Abstract
Adenosine signaling has been implicated in the pathophysiology of alcohol use disorders and other psychiatric disorders such as anxiety and depression. Numerous studies have indicated a role for A1 receptors (A1R) in acute ethanol-induced motor incoordination, while A2A receptors (A2AR) mainly regulate the rewarding effect of ethanol in mice. Recent findings have demonstrated that dampened A2AR-mediated signaling in the dorsomedial striatum (DMS) promotes ethanol-seeking behaviors. Moreover, decreased A2AR function is associated with decreased CREB activity in the DMS, which enhances goal-oriented behaviors and contributes to excessive ethanol drinking in mice. Interestingly, caffeine, the most commonly used psychoactive substance, is known to inhibit both the A1R and A2AR. This dampened adenosine receptor function may mask some of the acute intoxicating effects of ethanol. Furthermore, based on the fact that A2AR activity plays a role in goal-directed behavior, caffeine may also promote ethanol-seeking behavior. The A2AR is enriched in the striatum and exclusively expressed in striatopallidal neurons, which may be responsible for the regulation of inhibitory behavioral control over drug rewarding processes through the indirect pathway of the basal ganglia circuit. Furthermore, the antagonistic interactions between adenosine and dopamine receptors in the striatum also play an integral role in alcoholism and addiction-related disorders. This review focuses on regulation of adenosine signaling in striatal circuits and the possible implication of caffeine in goal-directed behaviors and addiction.
Keywords: A2AR, adenosine, alcohol use disorders, caffeine, ENT1, goal-directed behavior
INTRODUCTION
Adenosine, an inhibitory neuromodulator, has been implicated in the pathophysiology of alcohol use disorders (Asatryan et al., 2011; Dunwiddie and Masino, 2001; Ruby et al., 2010). Acute ethanol exposure in cultured neurons is known to increase extracellular adenosine. This spike in adenosine contributes to the intoxicating and/or rewarding effect of ethanol. In contrast, chronic ethanol exposure to cultured cells no longer increases extracellular adenosine levels. Furthermore, chronic exposure decreases type 1 equilibrative nucleoside transporter (ENT1) expression (Diamond et al., 1991). Specifically, mice lacking ENT1 present with reduced adenosine levels in the nucleus accumbens (NAc). The reduction in adenosine levels caused by the deletion of ENT1 may be one of the possible molecular mechanisms underlying functional ethanol tolerance and dependence (Nam et al., 2012). In addition, ethanol drinking impairs several striatal functions including reward evaluation, motor function and habit formation. Our recent studies have indicated that reduced adenosine A2A receptor (A2AR) signaling in the dorsomedial striatum (DMS) promote goal-directed behaviors in mice lacking the ethanol sensitive adenosine transporter, ENT1 (Nam et al., 2013). Furthermore, decreased DMS A2AR signaling contributes to excessive ethanol drinking (Nam et al., 2013). Therefore, a mechanistic understanding of adenosine-mediated signaling in the DMS will contribute to our understanding of the neurobiology of alcohol use disorders and will be helpful in the development of new therapeutic methods.
The complexity and heterogeneity of striatal neurons make it challenging to understand ethanol-induced neuroadaptation in reward circuitry. Despite these difficulties, recently developed bacterial artificial chromosome (BAC) transgenic mice expressing enhanced green fluorescent protein (EGFP) enable the examination of gene expression in these two distinct striatal neurons (Durieux et al., 2011; Ena et al., 2011; Gong et al., 2003; Surmeier et al., 2007). Morphologically indistinguishable striatopallidal and striatonigral neuronal subtypes comprise approximately 90–95% of striatal neurons (Graybiel, 2000; Humphries and Prescott, 2010). Striatopallidal neurons, which project to the ventral pallidum or globus pallidus, can be identified by the dopamine D2 receptor (D2R). In contrast, striatonigral neurons, which project axons to the substantia nigra pars reticulata, can be recognized by the cholinergic muscarinic M4 receptor (M4R) or dopamine D1 receptor (D1R) (Gong et al., 2003; Le Moine and Bloch, 1995; Lobo et al., 2006; Surmeier et al., 1996). This is especially important as striatal-specific neuronal circuits are implicated in several aspects of addictive behaviors (Koob and Volkow, 2010). Anatomically, the striatum can be divided into two distinct sub-regions, the dorsal striatum and the ventral striatum. While the ventral striatum (also known as the nucleus accumbens; NAc) mediates the rewarding and motivational effects of a substance or stimulus, the dorsal striatum (also known as the caudate-putamen; CPu) regulates voluntary movement and habitual behaviors (Everitt and Robbins, 2013; Voorn et al., 2004). Recent studies demonstrate that two different dorsal striatum regions, the dorsomedial striatum (DMS) and dorsolateral striatum (DLS), play distinctive roles in behavior development (Yin and Knowlton, 2006; Yin et al., 2007; 2009). Notably, the DMS primarily regulates goal directed (action-outcome) behavior, which is sensitive to outcome devaluation and instrumental learning, whereas the DLS is involved in habit formation (stimulus-response) (Balleine et al., 2007; Graybiel, 2008; Yin et al., 2004). The DMS (analogous to the caudate) is a part of the associative network, which is involved in ‘working memory’ and the anticipation of reward. By contrast, the DLS (similar to the putamen) receives input from the sensorimotor cortex and is involved in locomotor behavior and habit formation (Lovinger, 2010; Yin and Knowlton, 2006). Surprisingly, direct evidence for a role of striatal signaling in the behavioral shift from excessive ethanol drinking to habit development has not yet been established.
How alcohol-seeking behaviors are regulated by brain region or cell type specific adenosine signaling remains a key question in addiction research. This review will highlight the recent findings that have contributed to our understanding of habit formation development from excessive ethanol drinking and a potential role of caffeine consumption in the process.
ADENOSINE REGULATION IN THE BRAIN
Adenosine metabolism
Adenosine is produced both intracellularly and extracellularly in the central nervous system (CNS) from the breakdown of adenosine triphosphate (ATP). Dephosphorylation of adenine nucleotides ATP, adenosine diphosphate (ADP), and adenosine monophosphate (AMP) outside of cells account for the majority of extracellular adenosine. Extracellular concentrations can range from 25 to 250 nM, depending on the brain region of interest (Dunwiddie and Masino, 2001). In addition, the signal transduction molecule, cyclic AMP (cAMP) can also be degraded by phosphodiesterase to form adenosine (Brundege et al., 1997) (Fig. 1). Termination of adenosine action in the synapse is facilitated by the enzymatic conversion for adenosine to inosine, hypoxanthine, or xanthine by adenosine deaminase (Lloyd and Schrader, 1993; Pedata et al., 1990). This pathway becomes extremely important under conditions of high extracellular adenosine levels, such as hypoxia and ischemia, and can aid in clearing excess adenosine.
Fig. 1.
Purinergic signaling involved in alcohol use disorders. Adenosine is synthesized from AMP by nucleotidase activity in the cytosol and is then transported to extracellular region via a nucleoside transporter. Among several nucleoside transporters, ENT1 is known to regulate adenosine levels in response to ethanol. Adenosine can also be converted from ATP extracellularly by ecto-nucleotidase activity. Extracellular adenosine is able to bind to two main adenosine receptors, A1R and A2AR, which are expressed in two distinct neurons in the striatum. cAMP, cyclic adenosine monophosphate; ENT, equilibrative nucleoside transporter.
The location of adenosine metabolism appears to be mainly dependent upon CNS cell type, with astrocytes and neurons showing different expressions of nucleotide metabolizing enzymes and transporters (Parkinson et al., 2006); however, both neurons and astrocytes (Kulik et al., 2010) can ultimately function as a major source of adenosine in extracellular space. Specifically, the production of adenosine in astrocytes (Zamzow et al., 2008) implies that astrocytes play a diverse role in the regulation of neuronal adenosine, which appear to be dependent on pathophysiological conditions.
Adenosine transport
In addition to adenosine metabolism, the transport of adenosine into cells represents a crucial factor in determining the fate of the purinergic signaling system. Therefore, nucleoside transportation across the plasma membrane is a possible molecular pathway for adenosine recycling and redistribution (Dunwiddie and Masino, 2001). The identification of two adenosine transporter families encoding equilibrative and concentrative based transport in mammals provides a mechanism for the essential concept of homeostasis in adenosine regulation. For example, sodium-independent equilibrative nucleoside transporters (ENTs) mediate bi-directional nucleoside transport determined by the concentration gradient between membranes, and sodium-dependent concentrative nucleoside transporters (CNTs) mediate an inwardly directed transport that is driven by the sodium electrochemical gradient. Both ENTs and CNTs are expressed in highly metabolically active tissues, including the brain. However, they differ in selectivity, kinetics, regulation and subcellular distribution (King et al., 2006).
Four ENT and three CNT subtypes have been cloned (Baldwin et al., 2004). These types include ENT1, ENT2, ENT3, ENT4, and CNT1, CNT2, CNT3. ENT1 is sensitive to nanomolar concentrations of nitrobenzylthioinosine (nitrobenzylmercaptopurine riboside; NBMPR), whereas ENT2 is resistant to NBMPR up to 1 mM (Baldwin et al., 1999; 2004; Yao et al., 1997). Furthermore, ENT1 and ENT2 are widely expressed throughout the central nervous system (Jennings et al., 1998; 2001), while ENT3 appears to be expressed outside the CNS (Baldwin et al., 2005). The expression and pharmacological properties of ENT4 have yet to be fully characterized. The CNT family demonstrates a more distinct selectivity in nucleoside transporting than the ENT family. Most CNTs show a pyrimidine preference with similar affinity to adenosine molecules, yet the rate of adenosine transport by CNT1 is much lower than the rate of pyrimidine transport and CNT1 displays a much lower turnover number of molecules per second than ENT1 (King et al., 2006). Interestingly, acute ethanol treatment in neuronal cells is known to increase extracellular adenosine, whereas chronic exposure no longer increases adenosine. The normalized adenosine levels caused by chronic ethanol exposure could be attributed to the downregulation of ENT1 expression. Mice lacking ENT1 exhibit reduced adenosine levels in the NAc. This reduction in adenosine could be associated with the observed reduction in the acute intoxicating effects. of ethanol and the excessive alcohol consumption phenotype in these mice compared to wild-type littermates (Chen et al., 2010; Choi et al., 2004; Kim et al., 2011; Nam et al., 2011).
Adenosine receptor signaling
Adenosine is responsible for balancing neurotransmitter release, reducing neuronal excitability and regulating ion channel function through activation of four classes of G protein-coupled receptors: A1, A2A, A2B and A3, which all display a distinct affinity for adenosine (Fredholm et al., 2005a; 2005b; Huang et al., 2005). A1R and A2AR have 10 to 100 nM binding affinities, whereas A2BR and A3R have 1 to 5 μM binding affinities. As normal CNS adenosine levels are 25 to 250 nM, A1R and A2AR are the main subtypes thought to be involved in the regulation of psychiatric disorders. A1Rs are Gi coupled receptors, expressed ubiquitously in the CNS, and mediate the tonic inhibition of neuronal activity. In contrast, A2ARs are Gs coupled receptors and are primarily expressed in the striatum. A2ARs are known to activate adenylate cyclase, increase the levels of cAMP, and exert excitatory influences on striatal neurons. Despite their widespread distribution in the brain, little is known about A2BR and A3R because their binding affinity is too high to induce physiological responses. Inhibition of either A1R or A2AR appears to be responsible for the stimulating effects of adenosine receptor antagonists such as caffeine (Svenningsson et al., 1999).
ADENOSINE RECEPTOR SIGNALING IN ETHANOL DRINKING
Striatal A2AR signaling
Striatal adenosine levels play an important role in ethanol sensitivity, withdrawal, and drinking (Arolfo et al., 2004; Gordon and Diamond, 1993; Meng and Dar, 1995; Nagy and DeSilva, 1994). The A2AR is enriched in the striatum and exclusively expressed in striatopallidal neurons, while A1Rs are widely distributed throughout the CNS. In addition, A2AR in striatopallidal neurons are known to regulate inhibitory behavioral control over drug rewarding processes through the indirect basal ganglia circuit (Graybiel, 2000; 2008). Because the A2AR is able to couple with Gs proteins, decreased adenosine or inhibition of A2AR in the striatum dampens adenylyl cyclase activity (Kull et al., 2000). Furthermore, decreased cAMP activity within the cell inhibits PKA activity, and decreases CREB phosphorylation. The fact that A2AR and D2R are selectively coexpressed in striatopallidal GABAergic neurons suggests that A2AR-PKACREB signaling may be associated with the role of the encephalin and dopamine D2R, which could contribute to the addictive and drug-seeking responses to ethanol. Furthermore, recent studies suggest a direct role of the striatal A2AR in mediating many of the cellular and behavioral responses underlying ethanol- and heroin-seeking behavior (Arolfo et al., 2004; Yao et al., 2006). Because the A2AR has a higher binding affinity (Kd) for adenosine (150 nM) than A1R (70 nM), reduced striatal adenosine levels by ENT1 deletion (< 100 nM) may predominantly affect A2AR signaling (Ciruela et al., 2006a; 2006b), which may in turn contribute to excessive ethanol drinking in ENT1 null mice.
Similar to ENT1 null mice, striatal-specific A2AR inhibition and A2AR knockout (KO) mice consume more alcohol in comparison to wild-type littermates (Naassila et al., 2002). These data further support the theory that dampened A2AR signaling in the striatum plays an essential role in increased alcohol drinking in ENT1 null mice. Furthermore, A2AR signaling has been strongly implicated in many of the behavioral effects of ethanol consumption and withdrawal. For example, A2AR agonists are known to reduce ethanol-withdrawal responses as measured by hyper-excitability in response to handling (Kaplan et al., 1999). Additionally, daily treatment of A2AR antagonist, ZM241385 (20 mg/kg, 4 days, i.p.) increased ethanol consumption and preference in ENT1 wild-type mice to a level similar to that of ENT1 null mice in a two-bottle choice experiment (Nam et al., 2013), suggesting that ENT1 deletion or A2AR inhibition promotes increased ethanol drinking.
Furthermore, mice lacking A2AR (Chen et al., 1999; 2001; Ledent et al., 1997) have been shown to demonstrate reduced sensitivity to the hypnotic effects of alcohol, increased alcohol consumption, and reduced alcohol-withdrawal seizures (El Yacoubi et al., 2001; Naassila et al., 2002). As a result, antagonism of A2AR signaling could be useful in the treatment of alcohol withdrawal symptoms; however, since inhibition of A2AR also results in increased alcohol consumption, the mechanisms through which the A2AR mediates each of these effects should be further delineated to identify a more specific therapeutic approach.
A2AR inhibition in the DMS and goal directed drinking
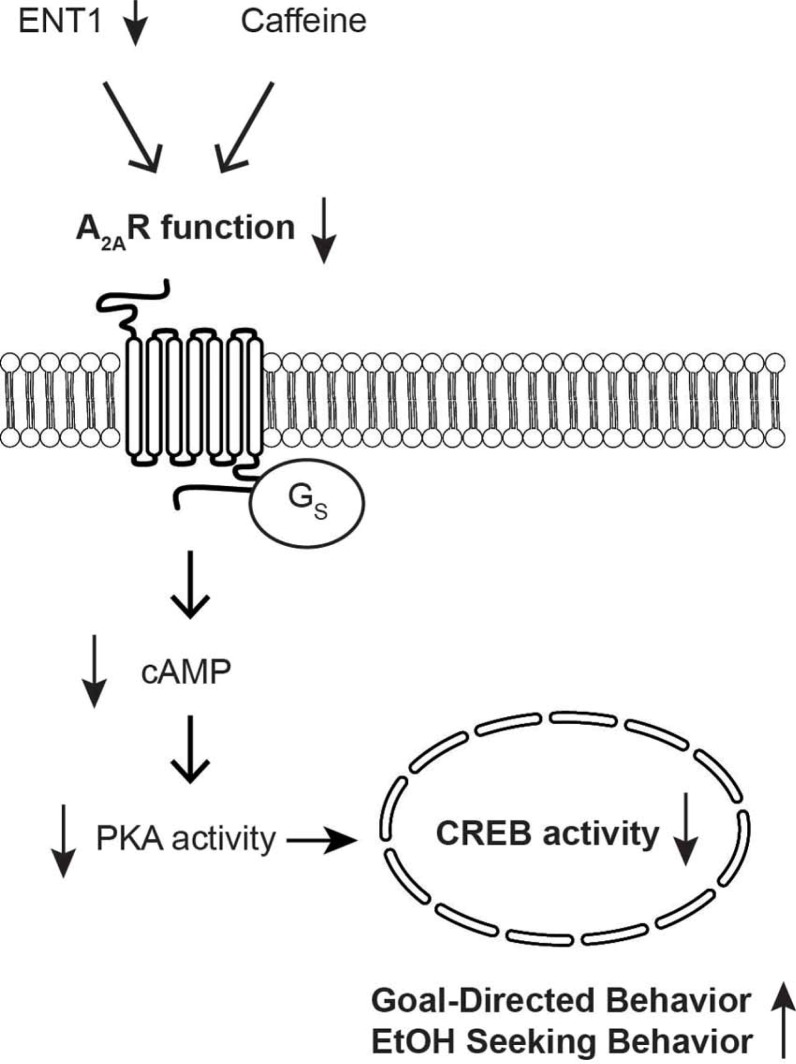
Recently, an essential role of the dorsal striatum in the development of habitual seeking behaviors, which include the regulation of voluntary movement, acquisition of goal-directed actions, and stimulus-driven habits, has been revealed (Lovinger, 2010; Yin and Knowlton, 2006). Recent studies have investigated the contribution of adenosine signaling in sub-regions of the dorsal striatum, the dorsomedial striatum (DMS; equivalent to caudate nucleus) and the dorsolateral striatum (DLS; equivalent to putamen) to habitual-seeking behaviors. Notably, the DMS primarily regulates goal-directed (action-outcome) behavior, which is sensitive to outcome devaluation and instrumental learning, whereas the DLS is more involved in habit (stimulus-response) formation (Yin and Knowlton, 2006; Yin et al., 2004; Yu et al., 2009). Since ethanol appears to impair several striatal functions including reward evaluation, motor function and habit formation (Corbit et al., 2012), the behavioral associations of adenosine signaling in habit formation have been examined. The lack of ENT1 function or inhibition of A2AR in the DMS reduces A2AR signaling through dampened PKA-driven CREB activity and accelerates the transition from goal-directed to habitual behaviors (Nam et al., 2013). Interestingly, inhibiting DMS A2AR promotes goal-directed behavior and increases both sucrose and ethanol seeking in operant self-administration experiments. Furthermore, reduced striatopallidal cAMP levels are correlated with faster acquisition of the reinforcer and a higher rate of lever pressing with extensive training in an instrumental conditioning experiment in mice (Lobo et al., 2007). This suggests that a reduction of cAMP-dependent PKA activity in A2AR expressing (mostly striatopallidal) neurons of the DMS could induce instrumental responding similar that of ENT1 null mice. Thus, hypo-A2AR function in the DMS of ENT1 null mice may lead to increased goal-oriented behavior. This is an early step towards habit formation in response to positive reinforcers and could render these mice more sensitive to the formation of compulsive habitual seeking behaviors (Fig. 2).
Fig. 2.
Possible mechanisms of hypo A2AR-CREB function in the dorsomedial striatum (DMS) via ENT1 deletion or A2AR inhibition or caffeine (Nam et al., 2013)
Reduced striatopallidal specific A2AR-CREB signaling may be associated with the reduction of enkephalin biosynthesis in the DMS. It is well known that the endogenous opioid system plays an important role in the development of ethanol reinforcement through repeated exposures (Herz, 1997). Furthermore, enkephalin is especially known to regulate the hedonic value and positive reinforcing properties of ethanol through the cortico-mesolimbic system. These lines of evidence support the hypothesis that reduction of A2AR-CREB activity in the DMS is critical to establish the reinforcing properties in the initial stages of habit formation. In addition, many alcohol preferring rodents exhibit lower enkephalin expression in the midbrain in comparison to non-preferring control animals and also have increased sensitivity to opioid alterations by ethanol treatment (Froehlich et al., 1991; Li et al., 1998; Mendez and Morales-Mulia, 2006; Ng et al., 1996). Thus, decreased CREB activity, possibly down-stream of A2AR in the DMS, also promotes excessive ethanol seeking, which is governed by positive reinforcement and sensitivity to goal-directed responses during the extinction stage. Furthermore, chronic ethanol exposure has been shown to induce hypo-CREB activity in the striatum, which could be one mechanism explaining how repeated ethanol exposure could lead to the development of habitual ethanol seeking (Lonze and Ginty, 2002; Yang et al., 1998).
Interactions between adenosine and dopamine receptors in the striatum
The interactions between signaling in the A2AR and the dopaminergic system appear to be an important striatal mechanism through which A2AR influences behavior. Adenosine and dopamine receptors are able to form several different combinations of adenosine-dopamine heterodimers throughout the brain; however, there are only two types of adenosine-dopamine heterodimers that are heavily concentrated in the striatum and thus relevant to addiction disorders: The A2AR-D2R heterodimer and A1R-D1R heterodimer (Fig. 3).
Fig. 3.
Interaction between adenosine and dopamine signaling in corticostriatal circuits for addictive behaviors. Striatal neurons in the GABAergic medium spiny neurons (MSN) which contain dopamine D1Rs co-express adenosine A1 receptors (A1R). These striatal neurons are able to activate the prefrontal cortex (PFC) or the sensory motor cortex (SMC) using thalamic neurons as an intermediary. In contrast, striatal neurons containing dopamine D2Rs co-express A2AR in the MSN, and inhibit the PFC or SMC by inhibiting thalamic neurons. Astrocytes are main source of adenosine and A1R and A2AR inhibit D1R and D2R respectively. Red line, glutamate neurotransmission; Blue line, GABA neurotransmission; Purple line, dopamine neurotransmission.
A2AR-D2R heterodimers are located on excitatory striatopallidal neurons. A2AR agonists are known to cause a conformational change in the shape of D2R, which in turn, leads to a reduction in dopamine binding to D2R, and thus prevents the effects of D2R signaling, such as phosphorylation of Ca2+ ion channels. Furthermore, A2AR agonism (as well as D2 antagonism) has been shown to increase the expression of c-fos (Fuxe et al., 2010) and inhibit the release of GABA. In contrast, D2R agonists and A2AR antagonists have the reverse effect and inhibit c-fos expression (Ferré et al., 1997; Fuxe et al., 2007; 2010). The D2R also has antagonistic effects on A2AR. D2R agonist signaling decreases cAMP levels that would have normally been stimulated by A2AR. Inhibition of the D2R and stimulation of A2AR results in an increase the activity of PKA, which in turn phosphorylates AMPA and NMDA glutamate receptors, and DARPP-32. This intracellular activity leads to an up-state of the striatopallidal neuron. Thus, activation of the D2R would bring the neuron back to a normal state. However, it is important to note that A2ARs can exert dopamine independent effects on neurotransmission. For example, caffeine, an A2A antagonist, is known to promote locomotor function in D2R KO mice (Zahniser et al., 2000). Due to the high concentration of A2AR-D2R heterodimers in the striatum, they may be able to be used as a specific target location in the treatment of addiction. Extensive studies have shown that A2ARs are up regulated in the ventral striatum (NAc) during extended cocaine use and are diminished during withdrawal periods. Interestingly, A2AR agonists are able to diminish the rewarding effects of cocaine, and counteract the sensitization to the locomotor effects of cocaine. Furthermore, A2AR antagonists have also been associated with reinstatement of cocaine self-administration, implying that the A2AR plays an important role in the mechanisms of addiction (Fuxe et al., 2010).
The A1R-D1R heterodimer is primarily found in striatonigral and striato-entopeduncular inhibitory GABA pathways. A1R agonists have been found to significantly decrease the binding affinity of D1R located in the NAc and the PFC (Fuxe et al., 2010). This suggests that, similar to A2AR-D2R heterodimers, a conformational change in the structure of the D1R is caused by adenosine binding to the A1R, and thus, decreases dopamine signaling. In contrast, A1R antagonists have been shown to have the reverse effect and increase dopaminergic signaling (Fuxe et al., 2010). Furthermore, A1R agonists have also demonstrated an ability to counteract the effects of D1R activation, indicating that the function of A1R in the A1R-D1R heterodimer is to inhibit dopamine signaling via the D1R. The ability of A1R to inhibit the D1R action could be useful in limiting the hijacking of the dopaminergic natural reward system by drugs and addictive substances. This in turn would eliminate the hedonic or pleasurable effects that can be experienced with drug usage. However, due to the large dispersal of A1R throughout the brain it is difficult to isolate the addiction centers of the brain without undesirable side effects. Therefore, manipulation of the A2AR-D2R heterodimer appear to be the most promising treatment for addiction related disorders. In any case, the antagonistic interactions between the A1-D1 and A2A-D2 receptors display strong implications for the molecular mechanisms of addiction (Fig. 3).
Caffeine and alcohol drinking
Interestingly, caffeine is known to inhibit both the A1R and A2AR. However, caffeine has a much higher binding affinity for A2AR, thus the effects of caffeine on the A1R are considered to be negligible. The antagonist effects of caffeine may provide the mechanism for its stimulant effects and its ability to promote excessive ethanol seeking in the CNS. Furthermore, specific actions of caffeine, including increased arousal (Huang et al., 2005), increased locomotor activity (El Yacoubi et al., 2000) and increased ethanol tolerance can be attributed to its inhibition of A2AR. In addition, a recent co-crystal structural study has revealed that ZM241385 (A2AR specific antagonist) and caffeine have similar pharmacological binding properties to A2AR (Dore et al., 2011), suggesting that inhibition of A2AR by either ZM241385 or caffeine might enhance goal-directed behavior and promote ethanol drinking (Fig. 2). Several investigations have demonstrated the importance of the interaction of ethanol with the A2AR system and why concurrent intake of ethanol and caffeine could lead to heavier drinking patterns and greater prevalence of goal-directed binge alcohol drinking (Butler and Prendergast, 2012). However, further investigation is needed to understand pharmacological regulation by caffeine and brain region-specific A2AR regulation in alcohol use disorders.
CONCLUSION
Although the alteration of extracellular adenosine levels at a given time remain somewhat constant, it is becoming increasingly clear that adenosine levels are modulated through neuroglial interactions and cell type specific adenosine transporters. Genetic and pharmacological approaches for adenosine signaling have been important in characterizing striatal signaling pathways involved in A2AR signaling and have been useful in behavioral analyses. Genetically engineered adenosine transporter ENT1 null and adenosine A2AR knockout mouse models have provided essential roles of adenosine signaling in alcohol seeking behaviors. Moreover, DMS adenosine signaling plays a large role in the orchestration of goal-oriented ethanol drinking. Furthermore, dampening of the A2AR-PKA-CREB signaling pathway in the DMS promotes goal-oriented seeking for both natural and hedonic stimuli, which suggests that A2AR inhibition is a salient feature for addiction development. However, further investigation on the involvement of caffeine in regulation of both A1R and A2AR signaling is still needed to fully understand how consuming caffeinated alcohol beverages may result in unintentional excessive ethanol consumption.
Acknowledgments
We thank all the current and previous laboratory members. Especially, we thank Mr. David Hinton for his comments and proofreading. This research was supported by the Samuel Johnson Foundation for Genomics of Addiction Program, Ulm Family Foundation, Center for Individualized Medicine, at Mayo Clinic and by grants from the National Institutes of Health (NIH) to DSC (AA018779, AA017830).
REFERENCES
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- Asatryan L, Nam HW, Lee MR, Thakkar MM, Dar MS, Davies DL, Choi DS. Implication of the Purinergic System in Alcohol Use Disorders. Alcohol Clin Exp Res. 2011;35:584–594. doi: 10.1111/j.1530-0277.2010.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SA, Mackey JR, Cass CE, Young JD. Nucleoside transporters: molecular biology and implications for therapeutic development. Mol. Med. Today. 1999;5:216–224. doi: 10.1016/S1357-4310(99)01459-8. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, Ritzel MW, Cass CE, Young JD. Functional characterisation of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem. 2005;280:15880–15887. doi: 10.1074/jbc.M414337200. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundege JM, Diao L, Proctor WR, Dunwiddie TV. The role of cyclic AMP as a precursor of extracellular adenosine in the rat hippocampus. Neuropharmacology. 1997;36:1201–1210. doi: 10.1016/s0028-3908(97)00102-0. [DOI] [PubMed] [Google Scholar]
- Butler TR, Prendergast MA. Neuroadaptations in adenosine receptor signaling following long-term ethanol exposure and withdrawal. Alcohol Clin Exp Res. 2012;36:4–13. doi: 10.1111/j.1530-0277.2011.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hackett E, Fink JS, Low MJ, et al. The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Nam HW, Lee MR, Hinton DJ, Choi S, Kim T, Kawamura T, Janak PH, Choi D-S. Altered glutamatergic neurotransmission in the striatum regulates ethanol sensitivity and intake in mice lacking ENT1. Behav Brain Res. 2010;208:636–642. doi: 10.1016/j.bbr.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci. 2006a;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Ferre S, Casado V, Cortes A, Cunha RA, Lluis C, Franco R. Heterodimeric adenosine receptors: a device to regulate neurotransmitter release. Cell Mol Life Sci. 2006b;63:2427–2431. doi: 10.1007/s00018-006-6216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol. Psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Nagy L, Mochly-Rosen D, Gordon A. The role of adenosine and adenosine transport in ethanol-induced cellular tolerance and dependence Possible biologic and genetic markers of alcoholism. Ann NY Acad Sci. 1991;625:473–487. doi: 10.1111/j.1749-6632.1991.tb33878.x. [DOI] [PubMed] [Google Scholar]
- Dore AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, Hurrell E, Bennett K, Congreve M, Magnani F, et al. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure. 2011;19:1283–1293. doi: 10.1016/j.str.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Durieux PF, Schiffmann SN, de Kerchove d’Exaerde A. Targeting neuronal populations of the striatum. Front Neuroanat. 2011;5:40. doi: 10.3389/fnana.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Menard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Daoust M, Costentin J, Vaugeois J. Absence of the adenosine A(2A) receptor or its chronic blockade decrease ethanol withdrawal-induced seizures in mice. Neuropharmacology. 2001;40:424–432. doi: 10.1016/s0028-3908(00)00173-8. [DOI] [PubMed] [Google Scholar]
- Ena S, de Kerchove d’Exaerde A, Schiffmann SN. Unraveling the differential functions and regulation of striatal neuron sub-populations in motor control, reward, and motivational processes. Front Behav Neurosci. 2011;5:47. doi: 10.3389/fnbeh.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.02.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005a;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005b;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology. 1991;103:467–472. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Borroto-Escuela DO, Guescini M, Fernandez-Duenas V, Tanganelli S, Rivera A, Ciruela F, Agnati LF. Adenosine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e18–42. doi: 10.1111/j.1755-5949.2009.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gordon AS, Diamond I. Adenosine mediates the effects of ethanol on the cAMP signal transduction system. Alcohol. Alcohol. 1993;2(Suppl):437–441. [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Jennings LL, Cass CE, Ritzel MWL, Yao SYM, Young JD, Griffiths M, Baldwin SA. Adenosine transport: Recent advances in the molecular biology of nucleoside transporter proteins. Drug Dev Res. 1998;45:277–287. [Google Scholar]
- Jennings LL, Hao C, Cabrita MA, Vickers MF, Baldwin SA, Young JD, Cass CE. Distinct regional distribution of human equilibrative nucleoside transporter proteins 1 and 2 (hENT1 and hENT2) in the central nervous system. Neuropharmacology. 2001;40:722–731. doi: 10.1016/s0028-3908(00)00207-0. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Bharmal NH, Leite-Morris KA, Adams WR. Role of adenosine A1 and A2A receptors in the alcohol withdrawal syndrome. Alcohol. 1999;19:157–162. doi: 10.1016/s0741-8329(99)00033-6. [DOI] [PubMed] [Google Scholar]
- Kim JH, Karpyak VM, Biernacka JM, Nam HW, Lee MR, Preuss UW, Zill P, Yoon G, Colby C, Mrazek DA, et al. Functional role of the polymorphic 647 T/C variant of ENT1 (SLC29A1) and its association with alcohol withdrawal seizures. PLoS One. 2011;6:e16331. doi: 10.1371/journal.pone.0016331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol Sci. 2006;27:416–425. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik TB, Aronhime SN, Echeverry G, Beylin A, Winn HR. The relationship between oxygen and adenosine in astrocytic cultures. Glia. 2010;58:1335–1344. doi: 10.1002/glia.21011. [DOI] [PubMed] [Google Scholar]
- Kull B, Svenningsson P, Fredholm BB. Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol Pharmacol. 2000;58:771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Li XW, Li TK, Froehlich JC. Enhanced sensitivity of the nucleus accumbens proenkephalin system to alcohol in rats selectively bred for alcohol preference. Brain Res. 1998;794:35–47. doi: 10.1016/s0006-8993(98)00191-7. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Schrader J. Adenosine metabolism in the guinea pig heart: the role of cytosolic S-adenosyl-L-homocysteine hydrolase, 5′-nucleotidase and adenosine kinase. Eur. Heart J. 1993;14(Suppl I):27–33. [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Cui Y, Ostlund SB, Balleine BW, Yang XW. Genetic control of instrumental conditioning by striatopallidal neuron-specific S1P receptor Gpr6. Nat Neurosci. 2007;10:1395–1397. doi: 10.1038/nn1987. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M, Morales-Mulia M. Ethanol exposure differentially alters pro-enkephalin mRNA expression in regions of the mesocorticolimbic system. Psychopharmacology. 2006;189:117–124. doi: 10.1007/s00213-006-0503-3. [DOI] [PubMed] [Google Scholar]
- Meng ZH, Dar MS. Possible role of striatal adenosine in the modulation of acute ethanol-induced motor incoordination in rats. Alcohol Clin Exp Res. 1995;19:892–901. doi: 10.1111/j.1530-0277.1995.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy LE, DeSilva SE. Adenosine A1 receptors mediate chronic ethanol-induced increases in receptor-stimulated cyclic AMP in cultured hepatocytes. Biochem. J. 1994;304(Pt 1):205–210. doi: 10.1042/bj3040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Lee MR, Zhu Y, Wu J, Hinton DJ, Choi S, Kim T, Hammack N, Yin JC, Choi DS. Type 1 equilibrative nucleoside transporter regulates ethanol drinking through accumbal N-methyl-D-aspartate receptor signaling. Biol Psychiatry. 2011;69:1043–1051. doi: 10.1016/j.biopsych.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, McIver SR, Hinton DJ, Thakkar MM, Sari Y, Parkinson FE, Haydon PG, Choi D-S. Adenosine and glutamate signaling in neuron-glial interactions: Implication in alcoholism and sleep disorders. Alcohol Clin Exp Ther. 2012;36:1117–1125. doi: 10.1111/j.1530-0277.2011.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Hinton DJ, Kang NY, Kim T, Lee MR, Oliveros A, Adams C, Ruby CL, Choi DS. Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. J Neurosci. 2013;33:4329–4338. doi: 10.1523/JNEUROSCI.3094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng GY, O’Dowd BF, George SR. Genotypic differences in mesolimbic enkephalin gene expression in DBA/2J and C57BL/6J inbred mice. Eur J Pharmacol. 1996;311:45–52. doi: 10.1016/0014-2999(96)00401-3. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Ferguson J, Zamzow CR, Xiong W. Gene expression for enzymes and transporters involved in regulating adenosine and inosine levels in rat forebrain neurons, astrocytes and C6 glioma cells. J Neurosci Res. 2006;84:801–808. doi: 10.1002/jnr.20988. [DOI] [PubMed] [Google Scholar]
- Pedata F, Pazzagli M, Tilli S, Pepeu G. Regional differences in the electrically stimulated release of endogenous and radioactive adenosine and purine derivatives from rat brain slices. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:447–453. doi: 10.1007/BF00169463. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Adams C, Knight EJ, Nam HW, Choi DS. An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev. 2010;3:163–174. doi: 10.2174/1874473711003030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yang X, Horn K, Wand GS. Chronic ethanol exposure impairs phosphorylation of CREB and CRE-binding activity in rat striatum. Alcohol Clin Exp Res. 1998;22:382–390. [PubMed] [Google Scholar]
- Yao SY, Ng AM, Muzyka WR, Griffiths M, Cass CE, Baldwin SA, Young JD. Molecular cloning and functional characterization of nitrobenzylthioinosine (NBMPR)-sensitive (es) and NBMPR-insensitive (ei) equilibrative nucleoside transporter proteins (rENT1 and rENT2) from rat tissues. J Biol Chem. 1997;272:28423–28430. doi: 10.1074/jbc.272.45.28423. [DOI] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Ueda T, Diamond I. Adenosine A2a blockade prevents synergy between muopiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proc. Natl. Acad. Sci. USA. 2006;103:7877–7882. doi: 10.1073/pnas.0602661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci. 2007;25:3226–3232. doi: 10.1111/j.1460-9568.2007.05606.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Gupta J, Chen JF, Yin HH. Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J Neurosci. 2009;29:15100–15103. doi: 10.1523/JNEUROSCI.4215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Simosky JK, Mayfield RD, Negri CA, Hanania T, Larson GA, Kelly MA, Grandy DK, Rubinstein M, Low MJ, et al. Functional uncoupling of adenosine A(2A) receptors and reduced responseto caffeine in mice lacking dopamine D2 receptors. J Neurosci. 2000;20:5949–5957. doi: 10.1523/JNEUROSCI.20-16-05949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzow CR, Xiong W, Parkinson FE. Astrocytes affect the profile of purines released from cultured cortical neurons. J Neurosci Res. 2008;86:2641–2649. doi: 10.1002/jnr.21718. [DOI] [PubMed] [Google Scholar]