Abstract
Lipid rafts are related to cell surface receptor function. Integrin is a major surface receptor protein in cell adhesion and migration on the extracellular matrix (ECM). Here, we showed that lipid rafts played a critical role in human melanoma A375 cell spreading and migration on fibronectin; an important component of the ECM that interacts with β1 integrin. We found that the disruption of lipid rafts did not markedly inhibit the expression and activation of β1 integrin. By coimmunoprecipitation and mass spectrometry, we investigated the influence of lipid rafts on the β1 integrin complex and identified nucleolin as a potential lipid-raft-dependent β1-integrin-interacting protein. Upon confirmation of the interaction between β1 integrin and nucleolin, further studies revealed that nucleolin colocalized with β1 integrin in lipid rafts and raft disruption interrupted their association. In addition, knockdown of nucleolin markedly attenuated A375 cell spreading and migration on fibronectin. Taken together, we demonstrated that nucleolin is a critical lipid-raft-dependent β1-integrin-interacting protein in A375 cell spreading and migration on fibronectin.
Keywords: β1 integrin, lipid rafts, mass spectrometry, melanoma cell spreading and migration, nucleolin
INTRODUCTION
There is a growing consensus that the plasma membrane contains specific microdomains, termed lipid rafts, which are enriched in cholesterol and sphingolipids (Simons and Gerl, 2011). These lipid rafts are thought to be essential for a wide variety of cellular processes, including membrane trafficking, and cell adhesion, polarity and motility (Ikonen, 2001; Mañes and Viola, 2006; You et al., 2007). During these processes, lipid rafts serve as platforms in which protein-protein or protein-lipid interactions take place, and facilitate efficient signal transduction and membrane dynamics (Rajendran and Simons, 2005). Several cell surface receptor proteins regulate cellular function through association with lipid rafts (Mañes and Viola, 2006). One such receptor protein, integrin, is a major surface receptor in cell adhesion and migration on extracellular matrix (ECM) (Holly et al., 2000).
The integrin family consists of 18α and 8β subunits, which combine into 24 different heterodimers depending on cell type and cellular function (Hehlgans et al., 2007). Yet, half of these heterodimers are made up by β1 integrin subunit (Riopel et al., 2013), which is widely expressed on the surface of most cells and can interact with a variety of membrane or intracellular proteins (Barczyk et al., 2010; Legate and Fässler, 2009; Porter and Hogg, 1998). It is generally accepted that β1 integrin subunit is crucial for cell adhesion and migration on fibronectin; one key component of the ECM. After interacting with fibronectin, β1 integrin subunit starts to move laterally in the plasma membrane (Li et al., 2011). Importantly, the lateral movement has been recently found to be lipid raft dependent (Leitinger and Hogg, 2002). Several researches showed that the disruption of lipid rafts by cholesterol sequestering agents, such as methyl-β-cyclodextrin (MβCD), inhibits the function of fibronectin-associated β1 integrin. For example, MβCD treatment leads to inability of β1-integrin-mediated Jurkat cell adhesion on fibronectin (Leitinger and Hogg, 2002), and MβCD treatment markedly attenuates the migration of B16 mouse melanoma cells on fibronectin by preventing ephrin-B2/β1-integrin interaction (Meyer et al., 2007). These studies point to the vital role of lipid rafts in the regulation of β1 integrin function that is critical for cell migration. Also, previous reports indicate that integrin-mediated cell adhesion and migration depend on its expression, activation and interaction with various proteins (Hood and Cheresh, 2002; Legate and Fässler, 2009; Maheshwari et al., 2000). On the basis of this evidence, one can hypothesize that lipid rafts contribute to cell migration by modulating the expression and activation of β1 integrin and its interaction with various proteins.
It is suggested that cell migration on 2D substrate requires integrin-mediated cell spreading in response to ECM (Holly et al., 2000; Maheshwari et al., 2000). Here, we showed that the disruption of lipid rafts severely impaired the spreading and migration of human malignant melanoma A375 cells on fibronectin. β1 integrin expression and activation were not changed by lipid raft disruption. However, by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), we demonstrated the effect of lipid raft disruption on the β1 integrin complex and identified potential β1-integrin-interacting proteins that are closely-related to lipid rafts. Among these proteins, nucleolin is proposed to be present in lipid rafts (Chen et al., 2011; Nisole et al., 2002; Said et al., 2002) and is implicated in adhesion and migration of cancer cells (Koutsioumpa et al., 2013; Reyes-Reyes and Akiyama, 2008). After further confirming the lipid-raft-dependent interaction between nucleolin and β1 integrin, we explored the biological role of nucleolin in β1-integrin-mediated A375 cell spreading and migration on fibronectin.
MATERIALS AND METHODS
Reagents and antibodies
MβCD, cholesterol, horseradish peroxidase (HRP)-conjugated cholera toxin subunit B (CTXB, C3741), and monoclonal antibodies (mAbs) to actin (AC-40) and tubulin (T4026) were purchased from Sigma-Aldrich. Human fibronectin, mAbs to β1 integrin (TDM29), and active β1 integrin (HUTS-4) were obtained from Millipore. Normal mouse or rabbit IgG, mAb to β1 integrin (JB1B) and polyclonal antibody to nucleolin (H-250) were purchased from Santa Cruz Biotechnology. Alexa-Fluor-488-conjugated CTXB was obtained from Molecular Probes. HRP, tetramethylrhodamine isothiocyanate (TRITC) or fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG and anti-rabbit IgG antibodies were obtained from Jackson ImmunoResearch Laboratories.
Cell culture
Human melanoma A375 cells were purchased from the cell bank of the type Culture Collection of the Chinese Academy of Sciences (China). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C in the presence of 5% CO2.
siRNA transfection
A375 cells were transfected with siRNA targeting nucleolin using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The sense and antisense sequences of nucleolin siRNA oligoribonucleotides were 5′-GGCAAAGCA UUGGUAGCAAdTdT-3′ and 5′-UUGCUACCAAUGCUUUG CCdTdT-3′, respectively. siRNA duplex oligoribonucleotides were synthesized by GenePharma.
Time-lapse videomicroscopy
A375 cells were resuspended in DMEM with or without 5 mM MβCD at 37°C for 30 min, or replenished with 1 mM cholesterol for 1 h after removal of MβCD. In siRNA experiments, A375 cells were transfected with nucleolin siRNA for 48 h prior to resuspension in DMEM. The cells were added to dishes with fibronectin-precoated glass bottoms and incubated in a temperature-controlled incubator at 37°C. Images were captured using a Nikon microscope (Eclipse 80i) with a CCD camera at 15s intervals over a 120-min time course. The recorded data were used for analysis of A375 cell spreading using ImageJ software. For kymograph analysis, kymographs were generated from randomly chosen cells along a one-pixel-wide line oriented in the direction of protrusions, using ImageJ with the Kymograph plugin. Kymographs were then analyzed by ImageJ to determine relative velocity of membrane protrusions.
Transwell assay
Cell migration was assayed using Transwell chamber inserts (polycarbonate membrane, 8 μm pore size; Costar). Control cells, MβCD-treated cells, cholesterol-replenished cells, and cells transfected with nucleolin-specific siRNA were added to the upper chamber, which was precoated with fibronectin on the undersurface of the membrane. The lower well was filled with 500 μl DMEM containing 2% FBS. After incubation for 16 h at 37°C, the cells on the upper surface of the membrane were mechanically removed, and the migrated cells on the undersurface of the membrane were fixed and stained with crystal violet. Cells were imaged using an inverted microscope (Olympus) and then crystal violet was solubilized in acetic acid to measure absorbance (495 nm) with a microplate reader (Bio Tek Instruments).
Cell migration in the agarose drop assay
Cell migration was quantified by measuring the extent of cell migration out of the agarose drops, as described by Ogier et al. (2006), with modifications. Briefly, A375 cells pretreated with 5 mM MβCD or replenished with 1 mM cholesterol, or cells transfected with nucleolin siRNA were resuspended in DMEM containing 2% FBS and 0.3% low melting-point agarose (Invitrogen). Two-microliter drops from the cell mixture were seeded in the centre of 35-mm culture dishes coated with fibronectin and then placed at 4°C for 20 min to solidify the agarose. After incubation at 37°C for 36 h, cell drops were imaged by phase-contrast microscopy (Olympus) and the cell migration distance was detected by measuring the radius.
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted using the TRIzol reagent (Invitrogen), cDNA was generated using reverse transcriptase, and PCR was performed. The PCR primers used were 5′-CCCTTGCA CAAGTGAACAGA-3′ and 5′-ACATTCCTCCAGCCAATCAG-3′ for β1 integrin, 5′-AGCAACTCCTGGTAAGAA-3′ and 5′-TTT CATCGCTGCTGGTTC-3′ for nucleolin, 5′-ATGCCAGGGTAC ATGGTGGT-3′ and 5′-TCGTGCGTGACATTAAGGAG-3′ for β-actin. The PCR products were analyzed on 1% agarose gel and quantified using imageJ software. Expression of β-actin was used as a control.
Immunofluorescence
A375 cells were pretreated with different drugs and then fixed with 5% formaldehyde for 10 min at room temperature. The cells were blocked in 3% bovine serum albumin and incubated with the indicated primary antibodies for 1 h followed by fluorochrome-conjugated secondary antibodies. All of the stained cells were observed under a confocal microscope (Olympus). The intensity of fluorescence was analyzed using the Olympus confocal software. The colocalization among GM1, nucleolin and β1 integrin was determined by the ImageJ plugin Colocalization Finder.
Immunoprecipitation and immunoblotting
A375 cells were resuspended in DMEM with or without 5 mM MβCD at 37°C for 30 min, or incubated with 1 mM cholesterol for 1 h after removal of MβCD. The cells were lysed in lysis buffer [50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.1% SDS, 2.5 mM sodium pyrophosphate, 1 mM NaF, 1 mM Na3VO4, 1 mM β-glycerophosphate, 1 mM phenylme-thylsulfonyl fluoride (PMSF), and 20 μg/ml aprotinin/leupeptin]. After incubation on ice for 30 min, the lysates were centrifuged at 12,000 × g for 30 min. The supernatant was incubated with the indicated antibodies at 4°C for 3 h, followed by incubation for another 3 h with 30 μl protein A/G-Sepharose beads. After washing with lysis buffer, the immunoprecipitates were resolved on SDS-PAGE, electrotransferred onto nitrocellulose membranes (Millipore), and probed with the indicated antibodies. Quantification was performed using ImageJ software.
Subcellular fractionation
Subcellular fractionation of A375 cells was performed as previously described (Villalba et al., 2000), with a slight modification. A375 cells (1 × 107) with or without 5 mM MβCD treatment were collected and resuspended in 500 μl ice-cold hypotonic buffer (42 mM KCl, 10 mM HEPES, pH 7.4, 5 mM MgCl2, 20 μg/ml aprotinin/leupeptin). After 20 min, the cells were sheared by repeated passage through a 22-gauge needle (30 times). The lysate was centrifuged at 200 g for 10 min and the supernatant (total fractions) was centrifuged at 13,000 × g for 60 min at 4°C. The supernatant (cytosol fractions) was collected and the pellets were lysed by adding 100 μl lysis buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 1 mM PMSF, and 20 μg/ml aprotinin/leupeptin, vortexed for 5 min at 4°C, and centrifuged again at 13,000 × g for 60 min at 4°C. The supernatant representing the membrane fractions was saved. The total, cytosol and membrane fractions were diluted with equal volume of 2× Laemmli buffer and separated by SDS-PAGE.
LC-MS/MS analysis
For LC-MS/MS, β1 integrin immunoprecipitates were resolved on SDS-PAGE. After visualization by silver staining, each gel lane was cut into two pieces of equal size and subjected to in-gel tryptic digestion. The extracted peptides from each gel piece were analyzed by LC-MS/MS as previously described (Mao et al., 2011). Protein identification results were extracted from the SEQUEST.out files with in-house software (BuildSummary). Subcellular location and molecular function of the identified proteins were analyzed by DAVID bioinformatics resources (http://david.abcc.ncifcrf.gov/home.jsp).
Flow cytometry
A375 cells (1 × 106) were harvested, fixed and then incubated with 2 μg isotype IgG or specific antibodies for 60 min, followed by staining with FITC-conjugated secondary antibody. After thorough washes with PBS, the cells were detected using a FACScan (Beckman-Coulter). At least 10,000 cells were counted per sample. The data were analyzed by FlowJo software.
Lipid raft purification
Lipid raft purification was performed by density gradient centrifugation at 4°C. A375 cells (2 × 107) were suspended in DMEM with or without 5 mM MβCD at 37°C for 30 min, centrifuged and lysed for 30 min on ice in lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 50 mM NaF, 10 mM sodium pyrophosphate and 1 mM Na3VO4). The lysates were homogenized with 10 strokes in a Dounce homogenizer and then repeatedly passed through a 22-gauge needle (30 times). To generate the density gradients for centrifugation, the homogenate (1 ml) was mixed with an equal volume of 80% sucrose in MNE buffer (25 mM 4-morpholineethanesulfonic acid, pH 6.5, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 1 mM PMSF, and 1 μg/μl of aprotinin), and then overlaid with 2 ml 30% sucrose followed by 1 ml 5% sucrose. The gradients were ultracentrifuged (200,000 × g at 4°C for 18 h) using a Beckman MLS50 rotor. Twelve fractions (400 μl/fraction) were obtained from the top to bottom.
RESULTS
Lipid rafts regulate human melanoma A375 cell spreading and migration on fibronectin
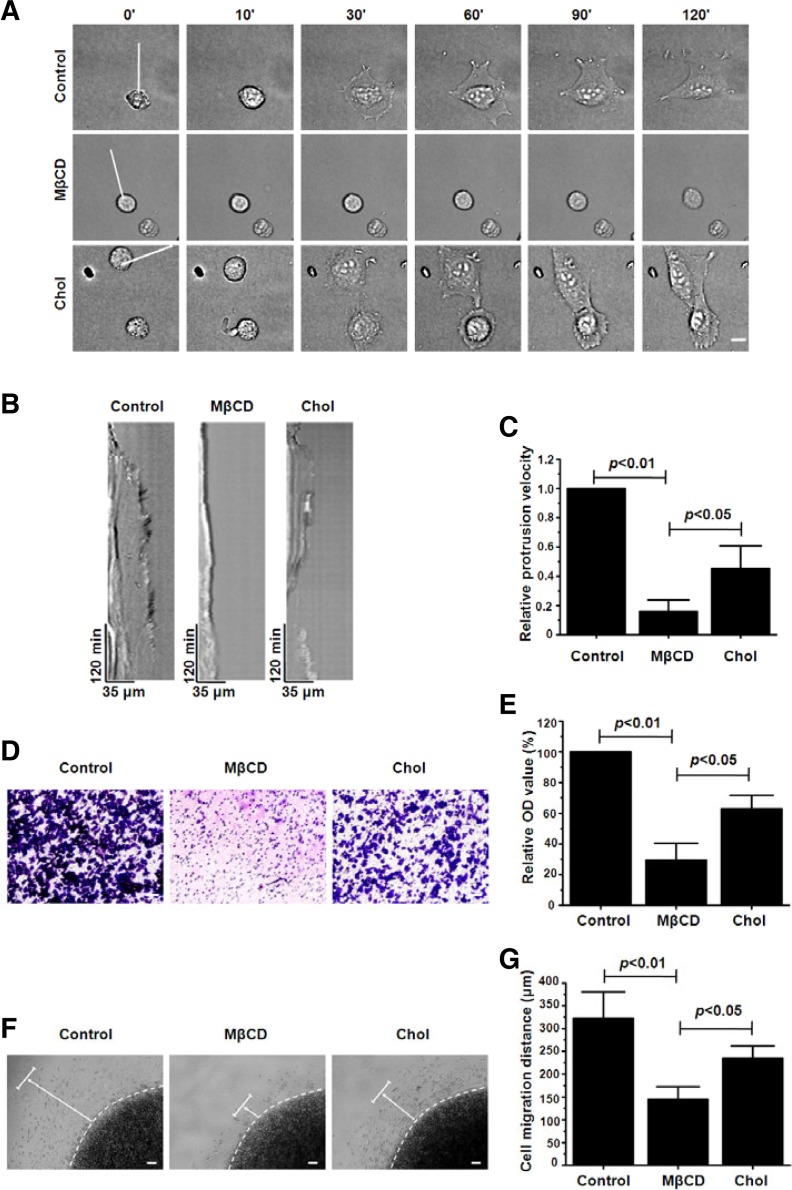
To investigate the role of lipid rafts in A375 cell spreading on fibronectin, we treated A375 cells with 5 mM MβCD, which can effectively disrupt the integrity of lipid rafts via depleting cholesterol (Wang et al., 2013), and then recorded cell spreading using time-lapse videomicroscopy. Almost all control cells exhibited a shape change from a round, spheroid morphology to that of an irregular flattened shape during the 120 min observation period. Conversely, the cells exposed to MβCD only exhibited slight changes in their shape and were hardly flattened. When cholesterol was replenished, the cells spread again (Fig. 1A, Supplementary Movies 1–3). Measurement of the movements of the lamellipodia during the spreading process using kymograph analysis indicated that A375 cells with MβCD treatment displayed greatly diminished membrane dynamics compared with the control cells, while cholesterol addition partly rescued the membrane dynamics (Fig. 1B). Histogram analyses of relative membrane protrusion velocity generated from kymographs revealed that the membrane protrusion velocity for MβCD-treated cells was 5 times lower than that of the control cells, but the velocity increased after cholesterol addition (Fig. 1C). These results confirm that lipid rafts are responsible for the spreading of A375 cells on fibronectin.
Fig. 1.
Integrity of lipid rafts is required for A375 cell spreading and migration on fibronectin. (A) Time-lapse images of spreading on fibronectin of control, 5 mM MβCD-treated and 1 mM cholesterol-replenished A375 cells. Scale bar = 10 μm. (B) Kymograph analysis showed the membrane protrusion dynamics along the white lines generated from the time-lapse images shown in (A). (C) Analyses of relative membrane protrusion velocity calculated from kymographs. Values are normalized to the membrane protrusion velocity of the control cells. (D) Photomicrographs show the effect of MβCD treatment or cholesterol replenishment on the migration of A375 cells 16 h after seeding in the Trans-well upper chamber. Scale bar = 50 μm. (E) Relative number of cells transmigrated onto membrane undersurface was analyzed. Values are normalized to the relative OD value of the control cells. (F) Photomicrographs show the migration of control, MβCD-treated and cholesterol-replenished A375 cells in the agarose drop model 36 h after seeding. The dashed lines indicate the edge of the agarose drop. The arrows point the direction and distance of cell migration. Scale bar = 100 μm. (G) The distance of cell migration was measured. Each bar represents the mean ± SD from three independent experiments (Student’s t-test).
Transwell assay was used to evaluate the effect of lipid rafts on the migration capacity of A375 cell on fibronectin. The relative number of cells transmigrated onto the membrane under-surface was determined by solubilizing crystal violet in acetic acid to measure the absorbance value. As shown in Figs. 1D and 1E, MβCD treatment markedly inhibited cell migration to the lower wells. In addition, by testing cell migration in an aga-rose drop model, we found that A375 cells without MβCD treatment were much more motile, and their migration distance was significantly longer than that of the treated cells (Figs. 1F and 1G). The addition of cholesterol reversed to a large extent the effect of MβCD on cell migration; both in the Transwell assay and agarose drop model (Figs. 1D–1G). The results above demonstrate that lipid rafts are indispensable for A375 cell migration on fibronectin. Taken together, these results indicate that the integrity of lipid rafts is required for A375 cell spreading and migration on fibronectin.
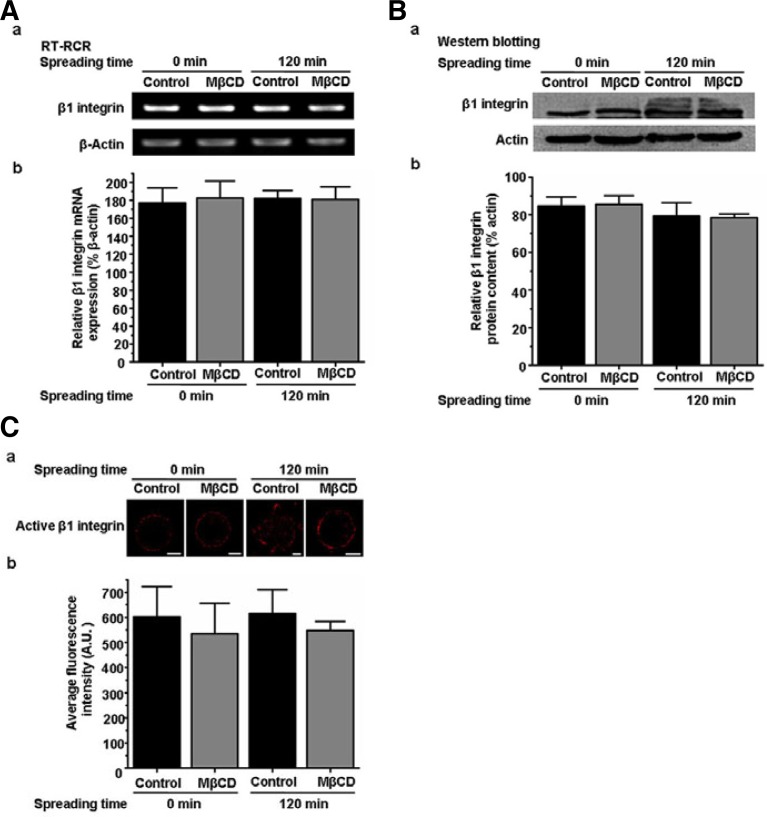
Disruption of lipid rafts does not influence expression and activation of β1 integrin
We previously demonstrated that initial spreading of A375 cells on fibronectin is mainly β1 integrin dependent (Wang et al., 2013). The expression and activation of integrin and its interaction with other proteins are thought to be key factors that affect integrin function (Hood and Cheresh, 2002; Legate and Fässler, 2009; Maheshwari et al., 2000). Thus, we investigated the influence of lipid rafts on the expression and activation of β1 integrin. RT-PCR and Western blotting indicated that β1 integrin mRNA and protein expression level in control and MβCD-treated (5 mM) suspension or spreading cells were similar (Figs. 2A and 2B), meaning that the disruption of lipid rafts does not affect expression of β1 integrin. We stained control and MβCD-treated suspension or spreading cells with antibody against active β1 integrin. The analysis of fluorescence intensity showed that there was no significant difference in the activation of β1 integrin between control and MβCD-treated cells (Fig. 2C). Together, these results suggest that the influence of lipid rafts on cell spreading and migration cannot be accomplished by regulating the expression and activation of β1 integrin.
Fig. 2.
Disruption of lipid rafts does not influence the expression and activation of β1 integrin. (A) a, RT-PCR analysis of β1 integrin gene expression in control or MβCD-treated suspension or spreading A375 cells. β-Actin was used as a loading control. b, Quantification of the relative expression of β1 integrin mRNA in a. Values are normalized to the corresponding actin. (B) a, Western blotting of β1 integrin protein expression normalized to actin. b, Quantification of the β1 integrin protein level in a. (C) a, Control or MβCD-treated suspension or spreading cells were fixed and labeled with antibody against active β1 integrin. Scale bar = 5 μm. b, Total immunofluorescence intensity of active β1 integrin was analyzed by Olympus confocal microscopy software to obtain a mean pixel intensity value (expressed in arbitrary units, A.U.). Thirty cells were analyzed per condition in each experiment. Each bar represents the mean ± SD from three independent experiments.
Identification and functional analysis of lipid-raft-dependent β1-integrin-interacting proteins
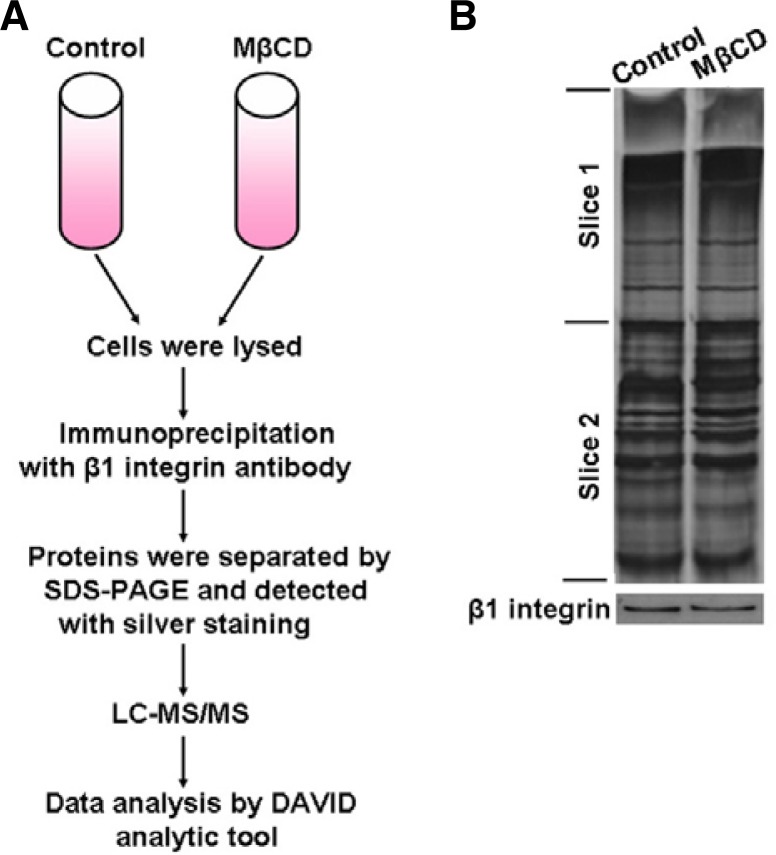
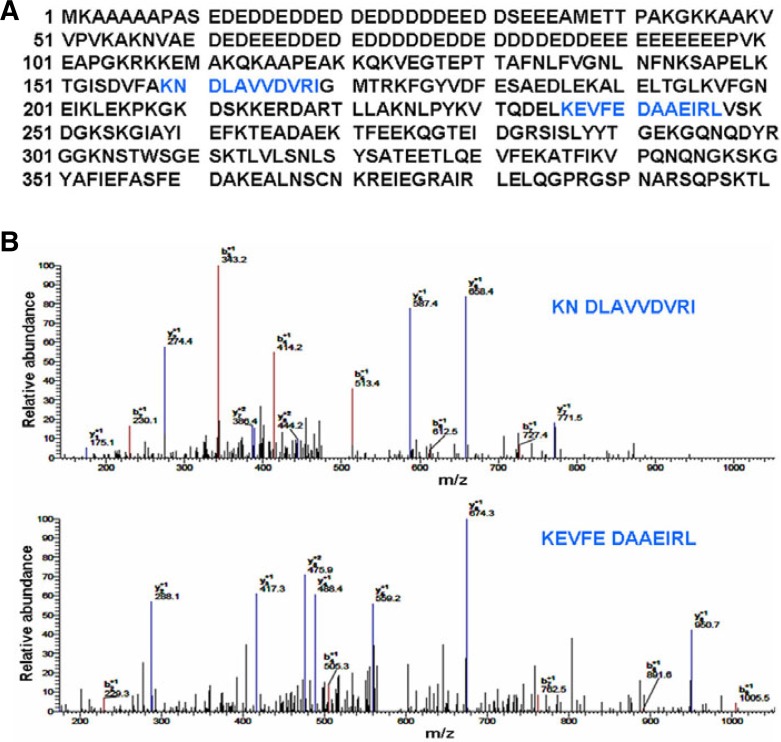
After eliminating the influence of lipid rafts on β1 integrin expression and activation, we focused on the effect of lipid rafts on the association between β1 integrin and other proteins. To screen potential β1-integrin-interacting proteins and detect the effect of lipid rafts on their association, we used coimmunoprecipitation combined with LC-MS/MS. Suspension cells treated with or without 5 mM MβCD were lysed and the lysates were incubated with β1 integrin antibody prior to adding protein G-Sepharose beads. The β1 integrin immunoprecipitates were separated by SDS-PAGE and visualized by silver staining. Each gel lane was cut into two pieces of equal size and subjected to trypsin digestion and LC-MS/MS protein identification (Figs. 3A and 3B). The identified proteins in both control and MβCD-treated cells, and the proteins that only contained one peptide, were filtered out during the analysis. To understand the function and subcellular location of the identified putative β1 integrin-associated proteins, we used DAVID bioinformatics resources to perform bioinformatics analysis. The results revealed that 8 proteins, whose relationship to β1 integrin remained unclear, were involved in cell adhesion, cell migration, actin skeleton rearrangement and cell membrane dynamics (Table 1). Among these proteins, nucleolin was the only one that localized in lipid rafts. Thus, we finally selected it for further investigation. The 2 specific peptides of nucleolin and their spectra are shown in Fig. 4.
Fig. 3.
Identification of lipid-raft-dependent β1-integrin-interacting proteins. (A) Schematic illustration of the strategy used to screen β1-integrin-interacting proteins. (B) β1-integrin immunoprecipitates from total lysates of A375 cells with or without 5 mM MβCD were fractionated by SDS-PAGE. The gels were either visualized by silver staining (upper panel) or blotted with anti-β1-integrin antibody (lower panel). Each gel lane was cut into two pieces of equal size and subjected to in-gel tryptic digestion, and the extracted peptides from each gel piece were analyzed by LC-MS/MS analysis.
Table 1.
List of potential β1-integrin-interacting proteins identified by LC-MS/MS analysis
| IPI | Protein name | Subcellular location | Molecular function (part) |
|---|---|---|---|
| 00936485 | EEF1A1 | Nucleus, cytosol | Actin dynamics, cell migration |
| 00382470 | Heat shock protein 90 kDa alpha | Cytosol, extracellular | Cell invasion and migration |
| 00014424 | EEF1A2 | Nucleus, cytosol | Actin remodeling, filopodia formation, cell invasion, migration |
| 00444262 | Nucleolin | Nucleus, cell membrane, lipid rafts | Cell adhesion, migration |
| 00022479 | HERC1 | Cytosol, intracellular membrane | Membrane traffic |
| 00218319 | Tropomyosin | Cytosol | Actin cytoskeleton dynamics, filopodia formation, cell migration |
| 00418875 | PREX2 | Cytosol | Rac activation, cell morphology change |
| 00024706 | SNX9 | Cytosol, cell membrane | Cell membrane remodeling |
Fig. 4.
Nucleolin is identified as a lipid-raft-fependent β1-integrin-interacting protein by LC-MS/MS. (A) The positions of the 2 peptides (blue) derived from LC-MS/MS is shown within the human nucleolin protein sequence, which led to the identification of the nucleolin protein. (B) The peptide spectra corresponding to KNDL AVVDVRI and KEVFEDAAEIRL are shown.
Lipid rafts are necessary for interaction between β1 integrin and nucleolin
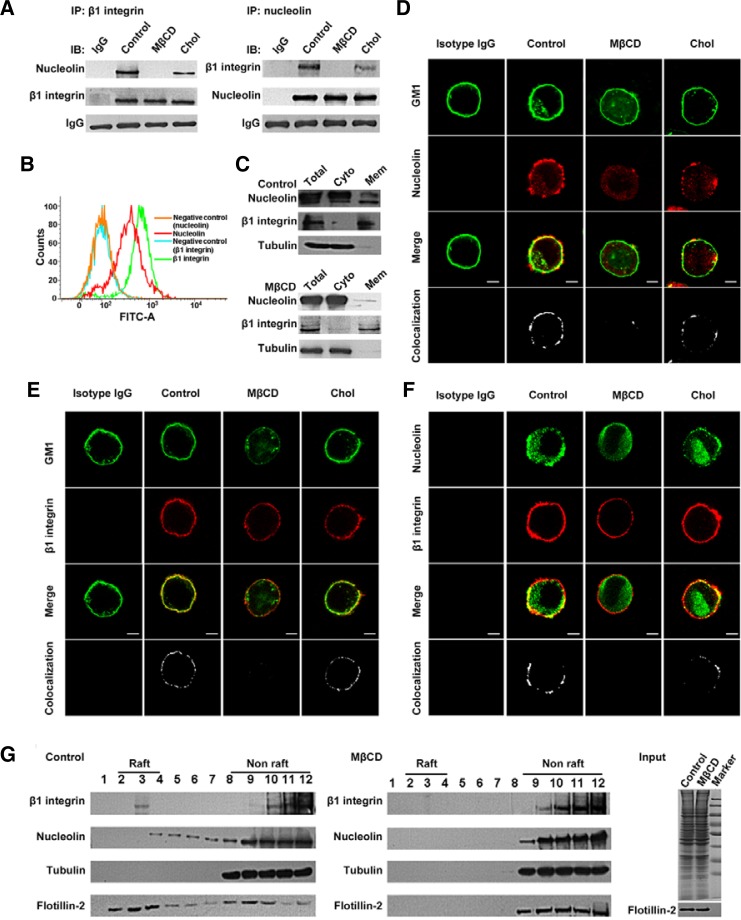
To validate the interaction between β1 integrin and nucleolin, we performed independent precipitations using anti-β1 integrin or anti-nucleolin antibody, respectively. As shown in Fig. 5A, endogenous nucleolin was coprecipitated with β1 integrin; β1 integrin also could be pulled down with anti-nucleolin antibody, and this association between β1 integrin and nucleolin was inhibited by 5 mM MβCD treatment and the inhibition could be reversed by 1 mM cholesterol replenishment. These data suggest that β1 integrin and nucleolin constitutively interact in the cells, and their association depends on intact lipid rafts.
Fig. 5.
Lipid rafts are necessary for the interaction between β1 integrin and nucleolin. (A) Confirmation of the interaction between β1 integrin and nucleolin by coimmunoprecipitation. (B) Expression of β1 integrin and nucleolin on A375 cell surface was determined by FACS analysis. Normal mouse or rabbit IgG served as a negative control. (C) Effect of lipid rafts on the distribution of nucleolin in the cytosol and membrane fractions of A375 cells were detected by subcellular fractionation and Western blotting. β1 Integrin and tubulin were used as markers of the membrane and cytosol fraction, respectively. (D–F) A375 cells treated with MβCD or replenished with cholesterol were fixed and double-stained for GM1 and nucleolin, GM1 and β1 integrin, nucleolin and β1 integrin, respectively. Their colocalization in merged images was determined and indicated by white dots (bottom panel). (G) Location of β1 integrin and nucleolin in lipid raft fractions. The equivalent amount of lysates from control and MβCD-treated cells were either stained with Coomassie blue R-250 or immunoblotted with anti-flotillin-2 antibody as input.
Nucleolin, a eukaryotic nucleolar protein, is located mainly in the nucleolus, but an increasing number of studies have confirmed that the localization of nucleolin in the membrane is important for various cell processes (Koutsioumpa et al., 2013; Reyes-Reyes and Akiyama, 2008; Said et al., 2002). This prompted us to investigate whether nucleolin is present on the A375 cell surface by flow cytometric analysis. Nucleolin, just like integrin, could be expressed on the A375 cell surface (Fig. 5B). To detect further the effect of lipid rafts on the distribution of nucleolin in A375 cells, we performed subcellular fractionation and Western blotting. Lipid raft disruption by MβCD treatment markedly reduced the distribution of nucleolin, but not β1 integrin, in the membrane fraction (Fig. 5C), suggesting that the location of nucleolin in the membrane depends on intact lipid rafts.
Based on our observation that both nucleolin and β1 integrin were expressed on the A375 cell surface, and that MβCD treatment weakened their interaction, we speculated that lipid rafts are critical for the spatial association between nucleolin and β1 integrin. To test this, we performed immunofluorescence experiments. As shown in Figs. 5D and 5E, in control cells, nucleolin and β1 integrin were mostly prominent at the cell periphery and colocalized with the lipid raft marker GM1, respectively. After lipid raft disruption by MβCD, the colocalization disappeared. Meanwhile, the tight colocalization between nucleolin and β1 integrin was also disturbed (Fig. 5F). However, with the replenishment of cholesterol, the colocalization among nucleolin, GM1 and β1 integrin was more or less recovered (Figs. 5D–5F). Next, we investigated the distribution of β1 integrin and nucleolin in lipid raft fractions. As illustrated in Fig. 5G, the fractions 2–4 were considered as lipid raft fractions because of the high level of the lipid raft marker flotillin-2. Tubulin, a protein known to reside in non-lipid raft fractions, was present in fractions 8–12. MβCD treatment resulted in the redistribution of flotillin-2 from lipid raft fractions to non-lipid raft fractions, indicating that the lipid raft fractions were isolated successfully. The results of immunoblotting with antibodies to nucleolin and β1 integrin showed that the two proteins resided together in lipid raft fractions, and MβCD treatment resulted in their delocalization from lipid raft fractions. Thus, we demonstrate that lipid rafts are necessary for the interaction between nucleolin and β1 integrin.
Nucleolin knockdown attenuates A375 cell spreading and migration
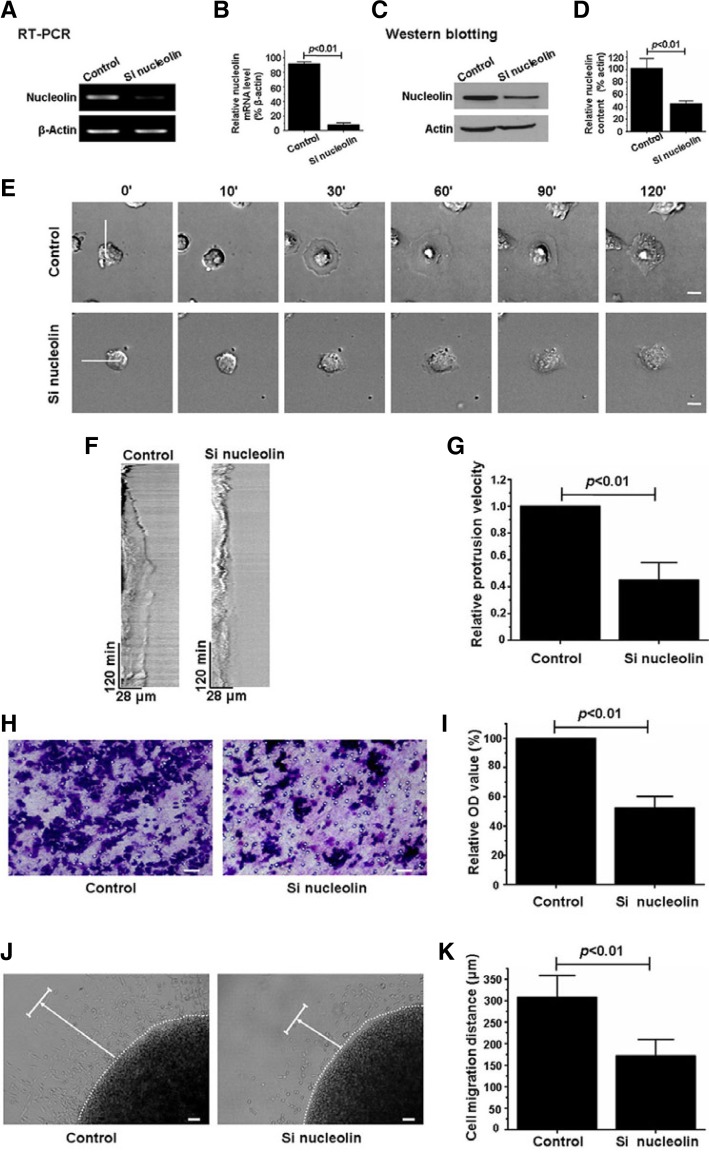
To determine further whether the lipid-raft-dependent association between nucleolin and β1 integrin plays essential roles in cell spreading and migration, we knocked down endogenous nucleolin using specific siRNA (Figs. 6A–6D) and examined the spreading and migration of A375 cells. Using time-lapse videomicroscopy, we found that compared with control cells, the spreading ability of nucleolin knockdown cells was significantly weakened (Fig. 6E, Supplementary Movies 4 and 5). Kymograph analysis indicated that siRNA-mediated nucleolin knockdown effectively blocked membrane protrusion during the A375 cell spreading (Fig. 6F). This finding was confirmed by measuring the relative membrane protrusion velocity. The measurements showed that the membrane protrusion velocity for nucleolin knockdown cells was 2.5 times lower than that of control cells (Fig. 6G). These results suggest that nucleolin is required for β1-integrin-dependent A375 cell spreading.
Fig. 6.
Nucleolin knockdown attenuates A375 cell spreading and migration. (A) The efficiency of nucleolin knockdown was determined by RT-PCR. β-Actin was used as a loading control. (B) Quantification of the relative mRNA level of nucleolin in (A). Values are normalized to the corresponding β-actin. (C) Nucleolin knockdown efficiency was monitored by Western blotting. (D) Quantification of the relative nucleolin content in (C). Values are normalized to the corresponding actin. (E) Time-lapse images of control and nucleolin-siRNA-treated A375 cells spreading on fibronectin. Scale bar = 10 μm. (F) Kymograph analysis showed membrane protrusion dynamics along the white lines generated from the time-lapse images shown in (E). (G) Analyses of relative membrane protrusion velocity calculated from the kymographs. Values are normalized to the membrane protrusion velocity of the control cells. (H) Photomicrographs showed the effects of nucleolin knockdown on the migration of A375 cell 16 h after seeding in the Transwell upper chamber. Scale bar = 50 μm. (I) Relative number of cells transmigrated onto membrane undersurface was analyzed. Values are normalized to the relative OD value of the control cells. (J) Photomicrographs show the control and the nucleolin siRNA treated A375 cells migration in the agarose drop model 36 h after seeding. The dashed lines indicate the edge of the agarose drop. The arrows point the direction and distance of cell migration. Scale bar = 100 μm. (K) The distance of cell migration was measured. Each bar represents the mean ± SD from three independent experiments (Student’s t-test).
Next, we investigated the effect of nucleolin knockdown on A375 cell migration using Transwell and agarose drop assays. The Transwell assay showed that compared with the control cells, the migration of nucleolin knockdown cells was reduced to approximately 50% (Figs. 6H and 6I). Similar results were obtained from the agarose drop experiments. As shown in Fig. 6J, nucleolin knockdown inhibited cell migration out of the drop. The migration distance of control cells was almost 2 times longer than that of nucleolin knockdown cells (Fig. 6K). These data reveal that nucleolin is indispensable for β1 integrin-dependent A375 cell migration.
DISCUSSION
Integrins are cell surface heterodimeric receptors that mediate cell-ECM interactions. Among integrin subunits, β1 integrin is widely expressed on the surface of various cell types and intimately correlated with cell polarity, spreading, migration, proliferation and survival on fibronectin (Chen et al., 2013; Danen et al., 2005; Mostafavi-Pour et al., 2003; Zhang et al., 1995). Thus, the regulation of β1 integrin-dependent cell behavior on fibronectin has received considerable attention over the past two decades. Several studies have shown that lipid rafts play important roles in cell adhesion and migration by modulating β1 integrin internalization, intracellular trafficking, and its movement within the plasma membrane (Vassilieva et al., 2008; Yanagisawa et al., 2004). In the present study, we showed that lipid rafts regulated A375 cell spreading and migration on fibronectin, suggesting that lipid rafts are tightly associated with β1 integrin function (Fig. 1).
It is generally accepted that integrin expression, activation and interaction with other proteins are responsible for its function in adhesion and migration of cells on ECM. Thus, we speculated that lipid rafts may be involved in the regulation of these three molecular events. Our results showed that lipid raft disruption did not directly affect the expression and activation of β1 integrin in A375 cells (Fig. 2). Next, we turned our attention to the possible effect of lipid raft disruption on the association between β1 integrin and its interacting proteins. Previous reports suggested that lipid rafts can concentrate or exclude some proteins to facilitate protein-protein or protein-lipid spatial interactions (Golub et al., 2004; Simons and Toomre, 2000). Importantly, recent evidence indicates that lipid rafts play crucial roles in regulating the interaction of integrin with other proteins, such as B-ephrins and platelet-derived growth factor receptor (Baron et al., 2003; Meyer et al., 2007). Using LC-MS/MS and bioinformatics analyses, we compared the difference in β1-integrin-interacting proteins between control and MβCD-treated A375 cells, and identified nucleolin as a lipid-raft-dependent β1-integrin-interacting protein (Figs. 3 and 4). The interaction of nucleolin and β1 integrin was confirmed by immunoprecipitation and Western blotting. The results are in agreement with the LC-MS/MS data, that is, nucleolin and β1 integrin coexisted in the same coimmunoprecipitates and lipid raft disruption by MβCD inhibited their association (Fig. 5A).
Nucleolin was originally thought to be a nuclear protein and play an important role in regulating ribosome biogenesis, cell proliferation and growth, cytokinesis, replication, embryogenesis, and nucleogenesis (Huang et al., 2006). Recently, nucleolin was also found to be present on the cell surface in a wide range of cells (Galzio et al., 2012) and serve as receptors for HIV-1, ECM, adhesion molecules and growth factors (El Khoury et al., 2010; Wang et al., 2011). Consistent with these reports, our results showed that nucleolin exists in the membrane fraction of A375 cells (Fig. 5B). Furthermore, MβCD treatment resulted in the translocation of nucleolin from membrane to cytosol fractions, suggesting that intact lipid rafts are required for the location of nucleolin in the membrane (Fig. 5C).
Previous studies indicated that cell-surface nucleolin could be incorporated into lipid rafts to mediate the binding of DNA nanoparticles and HIV to the cell membrane (Chen et al., 2011; Hovanessian et al., 2010). It has also been shown that the location of β1 integrin in lipid rafts is indispensable for its function (Leitinger and Hogg, 2002; Li et al., 2011). Notably, emerging evidences suggest that cell surface nucleolin has an intimate relationship with integrin. For example, Kang et al. (2009) reported that although only partially colocalized, αvβ3 integrin and nucleolin protein are highly coexpressed in HeLa cells. Interestingly, Koutsioumpa et al. (2013) recently indicated that the interplay between αvβ3 integrin and nucleolin regulates human endothelial and glioma cell migration. Reyes-Reyes and Akiyama (2008) have also shown the important role of surface nucleolin in p-selectin-mediated α5β1 integrin activation and colo-320 cell adhesion on fibronectin. Our results showed that nucleolin, β1 integrin and lipid raft marker GM1 colocalized with each other on the surface of A375 cells. MβCD treatment interrupted this colocalization and cholesterol replenishment partly abrogated the effect of MβCD treatment (Figs. 5D–5F). The location of nucleolin and β1 integrin in lipid rafts was confirmed by lipid raft isolation experiments (Fig. 5G). Overall, our data suggest that nucleolin and β1 integrin are associated within lipid rafts, which may favor their interaction. Indirect support for our opinion is provided by studies that documented the interaction of cell surface nucleolin with other membrane receptors, such as urokinase plasminogen activator receptor (Dumler et al., 1999) and ErbB receptors (Farin et al., 2009).
Cell spreading and migration on fibronectin is thought to be β1 integrin dependent. It has also been shown that cell surface nucleolin is implicated in the migration of endothelial and glioma cells (Koutsioumpa et al., 2013). Our results clearly indicate that the knockdown of nucleolin markedly attenuates the spreading and migration of A375 cells on fibronectin, suggesting a key role of lipid raft-dependent β1 integrin-nucleolin interaction in cell migration on fibronectin. However, the question of how the interaction of the two molecules influences A375 cell spreading and migration still remains to be answered. One possibility is that this new interaction between the cell surface β1 integrin and nucleolin in lipid rafts facilitates the formation of a β1 integrin functional complex, which is crucial for A375 cell migration on fibronectin.
In conclusion, we provide evidence that lipid rafts can effectively regulate β1-integrin-mediated spreading and migration of A375 cells on fibronectin by modulating β1 integrin-nucleolin interaction. We found that although MβCD treatment did not markedly alter the expression and activation of β1 integrin at the surface of A375 cells, the location and interaction of β1 integrin and nucleolin in lipid rafts were disrupted, which may be responsible for disturbing the spreading and migration of A375 cells on fibronectin. Further understanding of β1 integrin-nucleolin interaction in lipid rafts will provide novel insights into the mechanism of β1 integrin function regulation.
Acknowledgments
This study was supported by grants from the National Nature Science Foundation of China (81172014, 81071726 and 312 71509), the Specialized Research Fund for the Doctoral Program of Higher education (20100043110007) and the Fundamental Research Funds for the Central Universities (10SSXT129).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron W, Decker L, Colognato H, ffrench-Constant C. Regulation of integrin growth factor interactions in oligo-dendrocytes by lipid raft microdomains. Curr Biol. 2003;13:151–155. doi: 10.1016/s0960-9822(02)01437-9. [DOI] [PubMed] [Google Scholar]
- Chen X, Shank S, Davis PB, Ziady AG. Nucleolin-mediated cellular trafficking of DNA nanoparticle is lipid raft and microtubule dependent and can be modulated by glucocorticoid. Mol Ther. 2011;19:93–102. doi: 10.1038/mt.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HM, Lin YH, Cheng YM, Wing LY, Tsai SJ. Overexpression of integrin-β1 in leiomyoma promotes cell spreading and proliferation. J Clin Endocrinol Metab. 2013;98:E837–846. doi: 10.1210/jc.2012-3647. [DOI] [PubMed] [Google Scholar]
- Danen EH, van Rheenen J, Franken W, Huveneers S, Sonneveld P, Jalink K, Sonnenberg A. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol. 2005;169:515–526. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler I, Stepanova V, Jerke U, Mayboroda OA, Vogel F, Bouvet P, Tkachuk V, Haller H, Gulba DC. Urokinase-induced mitogenesis is mediated by casein kinase 2 and nucleolin. Curr Biol. 1999;9:1468–1476. doi: 10.1016/s0960-9822(00)80116-5. [DOI] [PubMed] [Google Scholar]
- El Khoury D, Destouches D, Lengagne R, Krust B, Hamma-Kourbali Y, Garcette M, Niro S, Kato M, Briand JP, Courty J, et al. Targeting surface nucleolin with a multivalent pseudopeptide delays development of spontaneous melanoma in RET transgenic mice. BMC Cancer. 2010;10:325. doi: 10.1186/1471-2407-10-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin K, Di Segni A, Mor A, Pinkas-Kramarski R. Structure-function analysis of nucleolin and ErbB receptors interactions. PLoS One. 2009;4:e6128. doi: 10.1371/journal.pone.0006128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzio R, Rosati F, Benedetti E, Cristiano L, Aldi S, Mei S, D’Angelo B, Gentile R, Laurenti G, Cifone MG, et al. Glycosilated nucleolin as marker for human gliomas. J Cell Biochem. 2012;113:571–579. doi: 10.1002/jcb.23381. [DOI] [PubMed] [Google Scholar]
- Golub T, Wacha S, Caroni P. Spatial and temporal control of signaling through lipid rafts. Curr Opin Neurobiol. 2004;14:542–550. doi: 10.1016/j.conb.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Holly SP, Larson MK, Parise LV. Multiple roles of integrins in cell motility. Exp Cell Res. 2000;261:69–74. doi: 10.1006/excr.2000.5040. [DOI] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Hovanessian AG, Soundaramourty C, El Khoury D, Nondier I, Svab J, Krust B. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS One. 2010;5:e15787. doi: 10.1371/journal.pone.0015787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Shi H, Zhou H, Song X, Yuan S, Luo Y. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood. 2006;107:3564–3571. doi: 10.1182/blood-2005-07-2961. [DOI] [PubMed] [Google Scholar]
- Ikonen E. Roles of lipid rafts in membrane transport. Curr Opin Cell Biol. 2001;13:470–477. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Kang WJ, Ko MH, Lee DS, Kim S. Bioimaging of geographically adjacent proteins in a single cell by quantum dot-based fluorescent resonance energy transfer. Proteomics Clin Appl. 2009;3:1383–1388. doi: 10.1002/prca.200900077. [DOI] [PubMed] [Google Scholar]
- Koutsioumpa M, Polytarchou C, Courty J, Zhang Y, Kieffer N, Mikelis C, Skandalis SS, Hellman U, Iliopoulos D, Papadimitriou E. Interplay between αvβ3 integrin and nucleolin regulates human endothelial and glioma cell migration. J Biol Chem. 2013;288:343–354. doi: 10.1074/jbc.M112.387076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate KR, Fässler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. J Cell Sci. 2002;115:963–972. doi: 10.1242/jcs.115.5.963. [DOI] [PubMed] [Google Scholar]
- Li C, Lu N, Qi Q, Li F, Ling Y, Chen Y, Qin Y, Li Z, Zhang H, You Q, et al. Gambogic acid inhibits tumor cell adhesion by suppressing integrin β1 and membrane lipid rafts-associated integrin signaling pathway. Biochem Pharmacol. 2011;82:1873–1883. doi: 10.1016/j.bcp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113:1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- Mañes S, Viola A. Lipid rafts in lymphocyte activation and migration. Mol Membr Biol. 2006;23:59–69. doi: 10.1080/09687860500430069. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang P, Hou S, Li F, Kijlstra A. Label-free proteomics reveals decreased expression of CD18 and AKNA in peripheral CD4+ T cells from patients with Vogt-Koyanagi-Harada syndrome. PLoS One. 2011;6:e14616. doi: 10.1371/journal.pone.0014616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Orsó E, Schmitz G, Landthaler M, Vogt T. Lubrol-RAFTs in melanoma cells: a molecular platform for tumor-promoting ephrin-B2-integrin-beta1 interaction. J Invest Dermatol. 2007;127:1615–1621. doi: 10.1038/sj.jid.5700778. [DOI] [PubMed] [Google Scholar]
- Mostafavi-Pour Z, Askari JA, Parkinson SJ, Parker PJ, Ng TT, Humphries MJ. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J Cell Biol. 2003;161:155–167. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S, Krust B, Hovanessian AG. Anchorage of HIV on permissive cells leads to coaggregation of viral particles with surface nucleolin at membrane raft microdomains. Exp Cell Res. 2002;276:155–173. doi: 10.1006/excr.2002.5522. [DOI] [PubMed] [Google Scholar]
- Ogier C, Bernard A, Chollet AM, LE Diguardher T, Hanessian S, Charton G, Khrestchatisky M, Rivera S. Matrix metalloproteinase-2 (MMP-2) regulates astrocyte motility in connection with the actin cytoskeleton and integrins. Glia. 2006;54:272–284. doi: 10.1002/glia.20349. [DOI] [PubMed] [Google Scholar]
- Porter JC, Hogg N. Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol. 1998;8:390–396. doi: 10.1016/s0962-8924(98)01344-0. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci. 2005;118:1099–1102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- Reyes-Reyes EM, Akiyama SK. Cell-surface nucleolin is a signal transducing P-selectin binding protein for human colon carcinoma cells. Exp Cell Res. 2008;314:2212–2223. doi: 10.1016/j.yexcr.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riopel MM, Li J, Liu S, Leask A, Wang R. β1 integrin-extracellular matrix interactions are essential for maintaining exocrine pancreas architecture and function. Lab Invest. 2013;93:31–40. doi: 10.1038/labinvest.2012.147. [DOI] [PubMed] [Google Scholar]
- Said EA, Krust B, Nisole S, Svab J, Briand JP, Hovanessian AG. The anti-HIV cytokine midkine binds the cell surface-expressed nucleolin as a low affinity receptor. J Biol Chem. 2002;277:37492–37502. doi: 10.1074/jbc.M201194200. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2011;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- Vassilieva EV, Gerner-Smidt K, Ivanov AI, Nusrat A. Lipid rafts mediate internalization of beta1-integrin in migrating intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G965–976. doi: 10.1152/ajpgi.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M, Coudronniere N, Deckert M, Teixeiro E, Mas P, Altman A. A Novel Functional Interaction between Vav and PKCu Is Required for TCR-Induced T Cell Activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mao M, Xu JC. Cell-surface nucleolin is involved in lipopolysaccharide internalization and signalling in alveolar macrophages. Cell Biol Int. 2011;35:677–685. doi: 10.1042/CBI20100625. [DOI] [PubMed] [Google Scholar]
- Wang R, Bi J, Ampah KK, Zhang C, Li Z, Jiao Y, Wang X, Ba X, Zeng X. Lipid raft regulates the initial spreading of melanoma A375 cells by modulating β1 integrin clustering. Int J Biochem Cell Biol. 2013;45:1679–1689. doi: 10.1016/j.biocel.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Nakamura K, Taga T. Roles of lipid rafts in integrin-dependent adhesion and gp130 signalling pathway in mouse embryonic neural precursor cells. Genes Cells. 2004;9:801–809. doi: 10.1111/j.1365-2443.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- You HJ, Seo JM, Moon JY, Han SS, Ko YG, Kim JH. Leukotriene synthesis in response to A23187 is inhibited by methyl-beta-cyclodextrin in RBL-2H3 cells. Mol Cells. 2007;23:57–63. [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]