Abstract
Although cancer stem cells (CSCs) play a crucial role in seeding the initiation of tumor progression, they do not always possess the same potent ability as tumor metastasis. Thus, precisely how migrating CSCs occur, still remains unclear. In the present study, we first comparatively analyzed a series of prostate CSCs, which exhibited a dynamically increasing and disseminating ability in nude mice. We observed that the transcriptional activity of HIF-1α and β-catenin became gradually elevated in these stem cells and their epithelial-mesenchymal transition (EMT) characteristic altered from an epithelial type to a mesenchymal type. Next, we further used cancer-associated fibroblasts (CAFs), which were cultured from surgically resected tissues of prostate cancer (PCa) to stimulate prostate CSCs. Similar results were reconfirmed and showed that the protein levels of both HIF-1α and β-catenin were markedly improved. In addition, the EMT phenotype displayed a homogenous mesenchymal type, accompanied with increased aggressive potency in vitro. Most importantly, the aforementioned promoting effect of CAFs on prostate CSCs was completely repressed after “silencing” the activity of β-catenin by transfection of stem cells with ShRNA. Taken together, our observations suggest that prostate migrating CSCs, with a mesenchymal phenotype, could be triggered by CAFs in a HIF-1α/β-catenin-dependent signaling pathway.
Keywords: cancer-associated fibroblast, epithelial-mesenchymal transition, migrating cancer stem cell, prostate cancer
INTRODUCTION
Several lines of evidence support the notion that the host reactive stroma could effectively improve the growth, and invasive potential of carcinoma cells (Joyce et al., 2009; Kalluri et al., 2006; Liotta et al., 2001). Among stromal host cells, activated fibroblasts are demonstrated to be involved in the growth and dissemination of several tumor cells through secretion of soluble growth factors or inflammatory cytokines, and following production of extracellular matrix proteins and their proteases (Chung et al., 2005; Kaminski et al., 2006; Silzle et al., 2004; Studebaker et al., 2008). Moreover, CAFs have been reported to stimulate cancer cells escaping from local growth control mechanisms, distant dissemination and angiogenesis (De Wever et al., 2003; Hwang et al., 2008). Nevertheless, whether CAFs propel CSCs to an aggressive state by modes of action that rely on altering their genotype and phenotype remains unclear. Particular attention should be devoted to the role of CAFs in the acquisition of migration by CSCs.
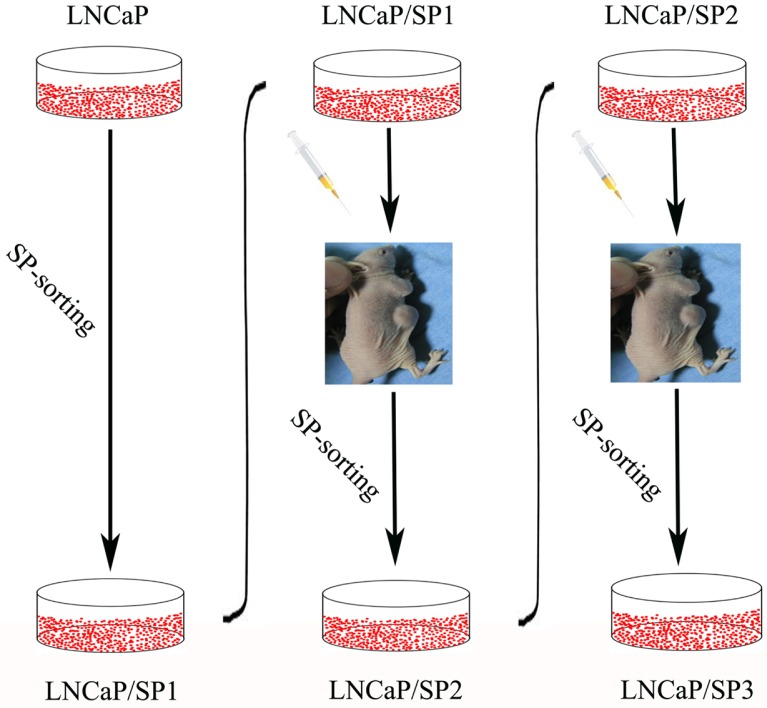
In previous studies, we have isolated three types of cell population with stem cell-like associated markers, self-renewal ability and typical holoclonal growth patterns based on a FACS analysis approach (Luo et al., 2012; 2013) (Fig. 1). The first was named LNCaP/SP1, which was derived from the LNCaP cell-line, the second was designated as LNCaP/SP2, and was sorted from subcutaneous tumor tissues produced by LNCaP/SP1 in nude mice, and the third was named LNCaP/SP3, which was separated from subcutaneous tumor tissues produced by LNCaP/SP2. It is worth noting that the increasing capability of tumorigenesis and metastasis was exhibited in these three types of prostate CSCs. To our knowledge, precisely how CSCs acquire migrating potency has not been previously elucidated. Our attention was therefore focused on CAFs, which have been identified to affect invasiveness and EMT characteristics in several cancer models. Additional studies are needed to determine whether CAFs could promote the migrating potency of CSCs and to investigate the underlying mechanisms. In this context, we have evaluated the proactive influence of CAFs on their prostate CSCs counterparts and further identified the role of the HIF-1α/β-catenin signaling pathway in this pathological process.
Fig. 1.
Schematic depiction showing the approach used to establish a series of prostate CSC models with different EMT phenotypes as determined by FACS analysis based on SP sorting.
MATERIALS AND METHODS
Cell culture
Following approval by the Committee on the Ethics of Clinical Experiments of the Capital Medical University, we obtained fibroblasts from surgically resected regions of PCa and benign prostatic hyperplasia (BPH) bearing patients in accordance with the following procedures. These isolated fibroblasts were referred to as CAFs and BPFs respectively. Tissues were sequentially cut with a scalpel into pieces 1–2 mm3, placed on plastic dishes and cultured with DMEM media, supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Fresh media were added daily until tissue fragments were attached to the plastic. After tissue attachment, the culture media were changed twice a week, and fibroblasts were explanted from tissue fragments while other cells were mostly retained within the tissue. When fibroblasts spread out on the culture dish with multiple dense colonies, they were briefly trypsinized and replated into new culture dishes. Additionally, the active fibroblasts, which we referred to as MFs, were produced by BPFs following stimulation with 10 ng/ml of TGF-β1 for 24 h (Kalluri et al., 2006). In the present study, MFs were used as functional control cells, and BPFs were used as dysfunctional control cells. All fibroblasts used in experiments were between passage 5 and 7. Conditioned media (CM) was obtained from 48 h serum-starved fibroblasts, which was clarified by centrifugation, and used freshly.
LNCaP cells were isolated from lymph node metastases of human PCa cells, and maintained in DMEM media supplemented with 1 mM sodium pyruvate, 2.5 mM L-glutamine, 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. Additionally, prostate CSCs were cultured in keratinocyte medium (Scien-Cell, USA) supplemented with 20 ng/ml epidermal growth factor (Sigma, USA), 50 μg/ml bovine pituitary extract (Sigma, USA), 2 ng/ml leukemia inhibitory factor (Sigma, USA), 2 ng/ml stem cell factor (Sigma, USA), and 100 ng/ml cholera toxin (Sigma, USA). Each of these cells were aseptically manipulated and propagated at 37°C in a humidified incubator containing 5% CO2.
Western immunoblot analysis
Twenty to eighty μg of clarified protein lysate were electrophoretically resolved on denaturing SDS-PAGE (8–12%) gels, and electro-transferred onto nitrocellulose membranes. The immunoblots were incubated in 3% bovine serum albumin, 10 mmol/L Tris-HCl (pH 7.5), 1 mmol/L EDTA, and 0.1% Tween-20 at room temperature for 2 h, and then probed with primary antibodies (Santa Cruz, USA) at 4°C overnight. Next, the membranes were hybridized with an appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (Boshide, China) for 2 h at room temperature. Finally, an enhanced chemiluminescence system (Amresco, USA) was used to detect the immunopositive protein bands.
Transfection of cells with ShRNA specific for β-catenin and HIF-1α
The recombinant plasmid pSUPER/β-catenin, which was constructed as described in our previous study (Zhao et al., 2011), and pSUPER/HIF-1α (Boshide, China) were first transfected into prostate CSCs using the Lipofectamine 2000 system (Life Technologies, USA) following the manufacturer’s instructions. Next, the transfected cells were cultured in medium containing 400 μg/ml puromycin until all non-transfected cells had died. Subsequently, we selected the silenced stem cells by treatment with 200 μg/ml puromycin.
Luciferase reporter assay
Cells were plated into 96-well plates. After overnight culture, the cells were transiently transfected with the TOPFlash and Renilla luciferase construct (Thermo Fisher Scientific, USA). And then cells were lysed after 24 h incubation and the activity of firefly luciferase and renilla luciferase were both determined by Dual-Lucy Assay Kit. The luciferase activity was normalized to the renilla luciferase activity.
Immunofluorescent staining
Cells were fixed in 10% paraformaldehyde for 30 min and blocked with goat serum for 30 min. Next, cells were incubated at 37°C for 1 h in mouse anti-human β-catenin monoclonal antibody (Santa Cruz, USA) at a dilution of 1:200. After washing three times in PBS, cells were co-incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Boshide, China) at 37°C for 1 h. The fluorescence staining intensity and intracellular localization were determined by fluorescence microscopy (Olympus, Japan).
In vitro transwell invasion assay
Eight-micrometer polycarbonate filters (Millipore, USA) were coated with 50 μg/cm2 of reconstituted Matrigel (Sigma, USA). Fifty thousand cells in 300 μl of serum-free growth medium were seeded into the upper chamber. Cells were incubated in normoxic conditions and allowed to migrate toward complete the growth medium for 24 h. Non-invading cells were removed mechanically using cotton swabs and the migrated cells, which were located on the lower surface, were fixed with methanol. The number of migrating cells was determined by counting 10 high-power fields of view on each membrane, and calculated as the mean number of cells per field. Each experiment was repeated three times for each cell-line.
Statistics
All data were presented as mean ± standard deviation (SD). The data were analyzed using the Statistical Package for Social Sciences (SPSS), version 13.0, for Windows. Statistical analysis was performed by Student’s t-test. P-values with an alphavalue of less than 0.05 were considered to be statistically significant.
RESULTS
Identification of the molecular and functional features of CAFs
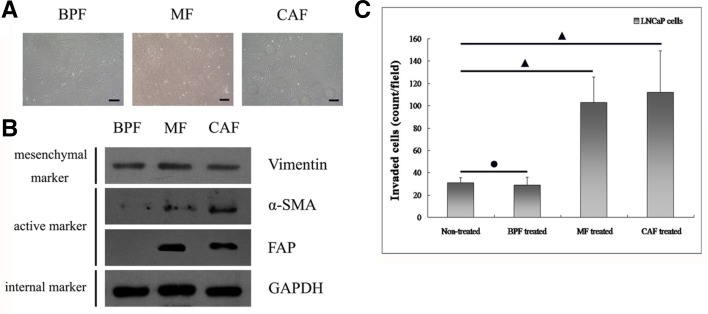
All three types of fibroblasts appeared as spindle shaped cells with loose intercellular connections (Fig. 2A). However, each type of cell showed different molecular and functional traits. To confirm the mesenchymal characteristics of the fibroblasts, we first detected the protein expression of epithelial markers so that we could exclude epithelial contamination. We observed that the three types of prostate fibroblasts all failed to express the epithelial proteins E-cadherin and CK18 (data not shown). Moreover, Western immunoblot analysis (Fig. 2B) verified that the prostate fibroblasts expressed the mesenchymal marker Vimentin. More importantly, the markers of active fibroblasts, α-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP), were positively expressed in both CAFs, and MFs but were absent in BPFs.
Fig. 2.
Showing the identification of the functional characteristics of CAFs. (A) The shape of CAFs has no apparent difference as compared with BPFs or MFs. In addition, they display a spindle-shaped appearance. The bar indicates 50 μm. (B) Although the general mesenchymal marker Vimentin was positively expressed in all three fibroblast populations, the expression of the active mesenchymal markers α-SMA and FAP, were both significantly heightened in both CAFs and MFs, and not in BPFs. (C) Comparative evaluation of the stimulatory effect of different fibroblasts on the invasive capability of human prostate cancer cells (LNCaP cells) in a three-dimensional transwell system. CAFs and MFs showed notably enhanced abilities to promote progression of prostate cancer as compared BPFs. (•) indicates P > 0.05 and (▴) indicates P < 0.05.
To determine the functional features of fibroblasts, we evaluated the invasiveness of LNCaP cells through three-dimensional matrigels following incubation of those cells with CM from BPFs, MFs and CAFs, respectively. The observations revealed that CAFs, and MFs, could dramatically increase the invasive ability of LNCaP cells (Fig. 2C). By contrast, BPFs did not assist in augmented the invasive capability of LNCaP cells. These observations suggest that CAFs, and MFs, could be active stromal components in the progression of PCa. In the following experiment, we attempted to further demonstrate the ability of CAFs to promote the migrating potency of prostate CSCs, and determine the possible molecular mechanism of this meaningful pathological process.
Regulation of HIF-1α/β-catenin pathway by CAFs in prostate CSCs
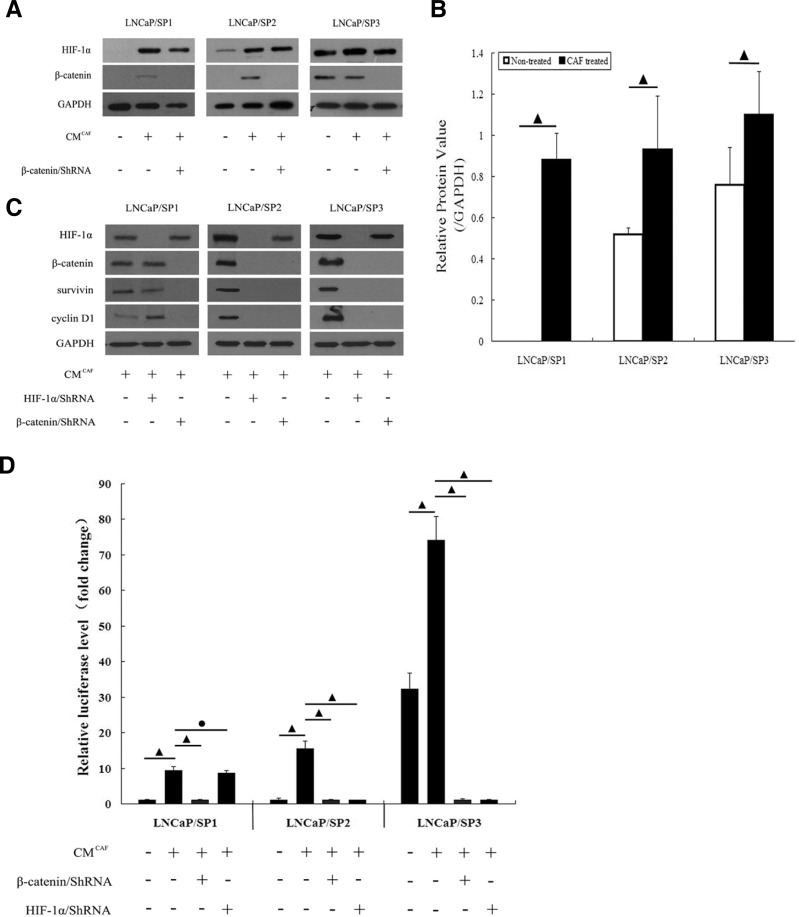
Our previous studies (Jiang et al., 2007; Luo et al., 2006; Zhao et al., 2011) verified that LNCaP cells underwent the EMT process following activation of the HIF-1α/β-catenin signaling pathway. To further identify whether CAFs could affect this pathway, we analyzed the corresponding CM for their activating effects. As shown by Western Immunoblot analysis (Fig. 3A), the HIF-1α/β-catenin signaling pathway displayed different activities in these prostate CSCs. This pathway was “closed” in LNCaP/SP1 cells, which failed to express either HIF-1α or β-catenin; was “partially-opened” in LNCaP/SP2 cells, which also positively expressed HIF-1α and failed to express β-catenin. By contrast, the HIF-1α/β-catenin signaling pathway was “opened” in LNCaP/SP3 cells, which were found to positively express both HIF-1α and β-catenin. However, the activity of the HIF-1α/β-catenin signaling pathway was opened in all prostate CSCs following exposure to CM from CAFs. Further quantitative analysis (Fig. 3B) also showed that the relative protein level of HIF-1α to GAPDH in cells treated with CAFs was significantly higher as compared with the corresponding cells not exposed to CM. These data preliminarily indicated that CAFs might activate the HIF-1α/β-catenin signaling pathway through regulating the expression of HIF-1α in prostate CSCs.
Fig. 3.
Showing that CAFs affected the activity of the HIF-1α/β-catenin pathway. (A) The expression of HIF-1α and β-catenin is dynamically and progressively increased in the prostate CSCs model. After incubation with CM from CAFs, the lower expression level of HIF-1α and β-catenin in both LNCaP/SP1 and LNCaP/SP2 stem cells became markedly elevated. Furthermore, the expression levels of both HIF-1α and β-catenin in LNCaP/SP3 stem cells were enhanced. Tansfection of ShRNA interfered with the expression of β-catenin induced by CAFs in these different prostate CSCs. (B) Quantitative analysis accurately demonstrated that the relative protein levels of HIF-1α in prostate CSCs was significantly increased by CAFs. (C) Western immunoblot analysis demonstrated that β-catenin/ShRNA effectively dampened the expression of survivin and cyclin D1, which were two typical downstream proteins of HIF-1α/β-catenin signaling pathway, in CAFs treated prostate CSCs. And HIF-1α/ShRNA exhibited the same interfering effect in LNCaP/SP2 and LNCaP/SP3 cells treated by CAFs. (D) Luciferase reporter assay further showed that the transcriptional activity of HIF-1α/β-catenin signaling pathway was elevated significantly by CM from CAFs. And comparing to HIF-1α/ShRNA, β-catenin/ShRNA could much more effectively repress luciferase activity in all three kinds of CAFs treated prostate CSCs. (•) indicates P > 0.05 and (▴) indicates P < 0.05.
Moreover, Western immunoblot analysis (Fig. 3C) further displayed that the expression of β-catenin and its two typical downstream proteins, survivin and cyclin D1, in CAF treated cells could be decreased by ShRNA target β-catenin; whereas the interfering effect of HIF-1α/ShRNA on above three proteins was noticeably exhibited in LNCaP/SP2 and LNCaP/SP3 cells treated by CAFs, except for CAFs treated LNCaP/SP1 cells. Additionally, luciferase reporter assay (Fig. 3D) also verified that the transcriptional activity of HIF-1α/β-catenin signaling pathway was significantly higher in CAFs treated cells than that in non-treated cells; more importantly, the transcriptional activity of β-catenin could be apparently repressed by transfection of ShRNA to specifically target β-catenin. Comparing to β-catenin/ShRNA, HIF-1α/ShRNA also exhibited perfect blocking effect on HIF-1α/β-catenin signaling pathway in LNCaP/SP2 and LNCaP/SP3 cells treated by CAFs, but not in CAF treated LNCaP/SP1 cells. These data accurately revealed that CAFs could control the activity of HIF-1α/β-catenin signaling pathway in three kinds of prostate CSCs.
In all, we conducted such experiments in order to determine whether HIF-1α/β-catenin signaling pathway was involved in the aggressive progress of prostate CSCs triggered by CAFs. And the blocking effect of β-catenin/ShRNA was much better than that of HIF-1α/ShRNA. Therefore, we applied β-catenin/ShRNA for following regulation of HIF-1α/β-catenin signaling pathway.
CAFs promoted the migrating ability of prostate CSCs with mesenchymal phenotype via the HIF-1α/β-catenin pathway
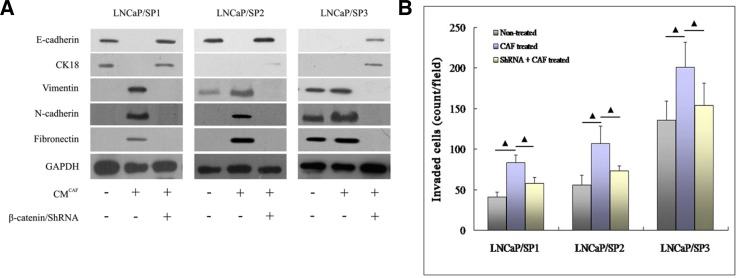
To assess the consequence of CAFs augmenting the activity of the HIF-1α/β-catenin signaling pathway, we determined alterations in the profile of the EMT phenotype and migration ability in prostate CSCs. As shown by Western immunoblot analysis (Fig. 4A), a dynamic EMT process was displayed by LNCaP/SP1, LNCaP/SP2 and LNCaP/SP3, and among them, LNCaP/SP1 cells showed the typical epithelial phenotype with complete absence of the EMT phenotype. In addition, LNCaP/SP3 cells exhibited a representative mesenchymal phenotype (EMT positive phenotype), while LNCaP/SP2 cells gained a partial mesenchymal phenotype with loss of CK18 and Vimentin expression.
Fig. 4.
The role of the HIF-1α/β-catenin pathway in the process of CAFs activating prostate CSCs. (A) Although three sources of prostate CSCs possessed different EMT phenotypes, all of them displayed the mesenchymal phenotype following treatment with CAFs. Following blockade of the HIF-1α/β-catenin signaling pathway with by transfection with ShRNA, the CAFs-treated prostate CSCs completely reversed to an epithelial phenotype. (B) The three- dimensional transwell assay showed that the migrating ability of prostate CSCs was markedly enhanced by stimulation with CAFs. However, the promoting effect of CAFs was effectively attenuated following suppression of the HIF-1α/β-catenin signaling pathway in prostate CSCs. (▴) indicates P < 0.05.
Although natural EMT characteristics were different, LNCaP/SP1, LNCaP/SP2 and LNCaP/SP3 all expressed mesenchymal phenotype after incubation in CM from CAFs. This was also characterized by loss of epithelial markers (E-cadherin and CK18) and acquisition of mensenchymal markers (Vimentin, N-cadherin and Fibronectin). Notably, LNCaP/SP1, LNCaP/SP2 and LNCaP/SP3 all regained epithelial phenotype after knockdown of β-catenin. Collectively, CAFs could indeed elicit EMT-associated molecular features of prostate CSCs and did so predominantly through activation of the HIF-1α/β-catenin signaling pathway. Moreover, blocking the HIF-1α/β-catenin signaling pathway dramatically reversed this important process.
More importantly, the results of the three-dimensional invasiveness assays (Fig. 4B) showed that CM from CAFs markedly promoted the invasiveness of prostate CSCs. In addition, after reverting to an epithelial phenotype following knockdown of β-catenin, the invasive potency of prostate CSCs was markedly reduced. Prostate CSCs also acquired a graded and increasing ability to migrate in vitro. This observation was concordant with their EMT characteristics, which shifted dynamically from an epithelial phenotype to a mesenchymal phenotype. Taken together, the above observations demonstrated that CAFs helped to trigger not only the EMT process but enhanced the aggressive potential of prostate CSCs by a mechanism that was dependent, at least in part, on activation of the HIF-1α/β-catenin signal transduction pathway.
DISCUSSION
Metastasis is a complicated multi-step process, which involves vascularization, invasion, detachment, survival in the circulation, extravasation, evasion of host immunity and progressive growth (Fidler et al., 2003). Although many hypotheses have been proposed to elucidate the underlying mechanisms, the process of metastasis remains incompletely understood.
It is now increasingly apparent that neoplasia is driven toward metastasis by CSCs. However, accumulating evidence has shown that not all CSCs exhibit the characteristic of migration. Hermann et al. (2007) reported that the CD133+ CXCR4+ subset determined the migrating phenotype of pancreatic cancer cells, although both CD133+CXCR4+ and CD133+ CXCR4− subsets possessed stem cell-like features associated with typical molecular and functional properties. An inhibitor of CXCR4 was found to significantly reduce metastasis in CD133+CXCR4+ subsets in a mouse model. Furthermore, removal of the CD133+ CXCR4+ subset from CD133+ CSCs inhibited the metastasis of pancreatic cancer, but did not affect tumorigenesis in the primary organ. Collectively, these data suggest that CD133+CXCR4+ CSCs determine the metastasis and identify the migrating CSCs of pancreatic cancer. Furthermore, Yang et al. (2008) also demonstrated that the CD90+CD44+ subpopulation displayed much greater capacity to metastasize than their CD90+CD44− counterparts in liver cancer. However, both subsets could form tumors effectively. In addition, the proportion of CD90+CD44+ subpopulations in the metastasis increased as compared with the primary cancer. Therefore, Yang considered that CD90+ CD44+ subpopulations may represent migrating CSCs. In 2005 (Brabletz et al., 2005), Kirchner first proposed the concept of migrating CSCs, which described a population of stem cells that were located predominantly at the tumor-host interface, and which were derived from stationary CSCs through the acquisition of a transient EMT in addition to stem cell-like features or “stemness”.
Regretfully, current studies assessing the behavior and mechanisms of migrating CSCs are very limited. This is mainly due to a lack of specific markers currently available to permit isolation of migrating CSCs. Interest in this study is three-fold. Firstly, we described a series of prostate CSC models with different EMT phenotypes, and EMT-positive CSCs showed a much greater aggressive potential as compared with EMT-negative CSCs. After EMT-negative CSCs underwent the EMT process, those new CSCs, which expressed the mesenchymal phenotype exhibited significantly increased abilities to migrate. Consistently, Mani et al. (2008) also isolated two stem-like subpopulations, namely CD44lowCD24high cells and CD44highCD24low cells. These cells were isolated from breast cancer tissues and revealed that the CD44highCD24low cell population expressed high levels of mesenchymal markers and low levels of E-cadherin by serial analysis of gene expression. On transplanting human mammary epithelial cells, which constitutively expressed EMT-inducible factor, either Snail or Twist, into immune-deficient mice, it was found that both had a higher tumorigenic capability, and that the number of CD44highCD24low subpopulations was elevated. The above studies indicated that EMT could be responsible for the generation of migrating CSCs, and thus the mesenchymal phenotype may represent a valid marker of migrating CSCs.
Secondly, we observed that CAFs could indeed promote the migrating ability of prostate CSCs by provoking the EMT process. Cancer cells and stromal cells interact through physical contact, soluble factors or insoluble extracellular matrix factors. Sung et al. (2008) verified that stromal fibroblasts from prostate and bone tissue expressed increased protein levels of several EMT-inducible factors, including brain-derived neurotropic factor, chemokines, stromal cell-derived factor-1, and HIF-1α. Lebret et al. (2007) also found that such factors known to induce the EMT process, are present at significantly higher levels in CAFs-CM as compared with normal mammary fibroblasts-CM in breast cancer. Additionally, following alterations in CM, the phenotype and behaviour of breast cancer cells also showed a corresponding change. Thus, Lebret provided important evidence suggesting a role for CAFs in increasing the migrating ability of breast cancer via an EMT process. Moreover, many studies have shown that CAFs contributed to enhanced cancer cell proliferation, survival, and invasive properties, and did so via secretion of inflammatory cytokines, chemokines, and growth factors, and thereby accelerated the invasive and metastatic process (Cat et al., 2006; Kalluri et al., 2009; Thiery et al., 2009). It transpires that CAFs are closely associated with not only bulk carcinoma cells but CSCs too. Recent work showed that CAFs enhanced the expression of stem cell-like markers by PCa cells and the formation of non-adherent prostaspheres; a property associated with prostate stem cells (Giannoni et al., 2010; Klarmann et al., 2009; Mani et al., 2008; Visvader et al., 2008). Giannoni confirmed that CAFs obtained from prostate carcinoma specimens, contributed to generating a population of prostate CSCs with a defined ability to form primary tumors and distant metastases (Giannoni et al., 2010). Graft experiments further showed that CSCs admixed with CAFs produced prostatic glandular structures, which were characterized by exhibiting more numerous lesions, a higher proliferative index, and tumor-like histopathology, as compared with those formed in the presence of normal prostate fibroblasts (Liao et al., 2010). Notably, we showed that prostate CSCs dynamically gained increased migrating potency accompanied with the acquisition of the EMT trait in the CAF co-culture system. Take together we believe that these findings have fundamental implications for our understanding of the role played by EMT between epithelial and stromal cells in the development and progression of malignant carcinoma.
Thirdly, we demonstrated for the first time, that the HIF-1α/β-catenin signaling pathway played a critical role during the EMT pathological process triggered by CAFs in prostate CSCs. Our previous work (Jiang et al., 2007; Luo et al., 2006; Zhao et al., 2011) showed that HIF-1α interfered with the protein expression of E-cadherin, which further caused depolymerization of the E-cadherin/β-catenin complex. Moreover, numerous down-stream target genes were activated due to the translocation of free β-catenin from the cytoplasm to the nucleus. Consequently, PCa underwent the EMT process and made further significant progress through activation of the HIF-1α/β-catenin signaling pathway. Recently, Giannoni et al. (2011) demonstrated that CAFs exerted the propelling role for EMT with strict dependence on HIF-1α, and further directed a migratory and aggressive phenotype of PCa cells. Tumor growth was abolished and metastasis formation was severely impaired by RNA interfering-mediated targeting of HIF-1α. Lebret et al. (2007) also reported that CM from CAF caused the depolymerization of the E-cadherin/β-catenin complex in human breast cancer cells. In addition, β-catenin exhibited a predominantly cytoplasmic expression profile, with E-cadherin localised predominantly to the cytoplasm. Although the activation of the HIF-1α/β-catenin signaling pathway could be involved in several tumor EMT process, rare data has discovered the biological link between this signaling pathway and CSCs. Recently, Conley et al. (2012) described the frequency of CSCs that could be increased by anti-angiogenic agents in a process that required generation of intratumoral hypoxia in a human breast cancer xenograft. Their in vitro xenograft models of treatment with the anti-angiogenic agents revealed that the occurrence of breast CSCs is primarily mediated by HIF-1α following activation of the Akt/β-catenin regulatory pathway. Our study further displayed that CAFs prompted CSCs to undergo the EMT process, which was strictly dependent on the activation of the HIF-1α/β-catenin signaling pathway in PCa.
In summary, the present study depicts a distinct process informing that CAFs drive prostate CSCs to undergo an EMT program of development and do so via activation of the HIF-1α/β-catenin signaling pathway, which ultimately promotes their motility. Therefore, a therapeutic strategy aimed at targeting the circuitry of HIF-1α/β-catenin might represent a valuable anti-metastatic tool affecting cancer cell malignancy.
Acknowledgments
This work was supported by National Natural Science Foundation of China (NO. 30700968 and 30901725).
REFERENCES
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. (2005). Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer 5, 744–749 [DOI] [PubMed] [Google Scholar]
- Cat B, Stuhlmann D, Steinbrenner H, Alili L, Holtkötter O, Sies H, Brenneisen P. (2006). Enhancement of tumor invasion depends on transdifferentiation of skin fibroblasts mediated by reactive oxygen species. J Cell Sci. 119, 2727–2738 [DOI] [PubMed] [Google Scholar]
- Chung LW, Baseman A, Assikis V, Zhau HE. (2005). Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 173, 10–20 [DOI] [PubMed] [Google Scholar]
- Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, Clouthier SG, Wicha MS. (2012). Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci USA 109, 2784–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wever O, Mareel M. (2003). Role of tissue stroma in cancer cell invasion. J Pathol. 200, 429–447 [DOI] [PubMed] [Google Scholar]
- Fidler IJ. (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3, 453–458 [DOI] [PubMed] [Google Scholar]
- Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. (2010). Reciprocal activation of prostate cancer cells and cancerassociated fibroblasts stimulates epithelialmesenchymal transition and cancer stemness. Cancer Res. 70, 6945–6956 [DOI] [PubMed] [Google Scholar]
- Giannoni E, Bianchini F, Calorini L, Chiarugi P. (2011). Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal. 14, 2361–2371 [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1, 313–323 [DOI] [PubMed] [Google Scholar]
- Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. (2008). Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68, 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T, Li MC, Lin YH. (2007). Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol. 14, 1034–1039 [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. (2009). Microenvironmental regulation of metastasis. Nat Rev Cancer 9, 239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R. (2009). EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 119, 1417–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. (2006). Fibroblasts in cancer. Nat Rev Cancer 6, 392–401 [DOI] [PubMed] [Google Scholar]
- Kaminski A, Hahne JC, Haddouti E, Florin A, Wellmann A, Wernert N. (2006). Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int J Mol Med. 18, 941–950 [PubMed] [Google Scholar]
- Klarmann GJ, Hurt EM, Mathews LA, Zhang X, Duhagon MA, Mistree T, Thomas SB, Farrar WL. (2009). Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis 26, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebret SC, Newgreen DF, Thompson EW, Ackland ML. (2007). Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors. Breast Cancer Res. 9, R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CP, Adisetiyo H, Liang M, Roy-Burman P. (2010). Cancer-associated fibroblasts enhance the gland-forming capability of prostate cancer stem cells. Cancer Res. 70, 7294–7303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. (2001). The microenvironment of the tumour-host interface. Nature 411, 375–379 [DOI] [PubMed] [Google Scholar]
- Luo Y, He DL, Ning L, Shen SL, Li L, Li X, Zhau HE, Chung LW. (2006). Over-expression of hypoxia-inducible factor-1alpha increases the invasive potency of LNCaP cells in vitro. BJU Int. 98, 1315–1319 [DOI] [PubMed] [Google Scholar]
- Luo Y, Cui XH, Jiang YG, Zhao JH, Zhao L, Chen YT, Li MC, Lin YH. (2012). Sorting and identification of cancer stem cells in human prostate cancer cell lines. Zhonghua Nan Ke Xue 18, 1062–1068 [PubMed] [Google Scholar]
- Luo Y, Cui XH, Jiang YG, He DL, Zhao JH, Zhao L, Chen YT, Li MC, Lin YH. (2013). Epithelial-mesenchymal transition of prostate cancer: cancer stem cells or bulk cancer cells. Zhonghua Yi Xue Za Zhi 93, 256–260 [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silzle T, Randolph GJ, Kreutz M, Kunz-Schughart LA. (2004). The fibroblast: sentinel cell and local immune modulator in tumor tissue. Int J Cancer 108, 173–180 [DOI] [PubMed] [Google Scholar]
- Studebaker AW, Storci G, Werbeck JL, Sansone P, Sasser AK, Tavolari S, Huang T, Chan MW, Marini FC, Rosol TJ, et al. (2008). Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 68, 9087–9095 [DOI] [PubMed] [Google Scholar]
- Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, et al. (2008). Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res. 68, 9996–10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. (2009). Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8, 755–768 [DOI] [PubMed] [Google Scholar]
- Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. (2008). Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 13, 153–166 [DOI] [PubMed] [Google Scholar]
- Zhao JH, Luo Y, Jiang YG, He DL, Wu CT. (2011). Knockdown of β-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1α. Cancer Invest. 29, 377–382 [DOI] [PubMed] [Google Scholar]