Abstract
Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5/GPR49) is highly expressed in adult stem cells of various tissues, such as intestine, hair follicles, and stomach. LGR5 is also overexpressed in some colon and ovarian tumors. Recent reports show that R-spondin (RSPO) family ligands bind to and activate LGR5, enhancing canonical Wnt signaling via the interaction with LRP5/6 and Frizzled. The identity of heterotrimeric G-proteins coupled to LGR5, however, remains unclear. Here, we show that Rho GTPase is a downstream target of LGR5. Overexpression of LGR5 induced SRF-RE luciferase activity, a reporter of Rho signaling. RSPOs, ligands for LGR4, LGR5, and LGR6, however, did not induce SRF-RE reporter activity in the presence of LGR5. Consistently, LGR5-induced activity of the SRF-RE reporter was inhibited by Rho inhibitor C3 transferase and RhoA N19 mutant, and knockdown of Gα12/13 genes blocked the reporter activity induced by LGR5. In addition, focal adhesion kinase, NF-κB and c-fos, targets of Rho GTPase, were shown to be regulated by LGR5. Here, we have demonstrated, for the first time, that LGR5 is coupled to the Rho pathway through G12/13 signaling.
Keywords: FAK, G12/13, LGR5, NFkappaB, Rho
INTRODUCTION
The G protein-coupled receptor (GPCR) family is a superfamily of cell membrane receptors that are encoded by approximately 800 genes and transduce extracellular cues to intracellular responses through interactions with G proteins (Alexander et al., 2011; Civelli et al., 2013). GPCRs transmit extracellular signals to heterotrimeric G proteins, such as Gs, Gq, Gi, and G12/13 (Alexander et al., 2011). These G proteins, in turn, interact with other membrane proteins to initiate intracellular signaling, for example, elevation of Ca2+ ion levels and regulation of cyclic AMP production (Alexander et al., 2011).
LGR5, first identified as a member of the glycoprotein hormone receptor family (Hsu et al., 1998), had been an orphan receptor until several groups showed that R-spondins (RSPOs) were able to bind and activate LGR5, enhancing canonical Wnt-β-catenin signaling together with LRP 5/6 and Frizzled (Carmon et al., 2011; 2012; de Lau et al., 2011; Glinka et al., 2011; Ruffner et al., 2012). LGR5 is expressed and plays an important role in various adult tissue stem cells in, for example, small intestine, hair follicles, and stomach, through regulation of Wnt signaling (Barker and Clevers, 2010). LGR5 also marks various cancer stem-like cells, implicating a role in tumorigenesis (Fan et al., 2010). It is intriguing, however, that RSPOs do not induce a change in Ca2+ ion and cAMP levels in the presence of LGR5 (Carmon et al., 2011). In addition, β-arrestin is not internalized by RSPOs, suggesting that conventional GPCR signaling may not take place downstream of LGR5 (Carmon et al., 2011).
In order to investigate downstream pathways of LGR5, we measured the activity of nuclear factor of activated T cell response element (NFAT-RE), cyclic AMP response element (CRE), serum response element (SRE), and serum response factor response element (SRF-RE) reporter genes, which are commonly used to assay GPCR activity, in the presence of LGR5 and RSPO1. Among these reporters, SRE and SRF-RE luciferase activities were induced by LGR5, but not by RSPO1. Because LGR5 activated both reporters, we hypothesized that the Rho pathway is involved in the downstream signaling of LGR5. To rule out an influence of ERK signaling, the SRF-RE reporter was used to evaluate the activity of Rho signaling induced by LGR5 in the remainder of the experiments.
Next, we showed that LGR4 and LGR6 did not induce SRFRE reporter activity, and that LGR5 used the G12/13-Rho pathway to induce SRF-RE reporter activity. Finally, focal adhesion kinase (FAK), nuclear factor-kappa B (NF-κB), and c-fos, all of which are downstream targets of Rho signaling, were shown to be regulated by the LGR5-G12/13-Rho pathway. In conclusion, we demonstrate for the first time that LGR5 is linked to G12/13-Rho signaling independently of RSPOs.
MATERIALS AND METHODS
Plasmids and reagents
Full-length human LGR5 cDNA and the TOPFLASH reporter plasmid were kindly provided by Dr. Hans Clevers (Hubrecht Institute, Netherlands) and cloned with a Myc tag into the vector pIRESneo3 (Clontech, USA). Human LGR4 cDNA was purchased from Missouri S&T cDNA Resource Center (USA). An expression construct for human LGR6 tagged with Myc and FLAG was purchased from Origene Technologies (USA). Recombinant RSPO 1, 2, 3, and 4 and Wnt3A were purchased from R&D Systems (USA). C3 transferase was purchased from Cytoskeleton (USA). CRE, SRE, SRF-RE, NFAT-RE, and NF- κB-RE-luciferase reporter plasmids were purchased from Promega (USA). The c-fos promoter-luciferase construct [Addgene plasmid 11983, kindly deposited by Dr. Ron Prywes (Cen et al., 2003)] was purchased from Addgene (USA).
Cell culture and transfection
293T and HEK293 cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) (Welgene, Korea) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin (Life Technologies, USA). HT-29 cells were grown in Roswell Park Memorial Institute (RPMI) medium (Welgene, Korea) supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were grown in a 37°C incubator with 95% humidity and 5% CO2, and transfected using Fugene 6 (Promega) according to the manufacturer’s protocol. The HEK293/ hLGR5- Myc stable cell line was selected and maintained in 0.5 mg/mL G418. Scrambled and LGR5 ON-TARGETplus SMARTpool siRNAs were purchased from Dharmacon (USA). siRNAs targeting Gα12 and Gα13 were purchased from Santa Cruz Biotechnology (USA). The cells were transfected with siRNAs using X-tremeGENE siRNA Transfection Reagent (Roche, Switzerland).
Antibodies and western analysis
Cells were lysed with RIPA buffer with protease inhibitor cocktail (Roche, Switzerland) and 1 mM DTT (Hwang et al., 2012). After centrifugation at 18,000 × g for 20 min, the cleared lysates were mixed with Laemmli SDS sample buffer. Proteins were separated by SDS-PAGE and transferred onto PROTRAN nitrocellulose membranes (Whatman, UK). Membranes were probed with anti-GPR49 (LGR5) (Abchem); anti-Myc (9E10) (Covance, USA); anti-Gα12, anti-Gα13 (both Santa Cruz Biotechnology); anti-phospho-ERK, anti-ERK, anti-FAK, or anti-phospho Tyr397-FAK (all Cell Signaling Technology, USA) antibodies. Protein bands were detected using HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, USA). The Westzol plus kit (Intron, Korea) was used for the enhanced chemiluminescence reaction.
Luciferase reporter assay
293T cells were transiently transfected with CRE, NFAT-RE, SRE, SRF-RE, NF-κB-RE, c-fos-Luc, or TOPFLASH reporter plasmids. Reporter plasmids were co-transfected with a thymidine kinase promoter-Renilla luciferase reporter plasmid (pRLTK) for normalization of transfection efficiency. Transfections were carried out using Fugene 6. Cells were serum-starved for 24 h, and luciferase activity was measured using the Dual- Luciferase assay kit (Promega). In the siRNA experiment, cells were co-transfected with plasmids and siRNAs using X-treme GENE siRNA Transfection Reagent for 48 h.
RESULTS AND DISCUSSION
Overexpression of LGR5 augments Rho GTPase-dependent reporter activities
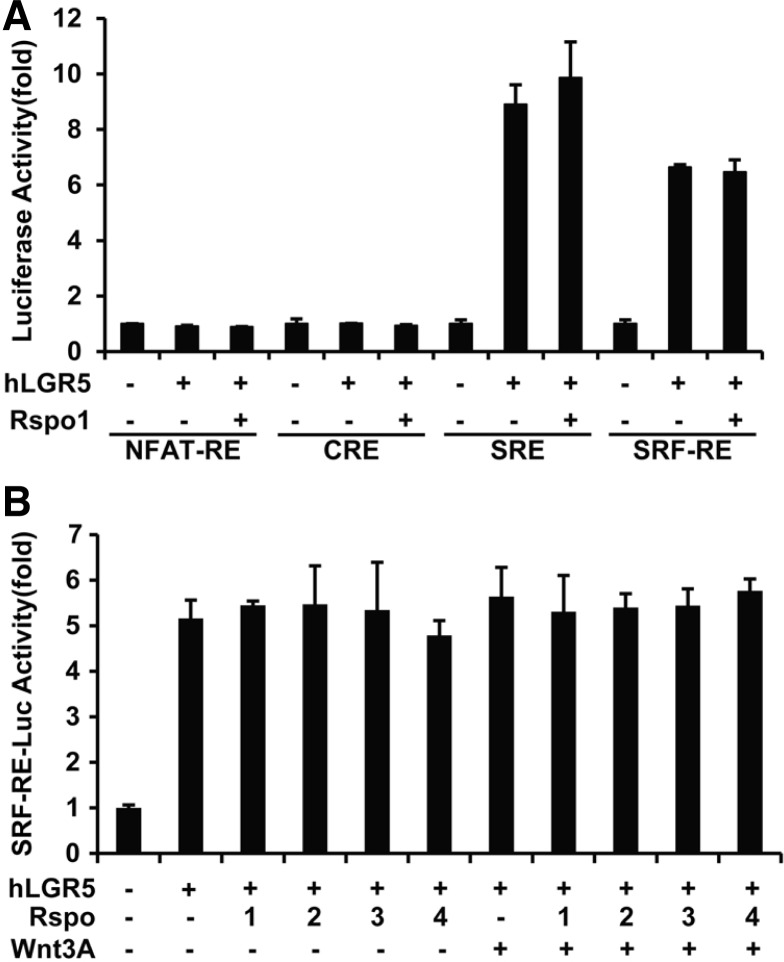
To investigate downstream signaling through heterotrimeric G proteins, we used luciferase reporter plasmids containing NFAT-RE, CRE, SRE, or SRF-RE. After transfection of 293T cells with reporter plasmids and LGR5, the serum-starved cells were treated with RSPO1 for 16 h. NFAT-RE and CRE reporter activities were not altered by overexpression of LGR5 and addition of RSPO1 (Fig. 1A). This result indicated that LGR5 may not be coupled to Gαq, Gαs, or Gαi, consistent with previous reports (Carmon et al., 2011; de Lau et al., 2011). Surprisingly, overexpression of LGR5 markedly induced the activities of SRE and SRF-RE reporters (Fig. 1A). The SRE reporter is induced by ERK and Rho GTPase signaling, whereas SRF-RE is augmented by Rho GTPase (Jaffe and Hall, 2005). Because LGR5 activated both reporters, we hypothesized that the Rho pathway is involved in the downstream signaling of LGR5. To rule out an influence of ERK signaling, the SRF-RE reporter was used to evaluate the activity of LGR5-induced Rho signaling in the remainder of the experiments. It was intriguing that RSPO1 did not stimulate the reporter activities in the presence of LGR5 (Fig. 1A).
Fig. 1.
R-spondin (RSPO)-independent induction of reporter activity by LGR5. 293T cells were transfected with indicated reporter plasmids (A) or SRF-RE (B), human LGR5-Myc, and pRL-TK as a transfection efficiency control. During the 24-h period of serum starvation, the cells were incubated with 25 ng/ml RSPOs in the presence or absence of 25 ng/ml Wnt3A for 16 h. Luciferase activity of reporters was normalized to pRL-TK Renilla luciferase activity. Values represent the mean and standard deviation from two independent experiments.
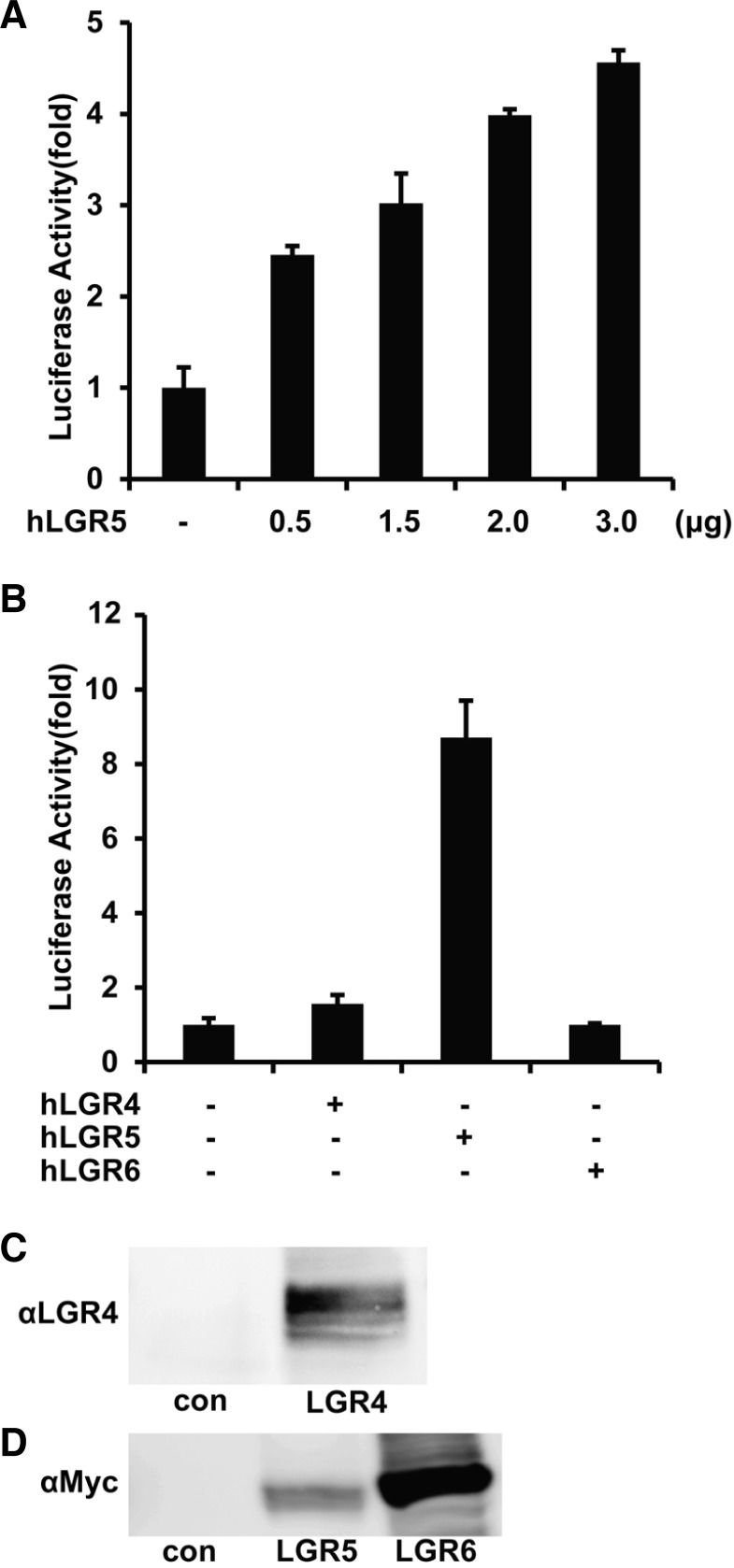
To test the effects of other RSPOs and Wnt, cells transfected with LGR5 and the SRF-RE or TOPFLASH (a reporter for Wnt/β-catenin pathway) reporters were treated with recombinant RSPO1, 2, 3, 4, and/or Wnt3A. RSPOs and Wnt3A, alone or together, stimulated TOPFLASH reporter activity (Supplementary Fig. S1A), but had no effect on luciferase activity of the SRF-RE reporter (Fig. 1B). Furthermore, when cells transfected with SRF-RE and LGR5 plasmids were incubated with each RSPO for different time periods, no significant changes were seen in the reporter activities (Supplementary Fig. S1B), suggesting that RSPOs are not ligands of LGR5 in terms of SRFRE-dependent signaling. To further confirm these data, 293T cells were transfected with increasing amounts of LGR5 expression construct together with the SRF-RE reporter. After serum-starvation for 24 h, luciferase activity was assayed. Luciferase activity was induced by LGR5 expression in a dose-dependent manner (Fig. 2A).
Fig. 2.
Dose-dependent activation of SRF-RE by LGR5, but not LGR4 or LGR6. (A) 293T cells were transfected with the SRF-RE reporter and the indicated amounts of hLGR5-Myc, and serumstarved for 24 h, followed by assay of luciferase activity. (B) hLGR4, hLGR5, and hLGR6 were overexpressed, together with the SRFRE reporter in 293T cells, as indicated. Serum-starved cells were lysed, and luciferase activity of reporters was assayed and normalized to pRL-TK Renilla luciferase activity. (C, D) 293T cells were transfected for 48 h with 2 μg plasmid encoding hLGR4, hLGR5, or hLGR6, lysed, and analyzed by western. Blots are representative of three independent experiments.
LGR5 and its close relatives, LGR4 and LGR6, belong to the family of GPCRs that are homologous to the glycoprotein hormone receptors (Barker and Clevers, 2010; Hsu et al., 1998; 2000). LGR5 and LGR6 have attracted much attention from the stem cell community as a result of their specific expression in proliferating adult stem cells in various tissues, including hair follicles, intestines, stomach, and skin, whereas LGR4 shows a broader expression pattern that extends beyond stem cells (Barker and Clevers, 2010; Van Schoore et al., 2005). Furthermore, LGR4 and LGR5 null mice show neonatal lethality, whereas LGR6 knockout mice are healthy and fertile (Mazerbourg et al., 2004; Morita et al., 2004; Snippert et al., 2010). These observations led us to investigate whether LGR4 and LGR6 regulate SRF-RE reporter activity. Intriguingly, only LGR5, and not LGR4 and LGR6, promoted luciferase reporter activity (Fig. 2B), although LGR4 (Fig. 2C) and LGR5 and LGR6 (Fig. 2D) were overexpressed at comparable levels. These results may explain, at least in part, the differences between in vivo phenotypes specifically mediated by LGR5 and those mediated by LGR4 and LGR6.
G12/13-Rho axis mediates LGR5 signaling
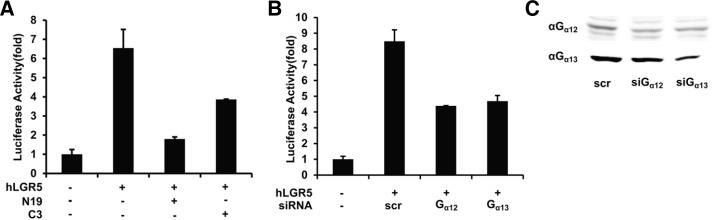
To test the hypothesis that Rho mediates LGR5 signaling, we used the Rho N19 mutant (N19), a dominant negative form of Rho GTPase, and exoenzyme C3 transferase, which covalently links ADP-ribose to and inactivates Rho GTPase. As shown in Fig. 3A, overexpression of the N19 almost completely downregulated the LGR5-induced activity of the SRF-RE reporter. C3 transferase also inhibited the reporter activity significantly, but not completely, probably due to incomplete diffusion of the protein into the cell. In addition, some reports had already shown that even overexpression of C3 transferase in cells does not abrogate all activity of Rho signaling in terms of SRF reporter activity (Medjkane et al., 2009; Merdek et al., 2008). These data collectively demonstrated that Rho GTPase is required for the activation of the SRF-RE reporter by LGR5. As described above, G12 and G13 among heterotrimeric G proteins are well known to regulate RhoGEF proteins to generate active Rho GTPase (Bian et al., 2006). To determine whether G12/13 mediate LGR5 signaling, 293T cells were transfected with siRNAs targeting Gα12/13, together with LGR5 and the SRF-RE reporter. Both siRNAs inhibited LGR5-induced luciferase activity by 50% (Fig. 3B).The knockdown efficiency of the siRNAs was approximately 50%, but this was sufficient to significantly reduce the level of Gα12/13 (Fig. 3C). These results indicate that LGR5 regulates Rho through G12/13.
Fig. 3.
G12/13-Rho axis mediates LGR5 signaling. 293T cells were transfected with plasmid encoding LGR5 and the SRF-RE reporter and serum- starved for 24 h, followed by incubation for 4 h in the presence or absence of 1 μg/ml C3 transferase and Rho N19 mutant (A) and control (scr) siRNA or siRNA targeting Gα12 or Gα13 (B). Luciferase activity of reporters was normalized to pRL-TK Renilla luciferase activity. Values represent the mean and standard deviation from two independent experiments. (C) Efficiency of Gα12 or Gα13 knockdown by the indicated siRNAs was assessed by western analysis. Blots are representative of three independent experiments.
Buch et al. (2008) demonstrated that the thyrotropin receptor activates ERK via the G12/13-Rho pathway, and our data showed that the SRE reporter, which is dependent on both ERK signaling, via ternary complex factor (TCF), and SRF, was induced by LGR5 (Fig. 1A). When the expression of LGR5 was reduced by siRNA in the LGR5/HEK293 stable cell line (Fig. 4A) and HT-29 cells (Fig. 4B), however, the level of ERK phosphorylation did not change. In addition, it has been suggested that FAK is a downstream target of the G12/13-Rho pathway and mediates the regulation of focal adhesion assembly in this pathway (Chikumi et al., 2002; Needham and Rozengurt, 1998; Torsoni et al., 2005). In the same LGR5/HEK293 (Fig. 4A) and HT-29 (Fig. 4B) cell lysates described above, phosphorylation of FAK at Tyr397 was significantly reduced in the presence of LGR5 siRNA.
Fig. 4.
Downstream targets of Rho GTPase are regulated by LGR5. (A, B) HEK293 cells stably expressing hLGR5-Myc (A) and HT-29 (B) cells were transfected with control (scr) siRNA or siRNA targeting LGR5 for 48 h, followed by lysis and western analysis. Blots are representative of three independent experiments. (C-F) 293T cells were transfected with reporter plasmids c-fos-luc (C, D) or NF-κB-luc (E, F). Cells were serum-starved for 24 h, followed by incubation for 4 h in the presence or absence of 1 μg/ml C3 transferase and Rho N19 mutant (C, E) and control (scr) siRNA or siRNA targeting Gα12 or Gα13 (D, F). Luciferase activity of reporters was normalized to pRL-TK Renilla luciferase activity. Values represent the mean and standard deviation from two independent experiments.
The promoter activity of c-fos, a target gene of the Rho pathway that includes SRE in its promoter region, was highly upregulated by overexpression of LGR5, and this induction was blocked by the N19 and C3 transferase (Fig. 4C) and siRNA targeting Gα12/13 (Fig. 4D). To further confirm the relationship between LGR5 and the G12/13-Rho pathway, we tested whether LGR5 regulates NF-κB reporter activity. NF-κB has long been known to be activated by the Rho pathway, although the underlying molecular mechanism remains unclear (Iguchi et al., 2008; Montaner et al., 1998; Perona et al., 1993; Shepard et al., 2001). As shown in Figs. 4E and 4F, NF-κB reporter activity was strongly induced by LGR5. Subsequent perturbation of the G12/13-Rho pathway by N19 or C3 (Fig. 4E) or LGR5 siRNA (Fig. 4F) abrogated LGR5-induced reporter activity. It has been suggested that NF-κB is a link between inflammation and tumor development induced by the Rho signaling pathway (Benitah et al., 2003; Karin et al., 2002; Lin and Karin, 2003; Segain et al., 2003). In addition, NF-κB plays a role in the regulation of stem cell self-renewal and differentiation (Schugar et al., 2008). In this sense, further study is needed to investigate the role of NF- κB in LGR5-expressing stem cells and cancer. Taken together, these data demonstrate that the G12/13-Rho pathway is coupled to LGR5.
Recent observations showed that LGR5 potentiates Wnt signaling in vivo, especially in adult stem cells (Schuijers and Clevers, 2012). Here, we clearly demonstrate for the first time that LGR5 is coupled to the heterotrimeric G proteins G12/13 and modulates Rho GTPase signaling. It has been known that Rho family GTPases, such as Rho, Rac, and Cdc42, are direct downstream components of the Wnt/Frizzled/Dishevelled pathway that regulates planar cell polarity, cell migration, and cell adhesion (Habas et al., 2001; Schlessinger et al., 2009). These pathways are, however, directly regulated by the interaction of Dishevelled and guanine nucleotide exchange factors (GEFs) for the Rho family GTPases. There is no evidence, to our knowledge, that the G12/13-Rho pathway is involved in Wnt signaling.
One intriguing missing link between Wnt signaling and the G12/13-Rho pathway is the role of phosphatidylinositol 4-kinase (PI4K), phosphatidylinositol 4-phosphate 5-kinase (PIP5K), and their product, phosphatidylinositol 4,5-phosphate (PIP2). PI4K, together with PIP5K, rapidly produces PIP2 after Wnt stimulation and enhances the phosphorylation of LRP5/6 and oligomerization of Dishevelled and LRP5/6 (Pan et al., 2008). Proteomic analysis also showed that Dishevelled interacts with PI4K, Rho, and even Gα13, after Wnt stimulation (Yokoyama, 2012). In addition, trafficking of PI4K and PIP5K to the plasma membrane is regulated by Rho GTPase (Weernink et al., 2004; Yang et al., 2004). Further study is needed to elucidate whether LGR5 enhances Wnt signaling through the G12/13-Rho pathway, as well as the RSPO-dependent pathway.
It is interesting to speculate about which functions in stem cells are regulated by LGR5 through the G12/13-Rho pathway. A decade ago, there was an intriguing report showing that mesenchymal stem cell shape, regulated by the RhoA-ROCK pathway, determines the commitment of the stem cell’s lineage to adipocytes or osteoblasts (McBeath et al., 2004). Stem cells decide the fate of daughter cells at the time of cell division, so the LGR5-Rho pathway might play a role in lineage commitment, as well as self-renewal of adult stem cells. We have also shown here that RSPOs are not ligands for the LGR5-G12/13-Rho pathway. In other words, RSPOs may be biased ligands for the Wnt pathway. Over the last decade, a variety of biased GPCR ligand have been discovered that selectively activate either G proteins or β-arrestins (Whalen et al., 2011). For example, CCL3, CCL4, CCL5, and CCL3L1, four different CCR5 ligands, show a relative bias for the processes of inositol-1- phosphate production and CCR5 internalization. In this case, CCL3L1 is 32.4-fold more active than CCL3 in inducing CCR5 internalization (Kenakin and Christopoulos, 2013). In addition, many synthetic compounds targeting GPCRs have a biased effect on G protein signaling and the β-arrestin pathway (Kenakin and Christopoulos, 2013). When a ligand biased toward LGR5-G12/13-Rho signaling is discovered, it will be a useful tool to elucidate the physiological relevance of this pathway.
Supplementary Material
Acknowledgments
This work was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (former Education, Science and Technology) (2010-0009162), and the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164:S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–1696. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Benitah SA, Valeron PF, Lacal JC. ROCK and nuclear factor-kappaB-dependent activation of cyclooxygenase- 2 by Rho GTPases: effects on tumor growth and therapeutic consequences. Mol. Biol. Cell. 2003;14:3041–3054. doi: 10.1091/mbc.E03-01-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian D, Mahanivong C, Yu J, Frisch SM, Pan ZK, Ye RD, Huang S. The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene. 2006;25:2234–2244. doi: 10.1038/sj.onc.1209261. [DOI] [PubMed] [Google Scholar]
- Buch TR, Biebermann H, Kalwa H, Pinkenburg O, Hager D, Barth H, Aktories K, Breit A, Gudermann T. G13-dependent activation of MAPK by thyrotropin. J Biol Chem. 2008;283:20330–20341. doi: 10.1074/jbc.M800211200. [DOI] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. Rspondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Lin Q, Gong X, Thomas A, Liu Q. LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/beta-catenin signaling. Mol Cell Biol. 2012;32:2054–2064. doi: 10.1128/MCB.00272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen B, Selvaraj A, Burgess RC, Hitzler JK, Ma Z, Morris SW, Prywes R. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol Cell Biol. 2003;23:6597–6608. doi: 10.1128/MCB.23.18.6597-6608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277:12463–12473. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- Civelli O, Reinscheid RK, Zhang Y, Wang Z, Fredriksson R, Schioth HB. G protein-coupled receptor deorphanizations. Ann Rev Pharmacol Toxicol. 2013;53:127–146. doi: 10.1146/annurev-pharmtox-010611-134548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- Fan XS, Wu HY, Yu HP, Zhou Q, Zhang YF, Huang Q. Expression of Lgr5 in human colorectal carcinogenesis and its potential correlation with beta-catenin. Int J Colorectal Dis. 2010;25:583–590. doi: 10.1007/s00384-010-0903-z. [DOI] [PubMed] [Google Scholar]
- Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/betacatenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Liang SG, Hsueh AJ. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol. 1998;12:1830–1845. doi: 10.1210/mend.12.12.0211. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol. 2000;14:1257–1271. doi: 10.1210/mend.14.8.0510. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Shin TJ, Choi SH, Cho HJ, Lee BH, Pyo MK, Lee JH, Kang J, Kim HJ, Park CW, et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G proteincoupled lysophosphatidic acid receptors with high affinity. Mol. Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi T, Sakata K, Yoshizaki K, Tago K, Mizuno N, Itoh H. Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J Biol Chem. 2008;283:14469–14478. doi: 10.1074/jbc.M708919200. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Ann Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107–114. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Bouley DM, Sudo S, Klein CA, Zhang JV, Kawamura K, Goodrich LV, Rayburn H, Tessier-Lavigne M, Hsueh AJ. Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol Endocrinol. 2004;18:2241–2254. doi: 10.1210/me.2004-0133. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Medjkane S, Perez-Sanchez C, Gaggioli C, Sahai E, Treisman R. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat Cell Biol. 2009;11:257–268. doi: 10.1038/ncb1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdek KD, Jaffe AB, Dutt P, Olson MF, Hall A, Fanburg BL, Kayyali US, Toksoz D. Alpha(E)-Catenin induces SRF-dependent transcriptional activity through its C-terminal region and is partly RhoA/ROCK-dependent. Biochem Biophys Res Commun. 2008;366:717–723. doi: 10.1016/j.bbrc.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner S, Perona R, Saniger L, Lacal JC. Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. doi: 10.1074/jbc.273.21.12779. [DOI] [PubMed] [Google Scholar]
- Morita H, Mazerbourg S, Bouley DM, Luo CW, Kawamura K, Kuwabara Y, Baribault H, Tian H, Hsueh AJ. Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Mol Cell Biol. 2004;24:9736–9743. doi: 10.1128/MCB.24.22.9736-9743.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LK, Rozengurt E. Galpha12 and Galpha13 stimulate Rho-dependent tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130 Crk-associated substrate. J Biol Chem. 1998;273:14626–14632. doi: 10.1074/jbc.273.23.14626. [DOI] [PubMed] [Google Scholar]
- Pan W, Choi SC, Wang H, Qin Y, Volpicelli-Daley L, Swan L, Lucast L, Khoo C, Zhang X, Li L, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008;321:1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona R, Esteve P, Jiménez B, Ballestero RP, Ramón y Cajal S, Lacal JC. Tumorigenic activity of rho genes from Aplysia californica. Oncogene. 1993;8:1285–1292. [PubMed] [Google Scholar]
- Ruffner H, Sprunger J, Charlat O, Leighton-Davies J, Grosshans B, Salathe A, Zietzling S, Beck V, Therier M, Isken A, et al. R-Spondin potentiates Wnt/beta-catenin signaling through orphan receptors LGR4 and LGR5. PLoS One. 2012;7:e40976. doi: 10.1371/journal.pone.0040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- Schugar RC, Robbins PD, Deasy BM. Small molecules in stem cell self-renewal and differentiation. Gene Ther. 2008;15:126–135. doi: 10.1038/sj.gt.3303062. [DOI] [PubMed] [Google Scholar]
- Schuijers J, Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31:2685–2696. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segain JP, Raingeard de la Bletiere D, Sauzeau V, Bourreille A, Hilaret G, Cario-Toumaniantz C, Pacaud P, Galmiche JP, Loirand G. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology. 2003;124:1180–1187. doi: 10.1016/s0016-5085(03)00283-x. [DOI] [PubMed] [Google Scholar]
- Shepard LW, Yang M, Xie P, Browning DD, Voyno-Yasenetskaya T, Kozasa T, Ye RD. Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus involve G alpha(13) and RhoA. J Biol Chem. 2001;276:45979–45987. doi: 10.1074/jbc.M104783200. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- Torsoni AS, Marin TM, Velloso LA, Franchini KG. RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H1488–1496. doi: 10.1152/ajpheart.00692.2004. [DOI] [PubMed] [Google Scholar]
- Van Schoore G, Mendive F, Pochet R, Vassart G. Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem Cell Biol. 2005;124:35–50. doi: 10.1007/s00418-005-0002-3. [DOI] [PubMed] [Google Scholar]
- Weernink PA, Meletiadis K, Hommeltenberg S, Hinz M, Ishihara H, Schmidt M, Jakobs KH. Activation of type I phosphatidylinositol 4-phosphate 5-kinase isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J Biol Chem. 2004;279:7840–7849. doi: 10.1074/jbc.M312737200. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SA, Carpenter CL, Abrams CS. Rho and Rho-kinase mediate thrombin-induced phosphatidylinositol 4- phosphate 5-kinase trafficking in platelets. J Biol Chem. 2004;279:42331–42336. doi: 10.1074/jbc.M404335200. [DOI] [PubMed] [Google Scholar]
- Yokoyama N. Proteomic analysis of Wnt-dependent dishevelled-based supermolecular complexes, Proteomics - Human diseases and protein functions. 2012. pp. 189–218. Prof. T.K. Man, (InTech)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.