Abstract
Ziprasidone is a benzisothiazolyl piperazine derivative that was developed from the chemically related antipsychotic drug tiospirone, and it improves neurological functions of the ischemic brain and is effective in treatment of schizophrenia. Mesenchymal stem cells (MSCs) are considered as a leading candidate for neurological regenerative therapy because of their neural differentiation properties in damaged brain. We investigated whether the transplantation of neural progenitor cells (NPCs) derived from adipose mesenchymal stem cells combined with ziprasidone enhances neuroprotective effects in an animal model of focal cerebral ischemia. In combination therapy groups, significant reduction of infarct volume and improvement of neurological functions were observed at 3 days after middle cerebral artery occlusion (MCAO) compared with monotherapy. Co-administration of ziprasidone and NPCs enhanced the anti-apoptotic effect and reduced the number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive apoptotic cells compared with the NPCs alone group at 7 days after MCAO. Ziprasidone or the combination of ziprasidone and NPCs induced the expression of endogenous neurotrophic factor gene brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and glial cell-derived neurotrophic factor (GDNF). The immunohistochemical investigation revealed that the ziprasidone and NPCs attenuated the increased intensity of microglial marker (Iba-1) in the infarcted cortical area. Moreover, the number of transplanted NPCs on day 7 with combination therapy was significantly higher than with NPCs alone. These effects might be responsible for improved functional behavior and increased survival of NPCs. Our finding indicates that combination therapy of ziprasidone and NPCs enhances neuroprotection against ischemic brain injury.
Keywords: combination therapy, ischemic brain, mesenchymal stem cell, ziprasidone
INTRODUCTION
Ischemic stroke is a leading cause of death and long-lasting disability (De Keyser et al., 1999a). In most cases, it results from a transient or permanent reduction in cerebral blood flow caused by the occlusion of a cerebral artery either by an embolus or by local thrombosis (Dirnagl et al., 1999). A target for acute intervention in ischemic brain is the penumbra, a zone of incomplete cerebral ischemia, where neurons are functionally inactive but still viable (De Keyser et al., 1999b).
Ziprasidone is a benzisothiazolyl piperazine derivative that was developed from the chemically related antipsychotic drug tiospirone (Rosa et al., 2008; Seeger et al., 1995). This atypical antipsychotic agent has been approved by the Food and Drug Administration (FDA) for the acute treatment of schizophrenia and schizoaffective disorder, and it has minimal adverse effects on motor, cognitive, prolactin-related, and anticholinergic functions and on weight (Daniel and Copeland, 2000). Ziprasidone has a unique pharmacological profile, as it has antagonist activity at dopamine (DA) D2 and serotonin (5-HT) 5-HT1D, 5-HT2A, and 5-HT2C receptors, and partial agonist activity at 5-HT1A receptors (Nemeroff et al., 2005; Rosa et al., 2008). Ziprasidone has two other interesting pharmacologic characteristics. First, it is an agonist at the 5-HT1A receptor, which is believed to occur pre- and post-synaptically (Caley and Cooper, 2002). Stimulation of 5-HT1A receptors is known to produce neuroprotection in vivo against ischemia and traumatic brain injury. 5-HT1A agonists also protect against NMDA-induced brain lesions. The mechanism underlying 5-HT1A agonist-induced neuroprotection is still not fully understood, but it might involve inhibition of glutamate release (Cosi et al., 2005). Recent studies have reported that atypical antipsychotics have neuroprotective effects against brain injury. Acute treatment with ziprasidone significantly improved neurological functions in ischemic brain injury and this provides a new insight for its clinical applications (Kam et al., 2012; Takahashi et al., 2008). Several mechanisms have been examined to explain the neuroprotective actions of atypical antipsychotics. Chronic administration of clozapine and olanzapine upregulates the levels of brain-derived neurotrophic factor (BDNF) in the rat brain (Bai et al., 2003).
Studies in animal models of ischemic stroke have shown that stem cells transplanted into the brain can lead to functional improvement (Bliss et al., 2007; Chen et al., 2001; Savitz et al., 2002). Mesenchymal stem cells (MSCs) are considered as a leading candidate for neurological regenerative therapy because of their immunological properties (Hoogduijn et al., 2010; Mauri et al., 2012). Recently it was reported that combined treatment with MSCs and neuroprotective agents enhanced amelioration of ischemic brain damage in rats. Combination therapy with antioxidants and MSCs caused a significant reduction of infarct volume, neurological defect, and apoptotic cells, enhanced MSCs migration into the ischemic brain, and increased the number of engrafted MSCs compared with MSCs transplanted alone (Chen et al., 2002; Kaengkan et al., 2013; Suda et al., 2011; Zhao et al., 2012). In the present study, we explored the combined effect of ziprasidone, an antipsychotic agent, and neural progenitor cells (NPCs) derived from mesenchymal stem cells from adipose tissue (AT-MSCs) on infarct volume, apoptotic cell, cell survival, and neurological function recovery by using a rat model of focal cerebral ischemia.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighing 250–300 g at the time of surgery were used for the study. The animals were housed in pairs in cages in a temperature-controlled room (22 ± 3°C) under a 12-h light-dark cycle. They had free access to food and tap water except the day before the operation. All the experimental procedures were approved by the Committee for Animal Experimentation and the Institutional Animal Laboratory Review Board of Inje University.
Isolation of AT-MSCs and induction of NPCs
We previously reported the isolation of AT-MSCs and the induction of NPCs (Hong et al., 2008). Human adipose tissue was collected from healthy donors by liposuction. Adipose tissues were transported to the laboratory in saline solution within 2 h post-surgery. Mesenchymal stem cells were collected using a stem cell collector (Huricell, model no. HRD-1500, Hurim Biocell Co. Korea). AT-MSCs were resuspended in the Dulbecco’s Modified Eagle Medium with low glucose (DMEM-LG, Gibco) supplemented with 100 U/ml penicillin, 100 ug/ml streptomycin, 3.7 mg/ml sodium bicarbonate, and 10% fetal bovine serum (FBS, Hyclone, USA). After cell counting, cell suspension was seeded in non-coated 75 cm2 culture flasks with a density of approximately 2 × 103 cells/cm2. A fresh complete culture medium was added every 3 days. AT-MSCs were induced to differentiate into neural progenitor cells by the modified method of Woodbury et al. (2000). Cells were cultivated in DMEM supplemented with 20% FBS, 0.1% β-mercaptoethanol (BME, Sigma), 1 × nonessential amino acid (Gibco), and 2 mM L-glutamine (Gibco). The cells were cultured for 2-3 days. Twenty four hours prior to neural induction, media were substituted with preinduction medium consisting of 1×N2 supplement (Gibco), 20 ng/ml epithelial growth factor (EGF, Sigma), and 20 ng/ml basic fibroblast growth factor (bFGF, Sigma) in the N2 medium (Gibco). For immunocytochemistry, cells were washed with phosphate buffered saline (PBS) and fixed by treating with 4% paraformaldehyde at room temperature for 30 min, followed by washing three times with PBS and PBS plus 1% Triton X-100. Cells were blocked with 5% BSA for 1 h and then were incubated with primary antibodies for 24 h at 4°C. The antibodies used in this study were β-tubulin III (1:2000, Sigma) and Nestin (1:200, Millipore). The cells were washed three times with PBS and incubated for 30 min at 4°C with the secondary antibodies TRITC (1:400, Sigma) and FITC (1:80, Sigma).
Experimental design
Ziprasidone (Pfizer Pharmaceuticals, USA) was dissolved in dimethyl sulfoxide (DMSO) and injected intraperitoneally. Four groups were studied, namely (1) vehicle group (5% DMSO, 1 ml/kg) (n = 3), (2) ziprasidone group (ziprasidone injection alone 5 mg/kg at 30 min after the onset of MCAO) (n = 3), (3) NPCs group (Neural progenitor cell 1 × 105 cell/rat injection alone, NPCs suspended in PBS) (n = 3), and (4) ziprasidone + NPCs group (combined injection of ziprasidone and neural progenitor cells) (n = 3). For combination therapy, animals were injected with ziprasidone at 30 min after the onset of MCAO, and this was followed by injection of NPCs via the internal carotid artery (ICA) 1 h after reperfusion.
Ischemic surgery and infarct analysis
MCAO was achieved according to the methods described by others, with modifications (Longa et al., 1989). Briefly, the right common carotid artery (CCA) was exposed and carefully dissected free of the vagus nerve. The right external carotid arteries (ECA) and internal carotid arteries (ICA) were also isolated. The external carotid artery was then ligated at the distal end, which was cut off. A 4-0 nylon thread precoated with silicon was aseptically introduced into the right carotid artery in an anterograde fashion toward the carotid bifurcation. It was then directed distally up through the right ICA to a distance of approximately 20 mm from the carotid bifurcation to occlude the origin of the middle cerebral artery. After 1 h, the thread was withdrawn for reperfusion. All the animals experienced ischemia for 1 h followed by reperfusion. The rectal temperature was maintained at 37 ± 0.5°C throughout the surgical procedure by using a thermostatically controlled warming plate and overhead lamp. Each group of animals was decapitated at 3 days after MCAO, and the brains were removed carefully and placed in a metallic brain matrix for tissue slicing immediately after decapitation. The brain was dissected into coronal sections of 2 mm in a metallic brain matrix, then immersed sequentially in a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC) in normal saline at 37°C for 10 min, and then fixed in 10% formalin for 10 s. The infarct area in the brain section was measured by National Institutes of Health (NIH) Image software (Image J). The ischemic lesion volume was calculated as the sum of ischemic lesions from five brain slices from half of the hemisphere.
Neurological deficit scores
The test was conducted by an investigator who was blind to the experimental groups. All the rats were evaluated using a modified neurological severity score (mNSS) (Chen et al., 2001). The mNSS was a composite of motor (muscle status and abnormal movement), sensory (visual, tactile, and proprioceptive), reflex, and balance tests. Neurological function is graded on a scale of 0 to 18 (normal score, 0; maximal deficit score, 18).
NPCs labeling, transplantation, and immunohistochemistry
Transplanted NPCs were infected by a recombinant adenovirus (Invitrogen, USA) containing green fluorescent protein gene (GFP). GFP (8 × 107 pfu/ml) virus was added to the media 3 days before transplantation. Resulting green fluorescence cells were visualized by fluorescence microscopy (Carl Zeiss Axioskop2+, Germany). Animals were injected with ziprasidone at 30 min after the onset of MCAO, and this was followed by injection of NPCs via the internal carotid artery 1 h after reperfusion. For intracarotid injections, the ipsilateral common carotid artery was exposed; NPCs (1 × 105 cell/5 μl) in PBS were injected into the ICA through a catheter located in the ECA. Rats were killed at 3 days and 7 days after MCAO for TTC staining and immunohistochemistry respectively. The brain was subjected to immunohistochemistry. The rats were sacrificed at 7 days after MCAO and then fixed by cardiac perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer. Samples were embedded in embedding medium for frozen tissue specimens (O.C.T. compound, Sakura Finetek USA, Inc.) and frozen. Then the brains were sliced into coronal sections (10 μm) by using a cryostat (HM 525, Thermo Scientific, USA). After blocking with the normal serum for 2 h at room temperature, the sections were incubated with primary antibodies for 24 h at 4°C. The antibodies used for immunohistochemistry in this study were against NeuN (1:700, Chemicon, USA) and Iba-1 (1:500, Wako, Japan), diluted in Tris-buffered saline (TBS) containing 1% bovine serum albumin (w/v) and 0.3% Triton-X 100. After rinsing in TBS, the sections were incubated with secondary antibody-conjugated FITC (1:300, Sigma, USA) and CY3 (1:500, Amersham) for 2 h at room temperature. The numbers of GFP-positive cells in the ischemic area were counted in three randomly selected microscopic fields under 200X magnification in a blind fashion from the bregma 0.00 mm to −2.00 mm (encompassing the majority of the lesion).
RT-PCR analysis
The lesioned side from each group was harvested for reverse transcription polymerase chain reaction (RT-PCR). Total RNA was extracted using the acid guanidinium isothiocyanate-phenol-chloroform method with TRI Reagent, followed by extraction and precipitation with isopropyl alcohol. The cDNA was synthesized from equal amounts of total RNA with superscript III reverse transcriptase, and polymerase chain reaction (PCR) was performed with high fidelity Taq DNA polymerase. The expression level of the gene of interest was corrected for that of the housekeeping gene, β-actin. The sequences of the primer pairs used were BDNF: 5′- GGT CAC AGC GGC AGA TAA AAA-3′, 5′- TTG GGT AGT TCG GCA TTG CGA-3′; NGF: 5′- ACA TCA AGG GCA AGG AGG TGA-3′, 5′-TGA CAA AGG TGT GAG TCG TGG-3′; GDNF: 5′- GAC TTG GGT TTG GGC TAC GA-3′, 5′-TGG TAA ACC AGG CTG TCG TC-3′; and β-actin: 5′-CCA TCA TGA AGT GTG ACG TT-3′, 5′-CCA CCA ATC CAC ACA GAG TA-3′. PCR products of different genes were detected by electrophoresis on a 1.5% agarose gel containing ethidium bromide. Band intensities were quantified by National Institutes of Health Image software (Image J) and normalized with β-actin.
TUNEL assay
To observe DNA strand breaks in nuclei, the apoptotic DNA fragmentation was analyzed with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using the In Situ Cell Death Detection Kit, POD (Roche Molecular Biochemicals, Inc., Germany) and was performed according to the manufacturer’s instructions. Briefly, the sections were fixed with 4% paraformaldehyde in phosphate buffer (pH 7.4) and treated with 0.3% H2O2 in methanol for 10 min, washed in PBS, and permeabilized in 0.1% Triton X-100 in 0.1% sodium citrate. Then the sections were incubated with terminal deoxynucleotidyl transferase (TdT) enzyme in a humidified chamber for 60 min. Further incubation with a peroxidase-conjugated antibody was performed for 30 min at 37°C. Finally, 3, 3′-Diaminobenzidine (DAB) was added, and the sections were incubated for 10 min followed by washing with PBS for the coloration of apoptotic cells. The numbers of TUNEL-positive cells in the ischemic area were counted in three randomly selected microscopic fields under 200 × magnification in a blind fashion.
Statistical analyses
Data are presented as the mean ± SE. Student t-tests were used to compare the difference between the treatments and the vehicle group. The difference was considered significant when p < 0.05.
RESULTS
Infarct volume
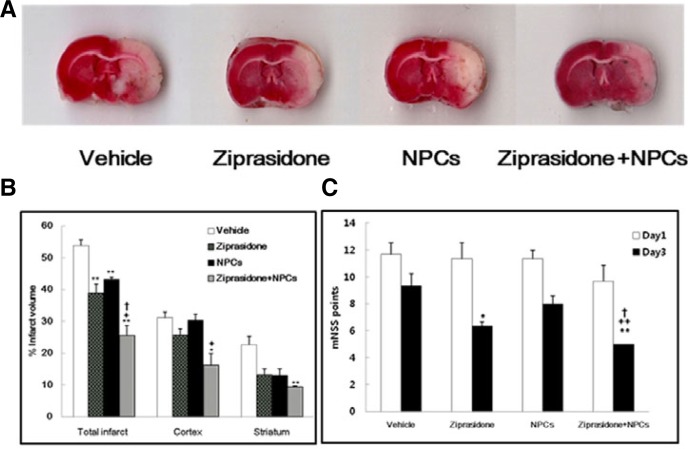
The borders of the TTC stain enclosing the white infarct area were readily distinguishable in contrast to the red color of the normal area. Quantitative measurement of the infarct volume was performed in the section at 4.00 mm to −6.00 mm according to the rat brain atlas (Paxinos and Watson, 2007). At 3 days after treatment, in the ziprasidone alone group, NPCs alone group, and ziprasidone + NPCs group, the total infarct volumes were significantly decreased compared with the vehicle group. The infarct volumes in the vehicle-treated group and the groups treated with ziprasidone, NPCs, and a combination of ziprasidone and NPCs were 53.87 ± 1.80, 38.82 ± 2.93, 43.27 ± 0.58, and 25.61 ± 3.09% respectively of the total area of the brain section. A significant decrease of the total infarct volume was also observed in the combination group of ziprasidone and NPCs compared with the vehicle, ziprasidone alone, and NPCs alone groups (p < 0.05 and p < 0.01; Figs. 1A and 1B).
Fig. 1.
Combination effects of ziprasidone and NPCs on infarct volume and functional behavioral recovery at 3 days after MCAO. (A) TTC-stained coronal section from vehicle, ziprasidone (5 mg/kg), NPCs (1 × 105), and combination of ziprasidone and NPCs from the bregma 0.00 mm to −2.00 mm (encompassing the majority of the lesion). (B) The infarct volume was calculated as the infarct areas × thickness (2 mm) and expressed as a percentage of the lesion half of the brain. The combination of ziprasidone and NPCs group shows a significantly lower infarct volume than the ziprasidone and NPCs alone groups 3 days after MCAO. (C) Neurological functional tests were performed at 1 and 3 days after MCAO. The combination of ziprasidone and NPCs group shows significantly lower mNSS score than the ziprasidone, NPCs, and vehicle groups 3 days after MCAO. Data are shown as mean S.E.M. (n = 3) * p < 0.05, **p < 0.01 vs. individual vehicle group, +p < 0.05, ++p < 0.01 vs. individual ziprasidone group, †p < 0.05 vs. individual NPCs group.
Functional recovery 3 days after MCAO
The mNSS test showed that motor and sensory functions were impaired by ischemic insult. All treated groups showed no significant improvement at 1 day after ischemia; however, at 3 days after treatment, there was significant functional recovery in the ziprasidone alone group and combination group of ziprasidone and NPCs compared with the vehicle group. Also, the combination group of ziprasidone and NPCs showed significant improvement compared with the ziprasidone and NPCs alone groups (p < 0.05; Fig. 1C).
Anti-apoptotic effects
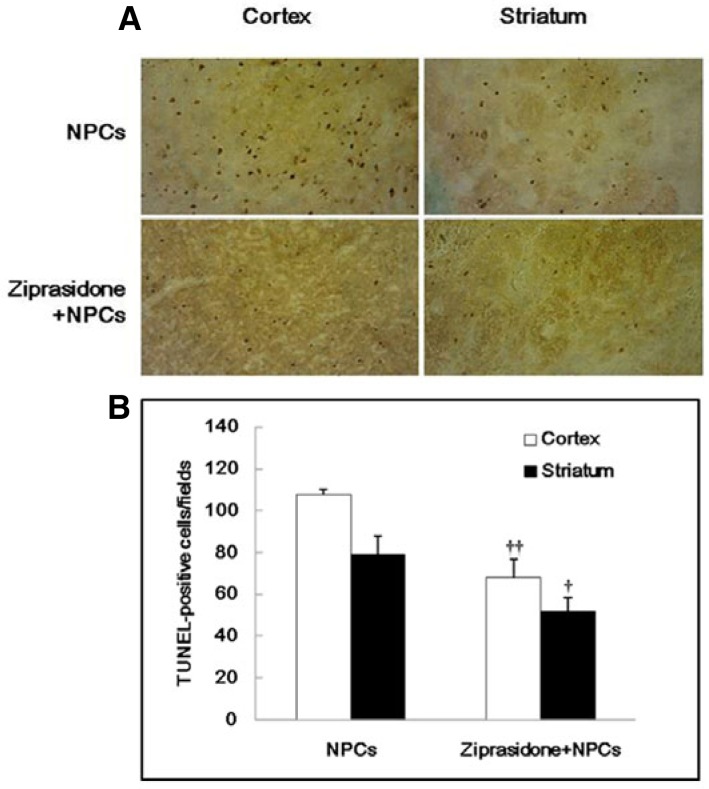
TUNEL-positive cells were frequently observed in the ischemic boundary area. Quantitative analysis of TUNEL-positive cells showed that the combination of ziprasidone + NPCs (68.00 ± 8.72) group displayed a significant decrease in TUNEL-positive cells in the cortical area compared with the NPCs group (107.67 ± 2.40) at 7 days after focal cerebral ischemia. Also quantitative analysis of striatal area TUNEL-positive cells showed that the combination group of ziprasidone and NPCs (51.67 ± 6.89) displayed a significant decrease in TUNEL-positive cells in that area compared with the NPCs group (79.33 ± 8.41) (p < 0.05 and p < 0.01; Figs. 2A and 2B).
Fig. 2.
Combination effects of ziprasidone and NPCs on TUNEL staining. (A) TUNEL staining of NPCs in the cortex and striatum area of the ischemic lesioned side of the brain with the combination of ziprasidone and NPCs treatment (200×). (B) Quantitative analysis of the number of TUNEL-positive cells. Data are the mean ± S.E.M. (n = 3). †p < 0.05 vs. individual NPCs group, ††p < 0.01 vs. individual NPCs group.
Survival rates of NPCs
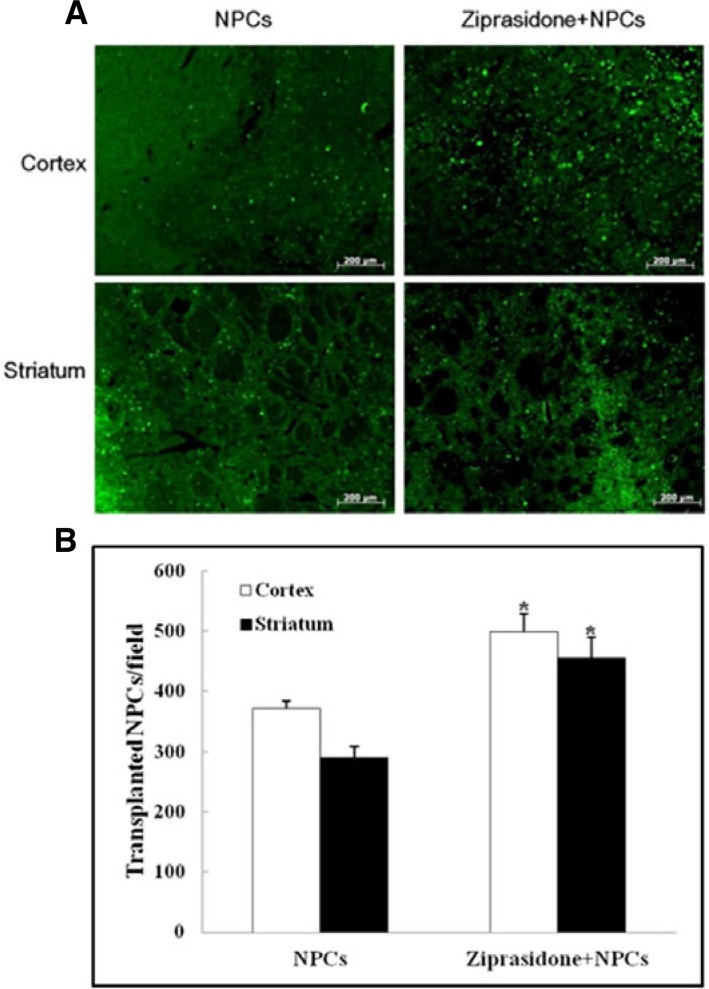
Immunohistochemistry was performed at 7 days after the MCAO. The GFP positive cells were evaluated in the ischemic zone of treated animals. Immunohistochemistry showed that GFP positive expression in the cortical area of the combination group of ziprasidone and NPCs (499.00 ± 29.28) was higher than in the NPCs (371.33 ± 13.17) alone group. Striatal area GFP positive cells showed that the combination group of ziprasidone and NPCs (456.00 ± 32.54) displayed a significant increase in GFP positive cells compared with the NPCs group (291.00 ± 16.86) (p < 0.05; Figs. 3A and 3B).
Fig. 3.
Survival rate of transplanted NPCs. (A) Immunohistochemistry showed GFP positive cells (green) in the cortical and striatal ischemic boundary zone at 7 days after MCAO (50×). (B) Quantitative analysis of the number of transplanted NPCs. GFP positive expression was significantly increased in the combination of ziprasidone and NPCs treated group compared with the individual NPCs group. Data are the mean ± S.E.M. (n = 3) *p < 0.05 vs. NPCs group.
Effect of ziprasidone and NPCs on gene expressions
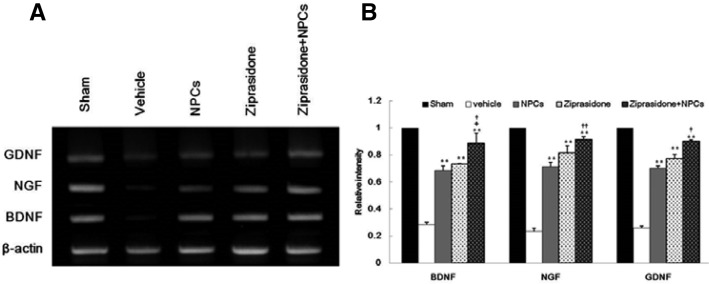
RT-PCR was performed to analyze the effects of ziprasidone and NPCs on the expression of endogenous neurotrophic factor BDNF, NGF, and GDNF genes. After a 1 h ischemic insult followed by 24 h of reperfusion, the ipsilateral whole brain of the lesioned area was subjected to RT-PCR analysis. The expression levels of BDNF, NGF, and GDNF genes were significantly elevated in the combination group of ziprasidone and NPCs compared with the vehicle, ziprasidone, and NPCs groups (p < 0.05 and p < 0.01; Figs. 4A and 4B).
Fig. 4.
Combination effects of ziprasidone and NPCs on the gene expressions of neurotrophic factors in the cortex and striatum. (A) Specific gene expressions were determined in relation to the expression of β-actin using RT-PCR. (B) The levels of BDNF, NGF, and GDNF gene expression were significantly increased in the combination of ziprasidone and NPCs treated group compared with the individual vehicle group. Experiments were repeated three times and individual values are expressed as the mean ± S.E.M. *p < 0.05, **p < 0.01 vs. individual vehicle group, +p < 0.05 vs. individual ziprasidone group, ††p < 0.01 vs. individual NPCs group.
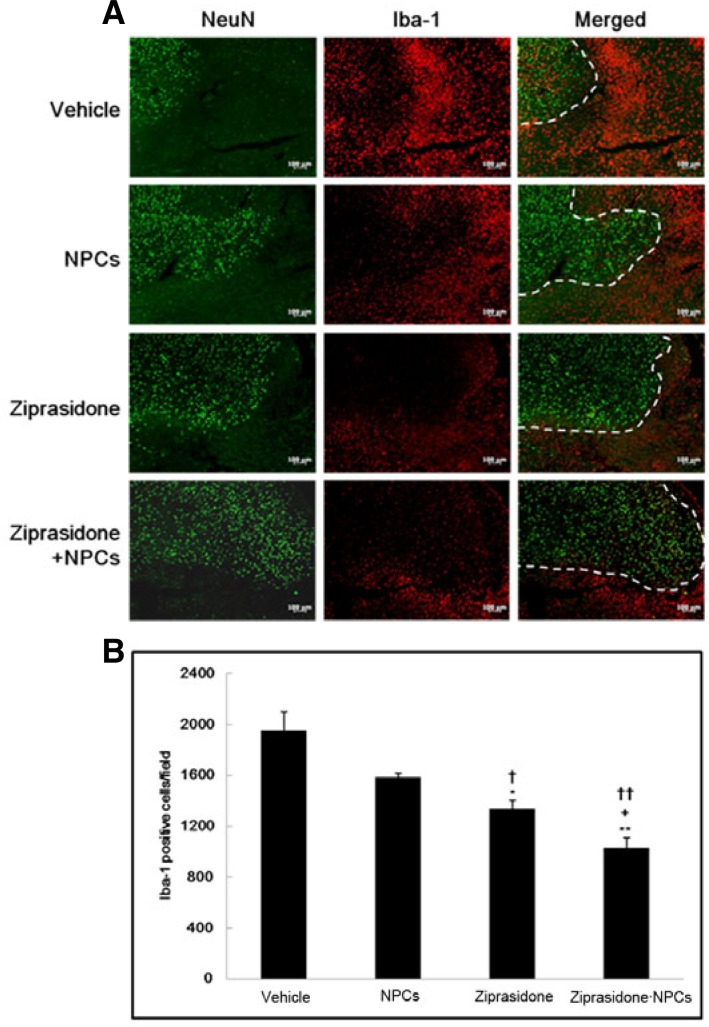
Immunohistochemistry of Iba-1 after NPCs transplantation
The positive neuronal marker NeuN and a microglial marker Iba-1 were evaluated in the cortical ischemic boundary zone of vehicle and treated animals. Immunohistochemistry showed that Iba-1 expression in the combination of ziprasidone and NPCs was lower than in the vehicle, NPCs alone, and ziprasidone alone groups (p < 0.05 and p < 0.01; Figs. 5A and 5B). However, the ziprasidone and combination of ziprasidone and NPCs treatment prevented the severe loss of NeuN signals and attenuated the increase in the Iba-1 signals intensity. Immunohistochemistry showed that Iba-1 expression in the combination of ziprasidone and NPCs (1030.00 ± 141.57) was lower than in the vehicle (1954.00 ± 256.84), NPCs alone (1584.00 ± 58.41), and ziprasidone (1337.00 ± 115.27) alone groups (p < 0.05 and p < 0.01; Figs. 5A and 5B).
Fig. 5.
Combination effects of ziprasidone and NPCs on the expression of Iba-1. (A) Immunohistochemistry showed NeuN positive neurons (green); a neuronal marker, Iba-1 positive (red); a microglial marker and the double-labeling of NeuN (green), Iba-1 (red) in the cortical ischemic boundary zone at 7 days after MCAO (50×). (B) Iba-1 expression was decreased in the combination of ziprasidone and NPCs treated group compared with the individual vehicle, NPCs, and ziprasidone groups. Data are the mean ± S.E.M. (n = 3) *p < 0.05, **p < 0.01 vs. individual vehicle group, +p < 0.05 vs. individual ziprasidone group, ††p < 0.01 vs. individual NPCs group.
DISCUSSION
Stroke occurs because of reduced perfusion to a brain region, resulting in death or permanent neurological deficits. Reduced perfusion of the brain initiates the ischemic cascade, leading to development of a reversible ischemic penumbra surrounding an irreversible area of infarction (Pandya et al., 2011). The newer pharmacological treatments for schizophrenia, now commonly referred to as second generation antipsychotics, offer several advantages over first generation antipsychotics such as greater improvements in negative symptoms, prevention of relapse, increased functional capacity and quality of life, and fewer movement-related side effects. Ziprasidone has a unique pharmacological profile with high affinity at a number of neurotransmitter receptors, including D2, 5HT1A, 5HT2A, and 5HT2C, as well as at 5HT1B/1D receptors. It has proven efficacy in schizophrenia and related disorders (Nemeroff et al., 2005; Rosa et al., 2008; Terry et al., 2006).
In the previous and present study, treatment with ziprasidone facilitated neurological recovery after MCAO as measured by mNSS, which scores severity in motor, sensory, reflex, and balance functions (Kam et al., 2012). The recovery may be attributed to reduction by ziprasidone treatment in the size of the infarct area induced by MCAO, which includes the cortex and the striatum that are mainly related to sensory perception and to motor control. Co-administration of ziprasidone and NPCs enhanced the anti-apoptotic effect and reduced the number of TUNEL-positive cells compared with the NPCs alone group at day 7 after MCAO. Apoptosis and necrosis in infarct areas of the brain occur in the early stage of cerebral ischemia. Recent research has revealed that many neurons in the ischemic penumbra or peri-infarct zone may undergo apoptosis after several hours or days, and thus they are potentially recoverable for some time after the onset of stroke (Broughton et al., 2009). Ziprasidone attenuated neuronal apoptosis in the cortex and striatum in the ischemic hemisphere as shown by a decrease in TUNEL -positive cells. Ziprasidone has reduced free radical production and lipid peroxidation and inhibited apoptosis, especially that induced by glutamate, Aβ, and MPP+. In light of its serotonin and norepinephrine reuptake inhibitor (SNRI) properties, ziprasidone may have the additional antiapoptotic property of monoamine oxidase B inhibition. 5-HT1A agonists have also demonstrated anti apoptotic effects (Lauterbach et al., 2010). In our study, ziprasidone injection increased the expression of neurotrophic factor genes BDNF, GDNF, and NGF after ischemia, and this might lead to a larger decrease in infarct volume and good functional recovery. Ziprasidone has been associated with marked increases in NGF and ChAT immunoreactivity in the DG, CA1, and CA3 regions of the hippocampus (Terry et al., 2006). SNRI properties of ziprasidone further suggest the capacity to reduce GSK-3β, pathogenic protein concentrations, nitric oxide synthesis, other free radicals, mitochondrial depolarization, apoptosis, microglial activation, and neuroinflammation while upregulating BDNF and GDNF (Lauterbach et al., 2010).
In our study, intracarotid transplanted NPCs can survive in the brain environment. Injected NPCs were localized to the ischemic boundary zone, and a combination of ziprasidone and NPCs produced an increase in cell survival of GFP-positive cells in comparison with the NPCs alone group. Injection of NPCs also evoked the expression of growth and trophic factors in the lesioned side and in turn, it might enhance plasticity of the remaining tissue within the boundary zone of an ischemic lesion. Meanwhile, ziprasidone treatment prevented the severe loss of NeuN signals and attenuated the increase in the Iba-1 signal intensity in the infarcted cortex. Ziprasidone inhibits the accumulation and activation of microglia. Microglial activation and the release of neurotoxic factors underlie inflammation-mediated neurodegeneration, and the inhibition of excessive microglial activation has been proposed as a neuroprotective mechanism (Choi et al., 2011; Liu and Hong, 2003). The present study showed that after stroke the combination of ziprasidone and NPCs resulted in a significant decrease in microglia activation compared with the NPCs alone group. This treatment might be exerting this effect through an anti-inflammatory action that leads to decreased microglia activation and protects the brain from delayed injury. We also speculate that treatment of ziprasidone injection may improve the viability of transplanted NPCs and thereby enhance neuroprotection against ischemic injury. Thus, these results suggest that combining ziprasidone and NPCs is a valuable strategy to enhance NPCs survival and to promote functional recovery.
Acknowledgments
This work was supported by the 2013 Inje University research grant.
REFERENCES
- Bai O, Chlan-Fourney J, Bowen R, Keegan D, Li X-M. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res. 2003;71:127–131. doi: 10.1002/jnr.10440. [DOI] [PubMed] [Google Scholar]
- Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38:817–826. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- Broughton BRS, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral Ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Caley CF, Cooper CK. Ziprasidone: the fifth atypical antipsychotic. Ann Pharmacother. 2002;36:839–851. doi: 10.1345/aph.1A053. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Chopp M. Caspase inhibition by Z-VAD increases the survival of grafted bone marrow cells and improves functional outcome after MCAO in rats. J Neurol Sci. 2002;199:17–24. doi: 10.1016/s0022-510x(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Choi DK, Koppula S, Suk K. Inhibitors of microglial neurotoxicity: focus on natural products. Molecules. 2011;16:1021–1043. doi: 10.3390/molecules16021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosi C, Waget A, Rollet K, Tesori V, Newman-Tancredi A. Clozapine, ziprasidone and aripiprazole but not haloperidol protect against kainic acid-induced lesion of the striatum in mice, in vivo: Role of 5-HT1A receptor activation. Brain Res. 2005;1043:32–41. doi: 10.1016/j.brainres.2005.02.072. [DOI] [PubMed] [Google Scholar]
- Daniel DG, Copeland LF. Ziprasidone: comprehensive overview and clinical use of a novel antipsychotic. Expert Opin. Invest. Drugs. 2000;9:819–828. doi: 10.1517/13543784.9.4.819. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing? Trends Neurosci. 1999a;22:535–540. doi: 10.1016/s0166-2236(99)01463-0. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing? Trends Neurosci. 1999b;22:535–540. doi: 10.1016/s0166-2236(99)01463-0. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Hong IK, Jeong NH, Kim JR, Do B-R, Kim HK, Kang SG. Differentiation of dopaminergic and cholinergic neurons from mesenchymal-like stem cells derived from the adipose tissue. Dev Reprod. 2008;12:31–39. [Google Scholar]
- Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C, Dahlke M-H. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 2010;10:1496–1500. doi: 10.1016/j.intimp.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Kaengkan P, Baek SE, Choi YW, Kam K-Y, Kim JY, Wu YR, Do B-R, Kang SG. Combination effect of p-hydroxybenzyl alcohol and mesenchymal stem cells on the recovery of brain damage in a rat model of brain ischemia. Animal Cell Syst. 2013;17:160–169. [Google Scholar]
- Kam K-Y, Jalin AMA, Choi YW, Kaengkan P, Park SW, Kim YH, Kang SG. Ziprasidone attenuates brain injury after focal cerebral ischemia induced by middle cerebral artery occlusion in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39:69–74. doi: 10.1016/j.pnpbp.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC, Shillcutt SD, Victoroff J, Coburn KL, Mendez MF. Psychopharmacological neuroprotection in neurodegenerative disease heuristic clinical applications. J Neuropsychiatry Clin Neurosci. 2010;22:130–154. doi: 10.1176/jnp.2010.22.2.130. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Mauri M, Lentini D, Gravati M, Foudah D, Biella G, Costa B, Toselli M, Parenti M, Coco S. Mesenchymal stem cells enhance GABAergic transmission in co-cultured hippocampal neurons. Mol Cell Neurosci. 2012;49:395–405. doi: 10.1016/j.mcn.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Lieberman JA, Weiden PJ, Harvey PD, Newcomer JW, Schatzberg AF, Kilts CD, Daniel DG. From clinical research to clinical practice: a 4-year review of ziprasidone. CNS Spectr. 2005;10:1–20. doi: 10.1017/s1092852900019842. [DOI] [PubMed] [Google Scholar]
- Pandya RS, Mao L, Zhou H, Zhou S, Zeng J, Popp AJ, Wang X. Central nervous system agents for ischemic stroke: neuroprotection mechanisms. Cent Nerv Syst Agents Med Chem. 2011;11:81–97. doi: 10.2174/187152411796011321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Rosa AR, Franco C, Torrent C, Comes M, Cruz N, Horga G, Benabarre A, Vieta E. Ziprasidone in the treatment of affective disorders: a review. CNS Neurosci Therapeutics. 2008;14:278–286. doi: 10.1111/j.1755-5949.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Rosenbaum DM, Dinsmore JH, Wechsler LR, Caplan LR. Cell transplantation for stroke. Ann Neurol. 2002;52:266–275. doi: 10.1002/ana.60000. [DOI] [PubMed] [Google Scholar]
- Seeger TF, Seymour PA, Schmidt AW, Zorn SH, Schulz DW, Lebel LA, McLean S, Guanowsky V, Howard HR, Lowe JA. Ziprasidone (CP-88,059): a new anti-psychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther. 1995;275:101–113. [PubMed] [Google Scholar]
- Suda S, Shimazaki K, Ueda M, Inaba T, Kamiya N, Katsura K-i, Katayama Y. Combination therapy with bone marrow stromal cells and FK506 enhanced amelioration of ischemic brain damage in rats. Life Sci. 2011;89:50–56. doi: 10.1016/j.lfs.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yasuhara T, Shingo T, Muraoka K, Kameda M, Takeuchi A, Yano A, Kurozumi K, Agari T, Miyoshi Y, et al. Embryonic neural stem cells transplanted in middle cerebral artery occlusion model of rats demonstrated potent therapeutic effects compared to adult neural stem cells. Brain Res. 2008;1234:172–182. doi: 10.1016/j.brainres.2008.07.086. [DOI] [PubMed] [Google Scholar]
- Terry AV, Parikh V, Gearhart DA, Pillai A, Hohnadel E, Warner S, Nasrallah HA, Mahadik SP. Time-dependent effects of haloperidol and ziprasidone on nerve growth factor, cholinergic neurons and spatial learning in rats. J Pharmacol Exp Ther. 2006;318:709–724. doi: 10.1124/jpet.105.099218. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Guan Y, Xu Y, Li Y, Wu W. Sodium Ferulate combined with bone marrow stromal cell treatment ameliorating rat brain ischemic injury after stroke. Brain Res. 2012;1450:157–165. doi: 10.1016/j.brainres.2012.02.053. [DOI] [PubMed] [Google Scholar]