Abstract
Interleukin 23 (IL-23) is an inflammatory cytokine that plays an important role in tumor promotion. Expression of IL-23 is increased in cancer cells and correlates with tumor progression. However, the mechanisms regulating IL-23 expression in cancer cells are still unclear. Here we report that tristetraprolin (TTP), an AU-rich element (ARE)-binding protein, inhibits IL-23 production in CT26 mouse colon cancer cells. Overexpression of TTP decreased the stability of IL-23 mRNA and the expression level of IL-23 in CT26 cells. Conversely, inhibition of TTP by siRNA increased IL-23 production. TTP destabilized a luciferase mRNA reporter containing the IL-23 mRNA 3’UTR, which contains five AREs. Analyses of deletion and point mutants of the IL-23 mRNA 3’UTR demonstrated that the ARE cluster between the third and fifth AREs was responsible for TTP-mediated destabilization of IL-23 mRNA. A RNA electrophoretic mobility shift assay confirmed that TTP binds to this ARE cluster. Taken together, these results demonstrate that TTP acts as a negative regulator of IL-23 gene expression in mouse colon cancer cells and suggest its potential application as a novel therapeutic target to control IL-23-mediated tumor promotion.
Keywords: ARE-binding protein, cancer cells, gene regulation, IL-23, TTP
INTRODUCTION
IL-23 is an important pro-inflammatory heterodimeric cytokine formed by linkage of p40 and p19 subunits that acts as a crucial regulator to drive a pathway that leads to the generation of IL-17–producing CD4 T cells (Aggarwal et al., 2003; Cua et al., 2003; Harrington et al., 2005; Langrish et al., 2004). The induction of IL-23–mediated processes leads to recruitment of a range of inflammatory cells in addition to T helper 17 cells, and has been shown to be crucial to the pathogenesis of a number of immune-mediated inflammatory diseases (Langrish et al., 2005). In a recent study, IL-23 emerged as a new player in the promotion of tumor growth and development through suppression of tumor infiltration of CD8+ T cells and promotion of tumor angiogenesis and metastases (Langowski et al., 2006; Teng et al., 2010). Moreover, anti-IL-23 monoclonal antibody acts synergistically with targeted therapies or IL-2 to suppress tumor growth and metastases (Teng et al., 2011), supporting the tumor-promoting activity of IL-23.
Many inflammatory cytokine and chemokine mRNAs are known to be unstable (Anderson, 2008). Expression of these mRNAs is modulated by post-transcriptional control, which is strongly dependent on AU-rich element (ARE)-mediated mechanisms (Stoecklin and Anderson, 2006). The AREs may destabilize inflammatory signaling mRNAs through specific binding of proteins that promote mRNA degradation (Caput et al., 1986; Shyu and Wilkinson 2000). Tristetraprolin (TTP) is a well-characterized ARE-binding protein that is involved in post-transcriptional regulation of inflammatory cytokines, proto-oncogenes, and growth regulatory genes (Cha et al., 2011; Lee et al., 2010a; 2011; 2012; 2013). TTP-knockout mice develop severe inflammatory arthritis and autoimmune dysfunction, indicating a role of TTP in limiting the inflammatory response (Taylor et al., 1996).
Macrophages and DCs are known to be the predominant producers of IL-23 (Hunter, 2005). The 3’UTR of IL-23 p19 mRNA contains multiple AREs and IL-23 p19 expression in macrophages is suppressed by TTP-mediated degradation of p19 mRNA (Qian et al., 2011). Recently, it has been reported that IL-23 is highly expressed in many tumors, including breast, prostate, liver, and skin cancers and head and neck squamous cell carcinoma (HNSCC), and that levels of IL-23 correlate with tumor progression (Kesselring et al., 2010; Langowski et al., 2006; Martin-Orozco et al., 2009). However, there are no convincing studies demonstrating the molecular basis for the regulation of IL-23 in cancer cells.
In the present study, we provide evidence that TTP inhibits IL-23 expression by enhancing the decay of IL-23 p19 mRNA in cancer cells. IL-23 transcript was detected in CT26 colon cancer cells. Overexpression of TTP decreased IL-23 expression level whereas inhibition of TTP by siRNA increased it. IL-23 p19 mRNA contains five AREs within the 3’UTR, and the direct binding of TTP to these AREs enhances decay of the mRNA. These results support a procarcinogenic role for IL-23 and suggest that TTP-mediated inhibition of IL-23 expression in cancer cells might provide a novel therapeutic strategy for the suppression of tumor-promoting inflammation.
MATERIALS AND METHODS
Cells
The CT26 and 293 EBNA cell lines were purchased from the American Type Culture Collection (USA) and maintained in RPMI 1640 media supplemented with 10% FBS (HyClone) at 37°C in a humidified atmosphere of 5% CO2.
Plasmids and siRNAs
The pcDNA6/V5-TTP construct has been described previously (Lee et al., 2010b). Mouse IL-23 p19 3′UTR was PCR amplified from cDNA of CT26 cells using Taq polymerase (SunGenetics, Korea) and the primers CCGCTCGAGGAGCTCTGGGGA GCCCACACT and ATAAGAATGCGGCCGCGGGCGAAAAT GGTTACGATGTG (underlined letters indicate restriction sites). PCR products were inserted into the XhoI/NotI sites of a psiCHECK2 Renilla/firefly dual-luciferase expression vector (Promega, USA) to generate the construct psiCHECK2/IL-23 3′UTR Full. The psiCHECK2/IL-23 3′UTR Full construct was used as a template to synthesize the deletion mutant construct psiCHECK2/IL-23 Frag-ΔARE. PCR primers used for mutant constructs were TAAGGGCTTTAAGTTATATGCCCTGAGAT AACT and AGTTATCTCAGGGCATATAACTTAAAGCCCTTA.
An oligonucleotide containing the five AUUUA motifs (ARE-1–5) of the IL-23 p19 mRNA 3′UTR was synthesized at Integrated DNA Technologies (USA) (Table 1). Mutant oligonucleotides in which single (ARE-M-1, ARE-M-2, ARE-M-3, ARE-M-4, or ARE-M-5) or all five AUUUA pentamers (ARE-M all) were sub-stituted with AGCA were also synthesized. The oligonucleotides were ligated into the XhoI/NotI site of the psiCHECK2 vector.
Small interfering RNAs (siRNAs) against mouse TTP (TTP-siRNA; sc-36760) and control siRNA (scRNA; sc-37007) were purchased from Santa Cruz Biotechnology (USA).
Transfections and luciferase assay
Cells (5 × 106) were electroporated with various plasmid constructs and siRNAs at 1,400 V and 30 ms using the Neon™ Transfection System (Invitrogen, USA) according to the manufacturer’s instructions.
Lysates of transfected cells were mixed with luciferase assay reagent (Promega, USA) and the chemiluminescent signal was measured in a Wallac Victor 1420 Multilabel Counter (EG&G Wallac, Finland). Renilla luciferase activity of psiCHECK2/IL-23 3′UTR Full was normalized to firefly luciferase for each sample.
Electrophoretic mobility shift assay (EMSA)
The biotinylated RNA probes for wild-type ARE (Oligo-ARE-3/4, 5′-CCUGUAUUUAUUUGAGCUAUUUAAGGAUCUAUUUAUG UUUAAGUAUUUAGAAAA-3′) and mutant ARE (Oligo-ARE-m3/4, 5′-CCUGUAGCAUUUGAGCUAGCAAGGAUCUAGCAU GUUUAAGUAGCAGAAAA-3′) were synthesized by Samchully Pharm. Co., LTD (Korea). A mutant RNA probe in which the AUUUA pentamers were all substituted with AGCA was used as a negative control. Cytoplasmic extracts were prepared from CT26 cells using NE-PER® Nuclear and Cytoplasmic extraction Reagent (Thermo Pierce Biotechnology Scientific, USA). RNA EMSA was performed using the Lightshift® Chemoluminescent EMSA Kit (Thermo Pierce Biotechnology Scientific) according to the manufacturer’s instructions. Briefly, 20 fmol of biotinylated RNA was combined with 4 μg of cytoplasmic cellular protein in a binding buffer. For the supershift EMSA, anti-TTP antibody (ab36558, Abcam, USA) or control antibody (I-5381, Sigma) was added to the reaction mixture. After the addition of antibodies, reaction mixtures were incubated overnight on ice. The reaction products were resolved on 5% nondenaturing polyacrylamide gels in 0.5× Tris borate/EDTA buffer. Gels were transferred to nylon membrane (Hybond™-N+) in 0.5× Tris borate/EDTA at 100 V and 4°C for 1 h. The RNAs were cross-linked to the membrane and detected using streptavidin-horseradish peroxidase binding and chemiluminescence.
SDS-PAGE analysis and immunoblotting
Proteins were resolved by SDS-PAGE, transferred to Hybond-P membranes (GE Healthcare Bio-Sciences Corp., USA), and probed with the appropriate dilution of anti-TTP antibody (ab36558, Abcam) and anti-V5 antibody (20-783-70389, Gen-Way). Immunoreactivity was detected using the ECL detection system (GE Healthcare Bio-Sciences Corp.). Films were exposed at multiple time points to ensure that images were not saturated.
Quantitative real-time PCR
For kinetic analysis of mRNA expression, we used actinomycin D to inhibit transcription and assessed IL-23 p19 mRNA expression by quantitative real-time PCR (qRT-PCR). qRT-PCR was performed by monitoring the increase in fluorescence of the SYBR Green dye (QIAGEN, Germany) in real-time using ABI Prism 7900 HT. Specificity of each primer pair was confirmed by melting curve analysis and agarose gel electrophoresis. PCR primer pairs were as follows: qIL-23: ACTACAACCG ATCCACCTCAC and ACTTTGCCTCCCAGATCACAG; qGAPDH: ATCTTCAAGCCATCCTGTGTGC and TGCGCTTGTCACAT TTTTCTTG.
Statistics
For statistical comparisons, p values were determined using Student’s t-test.
RESULTS
TTP regulates the expression level of IL-23
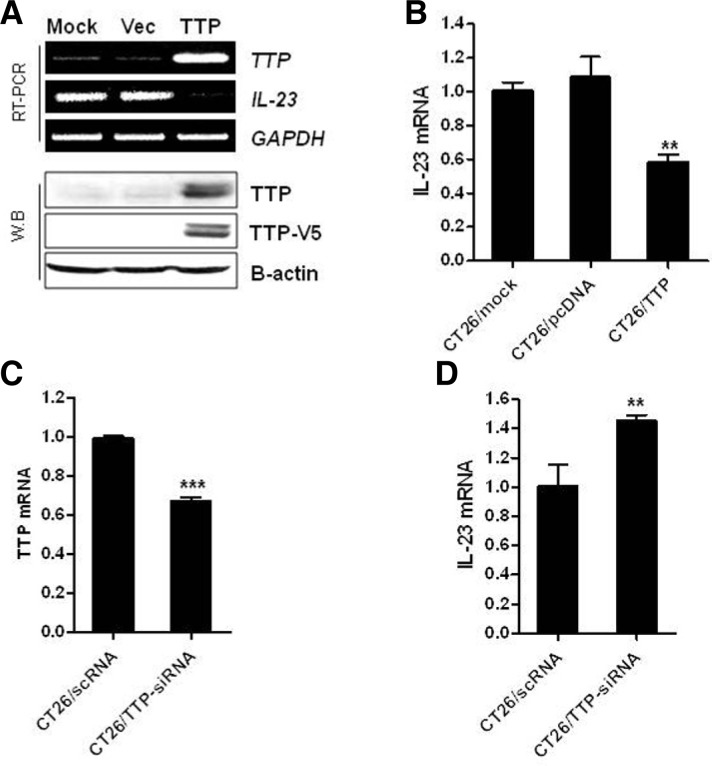
Chronic inflammation is thought to be the leading cause of many human cancers including colon cancer (Terzic et al., 2010). The proinflammatory cytokine IL-23 has emerged as a new player in the promotion of tumor growth and development. It was recently reported that IL-23 expression is highly expressed in many tumors and that the level of IL-23 correlates with tumor progression. Here, we investigated whether TTP regulates the expression of IL-23 in CT26 colon cancer cells. To determine the effect of TTP on IL-23 expression, the TTP expression vector pcDNA6/V5-TTP or pcDNA6/V6 as a vector control was transfected into CT26 cells. Overexpression of TTP was confirmed by RT-PCR and Western blot analyses. The expression of IL-23 was significantly reduced in pcDNA6/V5-TTP-transfected cells compared with cells transfected with empty vector (Figs. 1A and 1B).
Fig. 1.
TTP inhibits IL-23 expression in CT26 mouse colorectal cancer cells. CT26 cells were transfected with pcDNA6/V5-TTP or pcDNA6/V5 (A, B), and with TTP-siRNA (siTTP) or control-siRNA (scRNA) (C, D). Expression of TTP and IL-23 in cells was determined by (A) semiquantitative real-time PCR (top panel) and Western blot analysis (bottom panel), or (B, C, and D) quantitative real-time PCR. GAPDH and β-actin were included as loading controls for PCR and Western blot analysis, respectively. Results shown represent the mean ± SD of three independent experiments (**P < 0.01; ***P < 0.001).
Next, we determined whether specific inhibition of TTP by siRNA affected IL-23 expression in CT26 cells. Down-regulation of TTP by treatment with siRNA-TTP significantly increased the expression level of IL-23 (Figs. 1C and 1D). However, treatment with a non-specific siRNA (scRNA) did not decrease the expression level of endogenous TTP or induce a change in IL-23 expression. Collectively, these results indicate that the expression of IL-23 is regulated by changes in TTP expression in the CT26 mouse colon cancer cell line.
Overexpression of TTP decreases the expression level of luciferase mRNA containing the IL-23 3′UTR
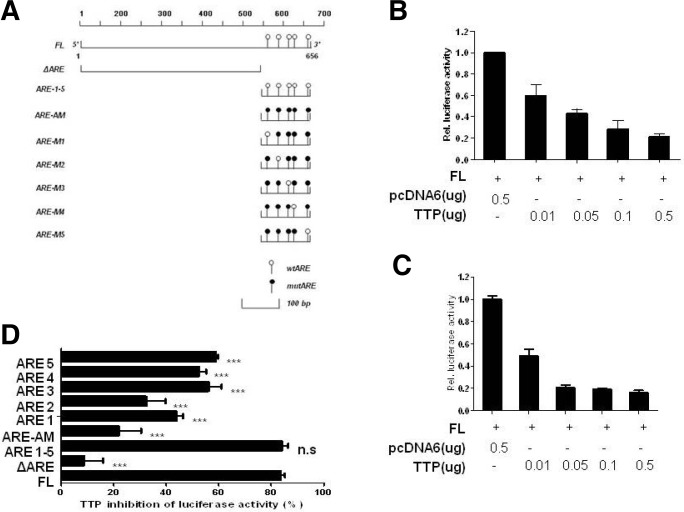
TTP protein regulates mRNA stability through binding to AREs within the mRNA 3′UTR. Analysis of the 655-bp human IL-23 3′UTR revealed the presence of five pentameric AUUUA motifs (Fig. 2A). To determine whether the TTP inhibitory effect is mediated through the IL-23 mRNA 3′UTR we prepared three IL-23 mRNA 3′UTR fragments: full length (containing full-length IL-23 mRNA 3′UTR), ΔARE (containing none of the pentameric AUUUA motifs, and ARE1-5 (containing all five motifs). To assay promoter activity we used a luciferase reporter gene linked to these fragments in the plasmid psiCHECK. First, we confirmed that transfection of 293 EBNA and CT26 cells with the human and mouse pcDNA6/TTP vector significantly suppressed the luciferase activity of a reporter gene containing full-length IL-23 mRNA 3’UTR (approximately 80% inhibition) compared with cells transfected with the empty vector pcDNA6/V5 (Figs. 2B and 2C). Although overexpression of TTP did not affect the luciferase activity of a reporter gene containing ΔARE (13% inhibition), it significantly inhibited activity of the ARE1-5 construct (85% inhibition) (Fig. 2D). These results demonstrated that the ARE1-5 region containing five AUUUA pentamers is important for TTP-mediated down-regulation of IL-23 expression.
Fig. 2.
AREs within the IL-23 mRNA 3′UTR are essential for the inhibitory effect of TTP. (A) Schematic representation of the luciferase reporter constructs used in this study. Fragments and oligonucleotides derived from the IL-23 mRNA 3′UTR were cloned upstream of the luciferase reporter gene in the psiCHECK2 luciferase expression vector. White circles represent the wild-type (w) pentameric motif, AUUUA, and black circles represent the mutated (m) motif, AGCA. (B, C) TTP overexpression inhibited activity of the luciferase reporter containing the IL-23 3′UTR. (B, C) 293 EBNA (B) and CT26 (C) cells were co-transfected with luciferase reporters containing full-length IL-23 mRNA 3′UTR (IL-23 3′UTR Full) and with either pcDNA/V5-TTP or the empty pcDNA/V5 control vector. Cells were harvested and Renilla luciferase activity was normalized to firefly activity. The luciferase values obtained from cells cotransfected with the luciferase construct and pcDNA6/V5 were set to 1. Results shown represent the mean ± SD of three independent experiments (p < 0.05). (D) Mapping of the region in the IL-23 mRNA 3′UTR that is required for TTP inhibition of luciferase activity. CT26 cells were cotransfected with 0.5 μg of a luciferase reporter construct as described in (A) and either pcDNA6/V5-TTP or the empty vector pcDNA6/V5. After normalization of luciferase activity, the TTP-induced inhibition of luciferase activity observed with each construct was compared with inhibition obtained with the full-length IL-23 mRNA 3′UTR. Results shown represent the mean ± SD of three independent experiments (P < 0.001).
To determine which AUUUA pentamer(s) is essential for the inhibitory effect of TTP, we prepared luciferase reporter genes containing wild-type or mutant oligonucleotides as follows: Oligo ARE 1–5 (containing wild-type AREs 1–5); Oligo ARE-AM (containing mutant AREs 1–5); Oligo ARE-M1 (containing mutant ARE 1 and wild-type ARE 2–5); Oligo ARE M2 (mutant ARE 2); Oligo ARE M3 (mutant ARE 3); Oligo ARE M4 (mutant ARE 4); Oligo ARE M5 (mutant ARE 5). As expected, Oligo ARE 1–5 MUT did not respond to TTP (23% inhibition) whereas TTP significantly inhibited activity of the ARE1–5 construct (85% inhibition). Any single mutation among the five ARE motifs attenuated the TTP inhibitory effect (34–60% inhibition). In particular, mutations ARE 3, 4, and 5 had a stronger effect than ARE 1 and ARE 2, indicating that the cluster of ARE 3–5 is required for efficient TTP inhibitory function (Fig. 2D).
TTP destabilizes IL-23 mRNA
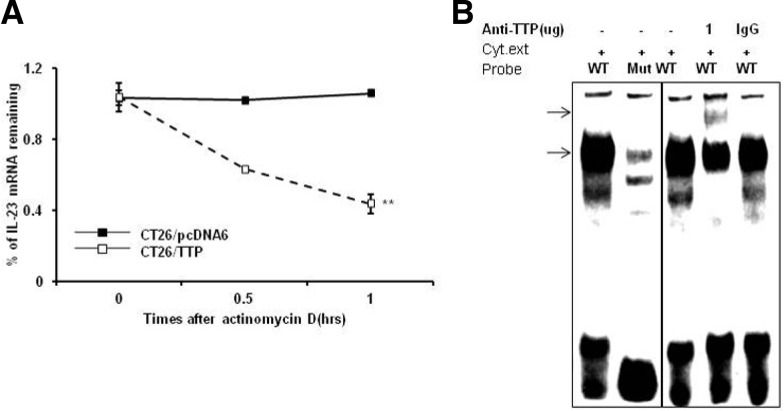
To determine whether the decreased expression of IL-23 resulted from changes in the stability of IL-23 mRNA, we measured the half-life of IL-23 mRNA by quantitative real-time PCR in actinomycin D-treated CT26 cells transfected with vector carrying TTP (CT26/TTP) or empty vector (CT26/pcDNA). In CT26/pcDNA cells, IL-23 mRNA was stable until 1 h after actinomycin D treatment. However, in CT26/TTP cells the half-life of IL-23 mRNA was 45 min (Fig. 3A), indicating that increased expression of TTP contributes to a decrease in IL-23 levels through destabilization of IL-23 mRNA.
Fig. 3.
(A) TTP destabilizes IL-23 mRNA. CT26 cells were transfected with pcDNA6/V5-TTP or pcDNA6/V5. Expression of IL-23 mRNA in CT26 cells was determined by quantitative real-time PCR at the indicated times after addition of 5 μg/ml actinomycin D. Data shown represent mean ± SD of three independent experiments. (**P < 0.01) (B) TTP binds to the ARE cluster within the IL-23 mRNA 3′UTR. An RNA EMSA was performed by mixing cytoplasmic extracts containing 4 μg of total protein from CT26 cells with 10 fmol of biotinylated wild-type or mutant probe. Anti-TTP or control antibody was added to the reaction mixtures. The binding reaction products were separated by electrophoresis on a 5% polyacrylamide gel under non-denaturing conditions. Arrows indicate the position of the TTP-containing band.
TTP binds to the ARE cluster within the IL-23 mRNA 3′UTR
To determine the interaction of TTP with the ARE cluster of IL-23-3′UTR, RNA EMSA was conducted using a biotinylated RNA probe containing the wild type or mutant ARE cluster of the IL-23 mRNA 3′UTR. Cytoplasmic extracts were prepared from CT26 cells transfected with pcDNA6/V5-TTP and incubated with the biotinylated RNA probes. When RNA EMSA was conducted using the wild type IL-23 probe, a dominant probe-protein complex was observed (Fig 3B). However, mutation of the IL-23 ARE prevented formation of this complex. The amount of this RNA-protein complex was reduced by preincubation with TTP-antibody in the reaction mixture in a concentration-dependent manner. There was no change in the complex when the mixture was preincubated with an anti-IgG as a negative control. These results showed that TTP directly interacts with the ARE cluster of IL-23 mRNA 3′UTR.
DISCUSSION
IL-23 is highly expressed in many tumors and the levels of IL-23 correlate with tumor progression (Kesselring et al., 2010; Langowski et al., 2006; Martin-Orozco et al., 2009). However, the molecular basis for the regulation of IL-23 in cancer cells is not fully understood. In this study, we provide evidence that TTP suppresses IL-23 expression by binding to the 3′UTR of IL-23 mRNA and promoting IL-23 mRNA degradation in cancer cells. TTP overexpression decreased the expression of a luciferase reporter gene containing the IL-23 mRNA 3′UTR and decreased IL-23 expression level whereas inhibition of TTP by siRNA increased IL-23 expression level.
Macrophages and DCs are the predominant producers of IL-23 (Hunter, 2005). In macrophages, IL-23 p19 expression has been reported to be suppressed by TTP-mediated degradation of p19 mRNA, and ARE clusters in the IL-23 3′UTR have been shown to participate in the regulation of mRNA stability (Qian et al., 2011). Consistent with this result, we found that TTP promoted IL-23 mRNA degradation through binding to a cluster of five ARE motifs within the IL-23 mRNA 3′UTR. Moreover, deletion and mutation analysis showed that these five AREs are required for efficient response to TTP in cancer cells. However, we found that the five AREs did not respond equally to TTP and that the cluster of the third to fifth AREs was primarily responsible for TTP inhibitory function. It has been reported that TTP-mediated mRNA decay requires the recruitment of exonucleases such as Dcp2 and Xrn1 (Lykke-Andersen and Wagner, 2005). We have previously reported that binding of TTP to the ARE motif is not sufficient for the recruitment of Dcp2 and Xrn1, but additional sequences surrounding the ARE motif are important for recruiting exonucleases and inducing TTP-mediated mRNA decay (Kim et al., 2010). The nucleotide sequences responsible for exonuclease recruitment have not yet been determined and we did not compare the nucleotide sequences surrounding the five AREs within the IL-23 mRNA 3′UTR. It is possible that the nucleotide sequences surrounding the first and second AREs may not suitable for recruitment of Dcp2 and Xrn1. Further studies on the nucleotide sequences surrounding the AREs and the recruitment of exonucleases to each AREs are necessary to understand the basic mechanisms.
TTP is one of the best-characterized proteins among the ARE-binding proteins that promote degradation of ARE-containing transcripts (Carballo et al., 1998; Lykke-Andersen and Wagner, 2005). TTP expression is significantly decreased in various cancers (Brennan et al., 2009), and this down-regulation correlates with increased expression of proto-oncogenes (Lee et al., 2010a; Young et al., 2009). The low level of TTP in cancer cells may not be sufficient to inhibit IL-23 expression, resulting in high IL-23 levels in cancer cells (Kesselring et al., 2010; Langowski et al., 2006; Martin-Orozco et al., 2009) which in turn may support tumor development. In this study, we provide evidence that ectopic expression of TTP decreased the IL-23 level in cancer cells. It has been reported that stimulation with LPS results in a strong and rapid induction of TTP mRNA in mouse macrophages (Mahtani et al., 2001). However, LPS stimulation does not enhance the TTP-mediated degradation of ARE-containing mRNA because it also activates p38 MAPK, which can phosphorylate and inactivate TTP (Chrestensen et al., 2004; Stoecklin et al., 2004). Stimulation with IFN-γ (Sauer et al., 2006), IL-10 (Schaljo et al., 2009) or nicotine (Joe et al., 2011) has been reported to induce TTP expression but not p38 MAPK activity and thus these agents increase the induction of mRNA decay by TTP. IFN-γ has been reported to increase both the level and the activity of TTP and enhance the TTP-mediated degradation of IL-23 mRNA in macrophages (Qian et al., 2011). The effects of these factors on TTP level and activity have been reported mostly in macrophages and it is not clear whether these factors are also involved in the induction of TTP in cancer cells. Even though TGF-β does not induce TTP expression, it can increase TTP activity by inhibition of p38 MAPK activity in cancer cells (Lee et al., 2011). Further studies are needed to determine whether these factors increase TTP expression and/or activity and lead to inhibition of IL-23 expression in cancer cells.
In conclusion, we demonstrate that TTP inhibits IL-23 production in cancer cells by promoting IL-23 mRNA degradation. Because IL-23 promotes tumor growth and metastasis, the inhibition of IL-23 production through enhanced TTP expression and activity may have important clinical implications.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korea government [Ministry of Education and Science Technolgoy (MEST)] (2008-0062611) and by the Ministry of Education, Science and Technology (2012R1A1A 1019768) and by the Korean government [Ministry of Education and Human Resources Development (MOEHRD)] (BRL-2009-0087350).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- Brennan SE, Kuwano Y, Alkharouf N, Blackshear PJ, Gorospe M, Wilson GM. The mRNA-destabilizing protein tristetraprolin is suppressed in many cancers, altering tumorigenic phenotypes and patient prognosis. Cancer Res. 2009;69:5168–5176. doi: 10.1158/0008-5472.CAN-08-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3-untranslated region of mRNA molecules specifying inflammatory mediators. Proc. Natl. Acad. Sci USA. 1986;83:1670. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HJ, Lee HH, Chae SW, Cho WJ, Kim YM, Choi HJ, Choi DH, Jung SW, Min YJ, Lee BJ, et al. Tristetraprolin downregulates the expression of both VEGF and COX-2 in human colon cancer. Hepatogastroenterology. 2011;58:790–795. [PubMed] [Google Scholar]
- Chrestensen CA, Schroeder MJ, Shabanowitz J, Hunt DF, Pelo JW, Worthington MT, Sturgill TW. MAPKAP kinase 2 phosphorylates tristetraprolin on in vivo sites including Ser178, a site required for 14-3-3 binding. J Biol Chem. 2004;279:10176–10184. doi: 10.1074/jbc.M310486200. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- Joe Y, Kim HJ, Kim S, Chung J, Ko MS, Lee WH, Chang KC, Park JW, Chung HT. Tristetraprolin mediates anti-inflammatory effects of nicotine in lipopolysaccharide-stimulated macrophages. J Biol Chem. 2011;286:24735–24742. doi: 10.1074/jbc.M110.204859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesselring R, Thiel A, Pries R, Trenkle T, Wollenberg B. Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. Br. J Cancer. 2010;103:1245–1254. doi: 10.1038/sj.bjc.6605891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Kim HK, Vo MT, Lee HH, Kim HJ, Min YJ, Cho WJ, Park JW. Tristetraprolin controls the stability of cIAP2 mRNA through binding to the 3′UTR of cIAP2 mRNA. Biochem Biophys Res Commun. 2010;400:46–52. doi: 10.1016/j.bbrc.2010.07.136. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Mckenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Son YJ, Lee WH, Park YW, Chae SW, Cho WJ, Kim YM, Choi HJ, Choi DH, Jung SW, et al. Tristetraprolin regulates expression of VEGF and tumorigenesis in human colon cancer. Int. J Cancer. 2010a;126:1817–1827. doi: 10.1002/ijc.24847. [DOI] [PubMed] [Google Scholar]
- Lee HH, Vo MT, Kim HJ, Lee UH, Kim CW, Kim HK, Ko MS, Lee WH, Cha SJ, Min YJ, et al. Stability of the LATS2 tumor suppressor gene is regulated by tristetraprolin. J Biol Chem. 2010b;285:17329–17337. doi: 10.1074/jbc.M109.094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Lee HH, Vo MT, Kim HJ, Ko MS, Im YC, Min YJ, Lee BJ, Cho WJ, Park JW. Casein kinase 2 regulates the mRNA-destabilizing activity of tristetraprolin. J Biol Chem. 2011;286:21577–21587. doi: 10.1074/jbc.M110.201137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Yoon NA, Vo MT, Kim CW, Woo JM, Cha HJ, Cho YW, Lee BJ, Cho WJ, Park JW. Tristetraprolin down-regulates IL-17 through mRNA destabilization. FEBS Lett. 2012;586:41–46. doi: 10.1016/j.febslet.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Lee HH, Kim WT, Kim DH, Park JW, Kang TH, Chung JW, Leem SH. Tristetraprolin suppresses AHRR expression through mRNA destabilization. FEBS Lett. 2013;587:1518–1523. doi: 10.1016/j.febslet.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen J, Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev. 2005;19:351–361. doi: 10.1101/gad.1282305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol. 2001;21:6461–6469. doi: 10.1128/MCB.21.9.6461-6469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N, Dong C. The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr. Opin. Invest Drugs. 2009;10:543–549. [PubMed] [Google Scholar]
- Qian X, Ning H, Zhang J, Hoft DF, Stumpo DJ, Blackshear PJ, Liu J. Posttranscriptional regulation of IL-23 expression by IFN-γ through tristetraprolin. J Immunol. 2011;185:6454–6464. doi: 10.4049/jimmunol.1002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer I, Schaljo B, Vogl C, Gattermeier I, Kolbe T, Müller M, Blackshear PJ, Kovarik P. Interferons limit inflammatory responses by induction of tristetraprolin. Blood. 2006;107:4790–4797. doi: 10.1182/blood-2005-07-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaljo B, Kratochvill F, Gratz N, Sadzak I, Sauer I, Hammer M, Vogl C, Strobl B, Müller M, Blackshear PJ, et al. Tristetraprolin is required for full anti-inflammatory response of murine macrophages to IL-10. J Immunol. 2009;183:1197–1206. doi: 10.4049/jimmunol.0803883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu AB, Wilkinson MF. The double lives of shuttling mRNA binding proteins. Cell. 2000;102:135–138. doi: 10.1016/s0092-8674(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Stoecklin G, Anderson P. Posttranscriptional mechanisms regulating the inflammatory response. Adv Immunol. 2006;89:1–37. doi: 10.1016/S0065-2776(05)89001-7. [DOI] [PubMed] [Google Scholar]
- Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P. MK2-induced triste-traprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, et al. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- Teng MWL, Andrews DM, McLaughlin N, von Scheidt B, Ngiow SF, Möller A, Hill GR, Iwakura Y, Oft M, Smyth MJ. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc. Natl. Acad. Sci USA. 2010;107:8328–8333. doi: 10.1073/pnas.1003251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MW, von Scheidt B, Duret H, Towne JE, Smyth MJ. Anti-IL-23 monoclonal antibody synergizes in combination with targeted therapies or IL-2 to suppress tumor growth and metastases. Cancer Res. 2011;71:2077–2086. doi: 10.1158/0008-5472.CAN-10-3994. [DOI] [PubMed] [Google Scholar]
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Young LE, Sanduja S, Bemis-Standoli K, Pena EA, Price RL, Dixon DA. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis. Gastroenterology. 2009;136:1669–1679. doi: 10.1053/j.gastro.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]