Abstract
CD137 is a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Interaction of CD137 with its ligand (CD137L) affects the apoptosis, proliferation and differentiation of immune cells. Interestingly, the CD137 receptor/ligand system involves the bi-directional transduction of signals. The expression of CD137 and its ligand is not restricted to immune organs, but can also be detected in a wide variety of tissues such as the brain, kidney, lung and heart. However, its role in brain is largely unknown. This study was performed to determine the role of CD137L reverse signaling in the apoptosis of neural stem cells. We identified the expression of CD137 and its ligand in C17.2 neural stem cells derived from mouse embryonic cerebellum. We found that the activation of CD137L reverse signaling by CD137 resulted in a decrease in cell adhesion to the fibronectin-coated culture basement, thus causing detachment-induced cell death. Furthermore, we showed that the cell death induced by CD137 was completely ameliorated by integrin activators and caspase inhibitors. Therefore we suggest that CD137L reverse signaling exerts a pro-apoptotic effect by suppressing integrin-mediated survival signals in neural stem cells.
Keywords: anoikis, CD137L reverse signal, cell adhesion, integrin-fibronectin interaction, neural stem cells
INTRODUCTION
CD137 (4-1BB) is a cytokine receptor that is a member of the tumor necrosis factor receptor (TNFR) superfamily, a group of cysteine-rich, cell surface molecules (Kwon et al., 1987; 2000). The interaction of CD137 with its ligand, CD137 ligand (CD137L), has been shown to play a crucial role in immune response (Pollok et al., 1993). For example, the cross-linking of CD137 by its ligand or by an agonistic antibody transmits a distinct and potent co-stimulatory signal. Proliferation, survival, cytokine production and cytotoxic killing activity are profoundly enhanced by CD137 signaling (Melero et al., 1997; Shuford et al., 1997).
Interestingly, the CD137 receptor/ligand system involves the bi-directional transduction of signals, a property shared with several other members of the TNF receptor/ligand family (Croft, 2003; Park et al., 2012). Reverse signaling through the CD137L (CD137L reverse signaling from hereon) activated by CD137 inhibits the proliferation of T lymphocytes and induces the cell death of oligodendrocytes (Senthilkumar and Lee, 2009; Yeo et al., 2012).
Though two studies have reported the presence of CD137 and CD137L in the central nervous system (CNS) (Reali et al., 2003; Yeo et al., 2012), very little is known about their functions in brain cells. This study was performed to determine the role of CD137 and CD137L in the CNS using C17.2, a neural stem cell (NSC) line.
NSCs are undifferentiated cells that symmetrically divide to generate identical cells and asymmetrically divide to produce progenitor cells giving rise to different cell types such as neurons and glia (Kriegstein and Alvarez-Buylla, 2009). NSCs shape the structural and functional layout of the brain in the developing CNS and continue to proliferate and generate new neurons in several areas of the adult brain including the subventricular zone and the dentate gyrus (Kokovay et al., 2008). This process of self-renewal plays a key role in the development as well as the maintenance of adult tissues. The regulation of this process is tightly coordinated and requires multiple pathways involved in the regulation of proliferation, apoptosis and the maintenance of the undifferentiated phenotype (Molofsky et al., 2004).
In this study, we tested the effects of activating CD137L reverse signaling by CD137 on the cellular responses in murine NSCs. We found that CD137L reverse signaling functions as a negative regulator of cell survival by inhibiting integrin-mediated cell attachment to fibronectin. Furthermore, we showed that the cell detachment from fibronectin by the activation of CD137L reverse signaling was completely compensated by integrin activators such as Mn2+ and dithiothreitol (DTT). Our data suggest that CD137L reverse signaling plays a causative role in the inhibition of the survival signal generated by integrinfibronectin interactions in NSCs.
MATERIALS AND METHODS
Cell culture and reagents
C17.2 cells were grown in DMEM supplemented with high glucose (4.5 g/L), 10% fetal bovine serum (FBS) and 5% horse serum at 37°C in a humidified atmosphere with 5% CO2 and 95% air. Fibronectin, laminin and poly-L-lysine were purchased from Sigma-Aldrich (USA). Z-DEVD-fmk (caspase 3 inhibitor) and Z-IETD-fmk (caspase 8 inhibitor) were purchased from Calbiochem (USA).
Flow cytometry (FACs)
The expression of cell-surface CD137 and CD137L were determined by FACs analysis. C17.2 cells were grown to confluence on 6-well plates. Cells were suspended in phosphate buffered saline (PBS)-EDTA buffer and washed in PBS. Cells were stained with the phycoerythrin (PE)-conjugated CD137 (eBiosciences, USA), CD137L (eBioscience) antibodies and isotype control antibodies (rat IgG2a) (eBioscience) in PBS containing 10% FBS for 1 h at 4°C and were washed again with PBS. Data were acquired using FACSCalibur (BD bioscience, USA) with CellQuest software (BD biosciences).
Activation of CD137L reverse signaling
To activate CD137L reverse signaling, we treated C17.2 cells with plate-bound CD137-Fc protein as previously described (Saito et al., 2004). Briefly, to immobilize recombinant CD137-Fc protein (Adipogen, Korea) or human Fc fragment (IgG-Fc) (Millipore, USA) on culture plates, IgG-Fc or CD137-Fc were incubated in 96-well plates or 60 mm dishes at 37°C for 1 h in a CO2 incubator and the wells were rinsed twice with PBS.
Cell adhesion assay
Assays to determine cell adhesion were performed as previously described (Mould et al., 1995). Extra cellular matrix (ECM) molecules such as fibronectin, laminin and poly-L-lysine, as well as IgG-Fc or CD137-Fc in various concentrations were coated on 96-well tissue culture dishes for 1–2 h at room temperature (RT). Wells were then blocked with 3% bovine serum albumin (BSA) in PBS for 2 h at RT. C17.2 cells were trypsinized, washed with DMEM and plated onto the prepared culture dishes. After 60 min of incubation, plates were washed three times with PBS before fixation. Fixed cells were stained with neutral red and counted. To inhibit integrin action, Mn2+ and DTT was added to the cell suspension before plating onto the culture dishes for the adhesion assays. Counted cells were visualized by using a Zeiss inverted microscope and a Canon Powershot digital camera.
Viability assays
C17.2 cells were plated onto 96-well plates coated with CD137-Fc protein or human IgG-Fc protein. After 24 h incubation, cell viability was measured using a CellTiter 96 Non-Radioactive Cell Proliferation Assay kit (Promega, USA) according to manufacture’s instructions. Briefly, MTS and phenazine methosulfate (PMS) were appropriately mixed and added to the cell culture medium. The plates were then incubated at 37°C in a humidified atmosphere for 1 h. Absorption readings were performed at 490 nm using a spectrophotomer. To determine the population of live and dead cells, cells were stained with trypan blue (Sigma-Aldrich). Live cell numbers were estimated by subtracting the trypan-blue-positive dying cell number from the total cell number.
Western blotting
Cells were homogenized in M-PER lysis buffer (Pierce Chemical CO., USA) containing a protease inhibitor cocktail (1 mM PMSF, 10 μg/ml leupeptin, and 3 mM aprotinin) and 1 mM sodium orthovanadate. Extracted protein (10–30 μg) was separated by SDS-polyacrylamide gel electrophoresis and was transferred to a nitrocellulose membrane by electrophoretic transfer. The membrane was incubated with rabbit anti-phospo-Akt (Ser473) antibody (Cell Signaling Technology Inc., USA), rabbit anti-Akt antibody (Cell Signaling), rabbit anti-phosphofocal adhesion kinase (FAK) (Tyr397) antibody (Sigma-Aldrich), and mouse anti-FAK antibody (Santa Cruz Biotechnology, USA). Immunoreactivity was detected with an enhanced chemiluminescence kit (Amersham Biosciences, UK).
P53 luciferase reporter assays
To analyze the transcriptional activity of p53, the p53-TA-luciferase reporter (Stratagene, USA) containing p53 response elements (tandem repeats of p53 enhancer element) and the β-galactosidase reporter plasmid (pCMV-β-gal; Clontech, USA) were cotransfected into C17.2 cells using Lipofectamine/PLUS (Invitrogen Life Technologies, USA). Cells were detached from culture dishes at 24 h after transfection, re-plated onto dishes coated with fibronectin and/or CD137-Fc protein and cultured under serum-starved conditions for 12 h. Both the attached and detached cells were then harvested and used for luciferase and β-galactosidase assays, as previously reported (Kim et al., 2002).
Statistical analysis
The Student’s t-test was used for comparison of the two groups. Differences among more than three groups were analyzed by one-way ANOVA with Dunnett’s multiple comparison post-hoc tests. Values of P < 0.05 were considered statistically significant.
RESULTS
Activation of CD137L reverse signaling decreased attachment of C17.2 cells
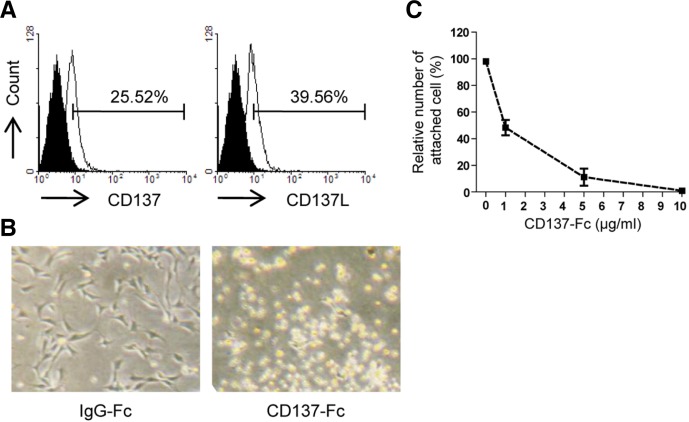
To investigate whether NSCs express CD137 and CD137L, we performed FACs analysis. As shown in Fig. 1A, both CD137 and CD137L were constitutively expressed in C17.2 cells, NSCs derived from the mouse cerebellum (Snyder et al., 1992).
Fig. 1.
Inhibition of cell attachment by CD137L reverse signaling. (A) Cell surface expression levels of CD137 and CD137L were detected by FACs. C17.2 cells were stained by PE-conjugated antibodies against CD137 (left, open curve), CD137L (right, open curve) or their isotype control antibodies (filled curve). Numbers in panels indicate the percentages of CD137- and CD137L-positive cells. Positive cells were defined as having a higher fluorescence signal than 95% of the cells stained by the isotype control. (B) Representative images showing morphological change of C17.2 cells cultured in the CD137-Fc-(10 μg/ml) or human IgG-Fc-(10 μg/ml) coated culture dishes. (C) Quantification of the number of attached cells using a cell adhesion assay.
To determine the physiological role of CD137/CD137L in NSCs, C17.2 cells were seeded onto plates coated with CD137-Fc protein (10 μg/ml). This CD137-Fc fusion protein was immobilized on tissue culture plates to allow it to cross link CD137L and thereby induce CD137L reverse signaling in the C17.2 cells, as previously reported (Yeo et al., 2012). Uncoated plates (data not shown) or plates coated with human IgG-Fc protein were used as negative controls. Interestingly, CD137-Fc protein induced C17.2 cells with round morphologies to float up from the culture basement, in contrast to the control cells treated with human IgG-Fc protein (Fig. 1B). However, the treatment of cells with soluble CD137-Fc had no effect on cell viability and cellular adherence to the culture dishes (data not shown), as previously reported in immune cells (Jiang et al., 2008).
To further investigate the inhibitory effect of CD137-Fc on the attachment of cells to the culture basement, we performed cell adhesion assays using C17.2 cells in the culture dish coated with CD137-Fc protein. The coated CD137-Fc protein induced the inhibition of cell adhesion to the culture basement in a dose-dependent manner (Fig. 1C). These results indicate that a functional CD137 signal inhibits cell adhesion through the activation of CD137L reverse signaling in NSCs.
Activation of CD137L reverse signaling-induced cell death
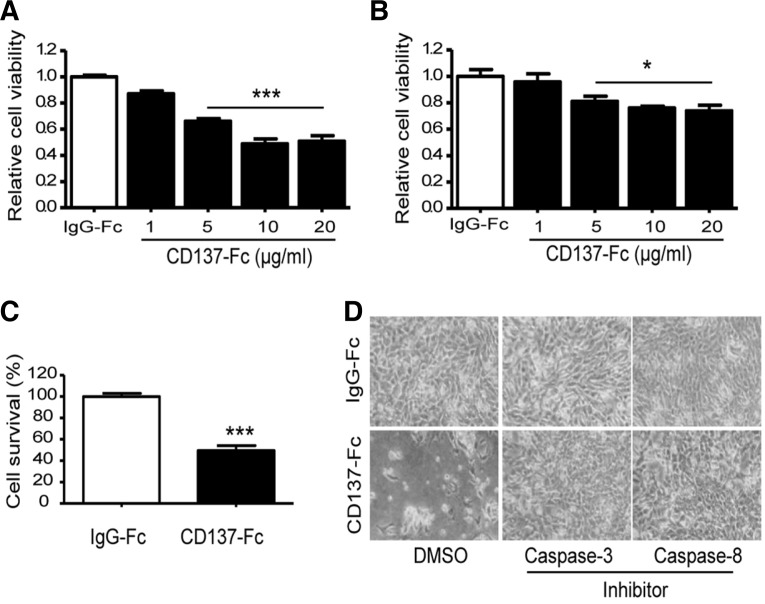
Many types of cells undergo apoptotic death when they are detached from the culture dish (Grossmann, 2002). We therefore investigated whether the activation of CD137L signaling could induce a death process in C17.2 cells. As shown in Fig. 2A, CD137-Fc protein coated onto culture dishes significantly decreased the viability of cells in serum-free media, resulting in the death of more than 50% of the cells 24 h after incubation. However, CD137-Fc protein induced only a minor decrease in cell viability in the complete media (Fig. 2B), suggesting that serum constituent(s) presumably act as survival factors to rescue the cells from death signals transmitted via CD137L reverse signaling.
Fig. 2.
Effect of CD137-Fc protein on cell death. (A, B) Cell viability after incubation with CD137-Fc. Trypsinized C17.2 cells were plated in CD137-Fc-coated (indicated concentration) dishes and cultured in the serum free medium (A) and complete medium (B). Each value represents mean ± SEM (n = 9). *p < 0.05; ***p < 0.001 versus human IgG-Fc-coated (10 μg/ml) controls. (C) Quantification of the relative number of live cells on the CD137-Fc-coated (10 μg/ml) plates. Cells were stained using trypan blue. Each value represents mean ± SEM (n = 9). ***p < 0.001 versus human IgG-Fc-coated control. (D) Representative images showing effect of caspase inhibitors on the CD137-Fc-induced changes in the morphology and number of attached C17.2 cells 48 h after plating. Cells were pretreated with 40 μM of a caspase 3 inhibitor (Z-DEVD-fmk) or 10 μM of a caspase 8 inhibitor (Z-IETD-fmk) at 30 min before being plated onto the CD137-Fc-coated dishes.
To reconfirm the cell death induced by CD137-Fc protein, live cells were counted using trypan blue staining. CD137-Fc protein significantly decreased the live cell numbers compared with the control human IgG group (Fig. 2C). However, the treatment of cells with soluble CD137-Fc had no effect on cell death or survival (data not shown), as it induced no significant changes in cell adherence.
To address if the activation of CD137L reverse signaling induced cell death via the action of caspase, a well-known death receptor-induced apoptotic pathway (Ceccatelli et al., 2004; Thorbum, 2004), we pretreated specific inhibitors of caspase 3 (Z-DEVD-fmk) and 8 (Z-IETD-fmk) in the suspended CD17.2 cells before seeding the cells in the CD137-Fc coated dishes. As shown in Fig. 2D, pretreatment with caspase 3 and caspase 8-specific inhibitors resulted in an increase in the number of attached cells on the plates coated with CD137-Fc protein after 48 h, which is essential for cell survival (Nicholson, 1999). These results suggest that CD137L reverse signaling is related with the death receptor-mediated cell death pathway.
Effect of CD137-Fc on cell attachment to ECM protein-coated plates
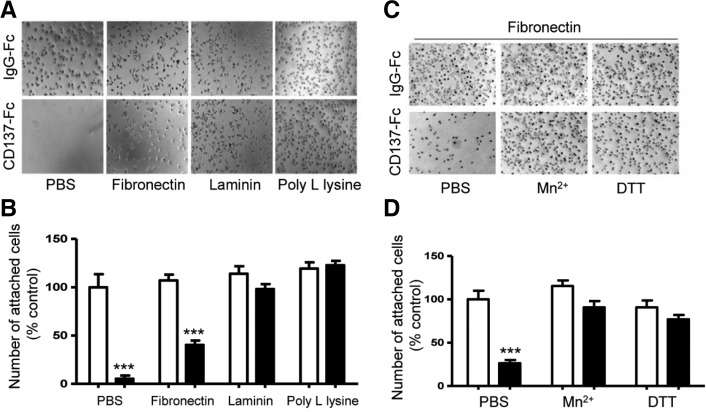
Next, we investigated the molecular basis of CD137-Fc protein-induced cell detachment. We first tested whether interference with cell-matrix interactions could account for this phenotype. Interestingly, CD137-Fc protein coated onto culture dishes markedly inhibited the adhesion of C17.2 cells to the plates coated with PBS and fibronectin, but not to the plates coated with laminin and poly-L-lysine (Figs. 3A and 3B). Thus, the activation of CD137L reverse signaling by CD137-Fc protein exerts a rather specific inhibition of the binding of cells with certain ECMs such as fibronectin.
Fig. 3.
Effect of CD137-Fc protein on the detachment of cells from ECM protein-coated plates. (A, B) C17.2 cells were plated on the culture dishes coated with fibronectin (1 μg/ml), laminin (40 μg/ml), and poly-L-lysine (100 μg/ml) in the presence of human IgG-FC (open bar) (10 μg/ ml) or CD137-Fc (filled bar) (10 μg/ml). The cells were then cultured in the serum free medium for 60 min before cell adhesion assays. (A) Representative figures showing the effect of ECM proteins on the CD137-Fc-induced detachment of cells from culture dishes. (B) Relative number of attached cells shown in (A). Each value represents mean ± SEM (n = 9). ***P < 0.001 versus human IgG-Fc-coated control. (C, D) C17.2 cells were plated onto fibronectin-coated (1 μg/ml) dishes in the presence of human IgG-Fc (open bar, 10 μg/ml) or CD137-Fc (filled bar, 10 μg/ml). Integrin activators such as Mn2+ (1 mM) and DTT (10 mM) were added into the cell suspensions for 30 min before plating. (C) Representative figures showing the effect of Mn2+ and DTT on the CD137-Fc-induced detachment of cells from fibronectin-coated dishes. (D) Relative number of attached cells shown in (C). Each value represents mean ± SEM (n = 9). ***p < 0.001 versus human IgG-Fc-coated control.
Integrins are well-known cellular membrane receptors for interaction with ECMs (Boudreau and Jones, 1999). Thus, we examined whether CD137L reverse signaling is involved in integrin-mediated binding to ECMs using activators of integrin action such as Mn2+ and DTT, which are known to increase the affinity of integrins for their ligand ECMs and thus maintain high-affinity interactions between them (Ni et al., 1998; Stuiver and O’Toole, 1995). Pre-treatment with Mn2+ and DTT abolished the inhibitory effect of CD137-Fc on the attachment of cells to the fibronectin-coated plates (Figs. 3C and 3D), further suggesting that the activation of CD137L reverse signaling specifically inhibits cell attachment by affecting the interaction between integrin and fibronectin.
Effect of CD137-Fc on the intracellular signaling molecules involved in attachment-induced cell survival
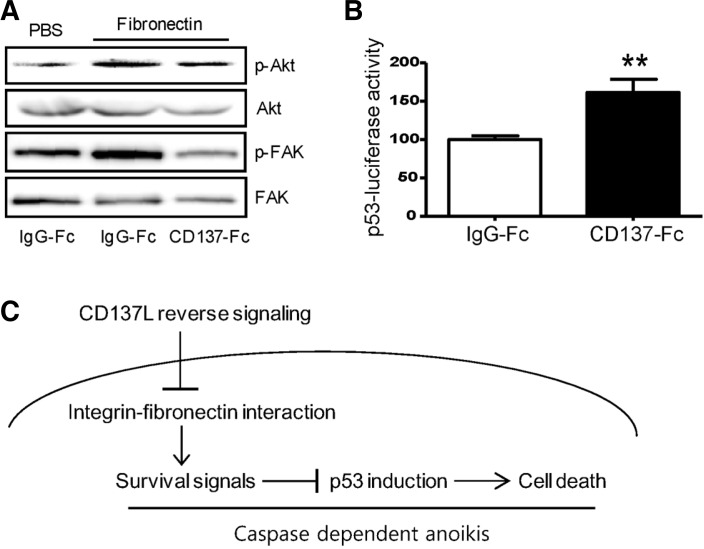
To further demonstrate that CD137L reverse signaling affects integrin-mediated cell adhesion and its associated pro-survival signaling, we assessed whether downstream components of the integrin signaling pathway are altered by CD137-Fc protein. FAK and Akt are phosphorylated when integrin is activated by ECM substrates (Hu and Luo, 2012). Interestingly, the activation of CD137L reverse signaling by CD137-Fc significantly suppressed fibronectin-induced phosphorylation of FAK and Akt in C17.2 cells compared with the human IgG-Fc treated group (Fig. 4A). Furthermore, the luciferase activity of the p53 reporter constructs was significantly increased even with a low dose of CD137-Fc protein (1 μg/ml) that resulted in only a minor decrease in cell survival (Fig. 2A). We were unable to measure luciferase activity using higher concentrations of CD137-Fc protein because these high concentrations along with the transfection process of the reporter vectors caused massive cell death. These results suggest that CD137L reverse signaling inhibits survival signal cascades triggered by integrin-fibronectin interactions (Fig. 4C).
Fig. 4.
Effect of CD137-Fc on the intracellular signaling molecules involved in attachment-induced cell survival. (A) Representative autoradiograms showing the effect of CD137-Fc on the phosphorylation of FAK and Akt. C17.2 cells were plated onto PBS or fibronectin-coated dishes in the presence of PBS, human IgG-Fc (10 μg/ml), or CD137-Fc (10 μg/ml) and cultured in serum free media for 30 min. Phosphorylation of FAK and Akt was detected by Western blot analysis. (B) Effect of CD137-FC on p53 reporter luciferase activity. C17.2 cells were transfected with p53-responsive elements conjugated with luciferase reporter plasmids and incubated for 24 h and then cultured in the CD137-Fc-coated (1 μg/ml) dishes for 12 h. The cells were harvested at the end of the incubation and subject to luciferase and β-galactosidase assays. Each value represents mean ± SEM (n = 6). **p < 0.01. (C) A model illustrating the mechanism by which CD137L reverse signaling induces apoptosis.
DISCUSSION
It has previously been reported that CD137 is expressed in a wide range of cell types including monocytes (Langstein et al., 1998), epithelial cells (Schwarz et al., 1995) and hematopoietic stem cells (Jiang et al., 2008). Recently, several reports have also shown that brain cells constitutively express CD137 and its ligand (Reali et al., 2003; Yeo et al., 2012). Here, we report that both CD137 and CD137L are expressed in C17.2 NSCs, and that CD137L reverse signaling is functional, as indicated by the activation of cell death signaling following ligation with CD137-Fc protein. We also found that CD137L reverse signaling decreased the attachment of C17.2 cells and induced cell death via the inhibition of integrin-mediated survival signals. These data suggest that CD137L reverse signaling plays a physiological role in detachment-induced cell death in NSCs.
In this study, we demonstrated that the stimulation of CD137L reverse signaling using CD137-Fc protein promotes apoptosis via the caspase pathway, consistent with the apoptotic role of CD137 on hematopoietic stem cells (Gullo et al., 2010). However, of particular interest is the finding that ligation of CD137 inhibits cell adhesion via the integrin signal pathway in NSCs. Previous studies have shown that integrin-mediated interactions with the ECM can induce pro-survival signals, whereas detachment from matrix proteins results in rapid apoptotic death in many cell types (Grossmann et al., 2002). Interestingly, in C17.2 cells, fibronectin-dependent adhesion is eliminated by CD137-Fc protein, but laminin-dependent adhesion is essentially unaffected. Fibronectin-dependent adhesion is mediated by integrin α5β1 (Wu et al., 1995). Therefore, the inhibitory effect of CD137 to cell adhesion is specific for certain integrin signaling pathways such as α5β1.
The loss of proper integrin-ligand interactions can lead to apoptosis. Anoikis, or apoptosis resulting from detachment from the ECM, is associated with the activation of caspase-8 or p53 (Frisch and Screaton, 2001; Grossmann, 2002), although a caspase-independent mechanism of anoikis also exists (Janes and Watt, 2004). Indeed, a specific caspase-8 inhibitor effectively rescued the neuroblastoma cells from anoikis (Bozzo et al., 2006), and furthermore, it attenuated the effect of CD137-Fc on the detachment of the cells in this study. Thus, the apoptosis-promoting caspase pathway seems to be closely related to the detachment-induced apoptosis, whereas it is unclear that the activation of caspase precedes the detachment of cells from ECM.
p53 is a transcription factor that responds to aberrant cell growth by inducing cell cycle arrest or apoptosis (Jin and Levine, 2001). Active p53 performs sequence-specific transactivation of several components in the apoptotic pathway. p21 and Bcl-2-associated X protein (Bax) are two primary p53-responsive genes (Haupt et al., 2003). The upregulation of Bax tends to induce apoptosis through the formation of monodimers that release cytochrome c from the mitochondria and activate caspase-9 (Cory and Adam, 2005). The down-regulation of FAK and Akt is associated with cell death induced by the detachment of cells from the ECM, as well as the induction of p53, which has been implicated in detachment-induced death in serum-starved conditions (Ilic et al., 1998). FAK is a well-known activator of the phosphatidylinositol 3-kinase Akt cell survival pathway (Xia et al., 2004). Furthermore, integrin activation promotes FAK phosphorylation and activity (Stupack et al., 2002). FAK activation up-regulates Akt and inhibits p53-regulated apoptosis (Ilic et al., 1998). Therefore, cell attachment to fibronectin suppresses this apoptosis through the activation of FAK (Almeida et al., 2000). The net effect on the intracellular environment is pro-survival. In this study, we demonstrated that the activation of integrin blocks the ability of CD137 to trigger cell detachment, and inhibitors of caspases block the ability of CD137 to regulate cell death. These results suggest that CD137-induced cell death is brought about through detachment-induced cell death or an anoikis mechanism.
Although several studies have reported an important contribution of the CD137L reverse signaling in the inflammatory response and cell death (Gullo et al., 2010; Shao and Schwarz, 2011), its role in the brain pathophysiology such as neuroinflammation-induced apoptosis has not yet been reported. Interestingly, CD137 null mutant mice have exhibited reduced apoptotic cell death in their newly generated neuroprogenitor cells in the hippocampal dentate gyrus (Yun and Lee, unpublished data), suggesting that the interaction between CD137-CD137L is important for the regulation of neural cell apoptosis.
The CNS contains multi-potent NSCs that are responsible for neurogenesis in specific regions, known as stem cell niches (Massirer et al., 2011). Though little is currently known about the in vivo function of integrins and ECMs in the NSC niche, in vitro data indicate that ECMs such as laminin and fibronectin are involved in the maintenance of NSCs via different integrin heterodimers (Campos, 2005). For example, fibronectin promotes survival and migration of NSCs via α5β1 integrin signal pathway (Tate et al., 2002). Further studies are required to clarify the role of CD137-CD137L in the interaction between integrins and ECMs in the NSC niches.
In this study, we showed that CD137L reverse signaling induced apoptosis by inhibiting cell adhesion in NSCs. We propose that the suppression of cell adhesion and the blockage of ECM survival signals might be specific mechanisms of apoptosis induced by CD137 reverse signaling (Fig. 4C).
Taken together, the results show that CD137L reverse signaling functions as a cell death inducer by modulating the integrin-FAK-Akt-p53 pathway in NSCs. CD137L reverse signaling also plays a pro-apoptotic role through the p53 transcription pathway in NSCs. By demonstrating the involvement of the CD137 receptor/ligand system in NSCs, this study raises the implication that CD137-CD137L activity may contribute to neurodegenerative diseases.
Acknowledgments
This work was supported by the Research Fund of University of Ulsan.
REFERENCES
- Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem. J. 1999;339(Pt 3):481–488. [PMC free article] [PubMed] [Google Scholar]
- Bozzo C, Sabbatini M, Tiberio R, Piffanelli V, Santoro C, Cannas M. Activation of caspase-8 triggers anoikis in human neuroblastoma cells. Neurosci Res. 2006;56:145–153. doi: 10.1016/j.neures.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Campos LS. Beta1 integrins and neural stem cells: making sense of the extracellular environment. Bioessays. 2005;27:698–707. doi: 10.1002/bies.20256. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Tamm C, Sleeper E, Orrenius S. Neural stem cells and cell death. Toxicol Lett. 2004;149:59–66. doi: 10.1016/j.toxlet.2003.12.060. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. Killing cancer cells by flipping the Bcl-2/Bax switch. Cancer Cell. 2005;8:5–6. doi: 10.1016/j.ccr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Grossmann J. Molecular mechanisms of “detachment-induced apoptosis--Anoikis”. Apoptosis. 2002;7:247–260. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- Gullo C, Koh LK, Pang WL, Ho KT, Tan SH, Schwarz H. Inhibition of proliferation and induction of apoptosis in multiple myeloma cell lines by CD137 ligand signaling. PLoS One. 2010;5:e10845. doi: 10.1371/journal.pone.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis - the p53 network. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- Hu P, Luo BH. Integrin bi-directional signaling across the plasma membrane. J Cell Physiol. 2012;228:306–312. doi: 10.1002/jcp.24154. [DOI] [PubMed] [Google Scholar]
- Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes SM, Watt FM. Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol. 2004;166:419–431. doi: 10.1083/jcb.200312074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Chen Y, Schwarz H. CD137 induces proliferation of murine hematopoietic progenitor cells and differentiation to macrophages. J Immunol. 2008;181:3923–3932. doi: 10.4049/jimmunol.181.6.3923. [DOI] [PubMed] [Google Scholar]
- Jin S, Levine AJ. The p53 functional circuit. J Cell Sci. 2001;114:4139–4140. doi: 10.1242/jcs.114.23.4139. [DOI] [PubMed] [Google Scholar]
- Kim MS, Hur MK, Son YJ, Park JI, Chun SY, D’Elia AV, Damante G, Cho S, Kim K, Lee BJ. Regulation of pituitary adenylate cyclase-activating polypeptide gene transcription by TTF-1, a homeodomain-containing transcription factor. J Biol Chem. 2002;277:36863–36871. doi: 10.1074/jbc.M206443200. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Shen Q, Temple S. The incredible elastic brain: how neural stem cells expand our minds. Neuron. 2008;60:420–429. doi: 10.1016/j.neuron.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BS, Kim GS, Prystowsky MB, Lancki DW, Sabath DE, Pan JL, Weissman SM. Isolation and initial characterization of multiple species of T-lymphocyte subset cDNA clones. Proc. Natl. Acad. Sci. USA. 1987;84:2896–2900. doi: 10.1073/pnas.84.9.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B, Moon CH, Kang S, Seo SK, Kwon BS. 4-1BB: still in the midst of darkness. Mol. Cells. 2000;10:119–126. doi: 10.1007/s10059-000-0119-0. [DOI] [PubMed] [Google Scholar]
- Langstein J, Michel J, Fritsche J, Kreutz M, Andreesen R, Schwarz H. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol. 1998;160:2488–2494. [PubMed] [Google Scholar]
- Massirer KB, Carromeu C, Griesi-Oliveira K, Muotri AR. Maintenance and differentiation of neural stem cells. Wiley Interdiscip Rev Syst Biol Med. 2011;3:107–114. doi: 10.1002/wsbm.100. [DOI] [PubMed] [Google Scholar]
- Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Mould AP, Akiyama SK, Humphries MJ. Regulation of integrin alpha 5 beta 1-fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+ J Biol Chem. 1995;270:26270–26277. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- Ni H, Li A, Simonsen N, Wilkins JA. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the beta1 integrin chain. J Biol Chem. 1998;273:7981–7987. doi: 10.1074/jbc.273.14.7981. [DOI] [PubMed] [Google Scholar]
- Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- Park SJ, Kim HJ, Lee JS, Cho HR, Kwon B. Reverse signaling through the co-stimulatory ligand, CD137L, as a critical mediator of sterile inflammation. Mol. Cells. 2012;33:533–537. doi: 10.1007/s10059-012-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- Reali C, Curto M, Sogos V, Scintu F, Pauly S, Schwarz H, Gremo F. Expression of CD137 and its ligand in human neurons, astrocytes, and microglia: modulation by FGF-2. J Neurosci Res. 2003;74:67–73. doi: 10.1002/jnr.10727. [DOI] [PubMed] [Google Scholar]
- Saito K, Ohara N, Hotokezaka H, Fukumoto S, Yuasa K, Naito M, Fujiwara T, Nakayama K. Infection-induced up-regulation of the costimulatory molecule 4-1BB in osteoblastic cells and its inhibitory effect on M-CSF/RANKL-induced in vitro osteoclastogenesis. J Biol Chem. 2004;279:13555–13563. doi: 10.1074/jbc.M303791200. [DOI] [PubMed] [Google Scholar]
- Schwarz H, Valbracht J, Tuckwell J, von Kempis J, Lotz M. ILA, the human 4-1BB homologue, is inducible in lymphoid and other cell lineages. Blood. 1995;85:1043–1052. [PubMed] [Google Scholar]
- Senthilkumar R, Lee HW. CD137L- and RANKL-mediated reverse signals inhibit osteoclastogenesis and T lymphocyte proliferation. Immunobiology. 2009;214:153–161. doi: 10.1016/j.imbio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol. 2011;89:21–29. doi: 10.1189/jlb.0510315. [DOI] [PubMed] [Google Scholar]
- Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- Stuiver I, O’Toole TE. Regulation of integrin function and cellular adhesion. Stem Cells. 1995;13:250–262. doi: 10.1002/stem.5530130306. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729–3738. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- Tate MC, Shear DA, Hoffman SW, Stein DG, Archer DR, LaPlaca MC. Fibronectin promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain. Cell Transplant. 2002;11:283–295. [PubMed] [Google Scholar]
- Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004;279:33024–33034. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- Yeo YA, Martinez Gomez JM, Croxford JL, Gasser S, Ling EA, Schwarz H. CD137 ligand activated microglia induces oligodendrocyte apoptosis via reactive oxygen species. J. Neuroinflammation. 2012;9:173. doi: 10.1186/1742-2094-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]