scholar]