Abstract
Midazolam is a widely used anesthetic of the benzodiazepine class that has shown cytotoxicity and apoptosis-inducing activity in neuronal cells and lymphocytes. This study aims to evaluate the effect of midazolam on growth of K562 human leukemia cells and HT29 colon cancer cells. The in vivo effect of midazolam was investigated in BALB/c-nu mice bearing K562 and HT29 cells human tumor xenografts. The results show that midazolam decreased the viability of K562 and HT29 cells by inducing apoptosis and S phase cell-cycle arrest in a concentration-dependent manner. Midazolam activated caspase-9, capspase-3 and PARP indicating induction of the mitochondrial intrinsic pathway of apoptosis. Midazolam lowered mitochondrial membrane potential and increased apoptotic DNA fragmentation. Midazolam showed reactive oxygen species (ROS) scavenging activity through inhibition of NADPH oxidase 2 (Nox2) enzyme activity in K562 cells. Midazolam caused inhibition of pERK1/2 signaling which led to inhibition of the anti-apoptotic proteins Bcl-XL and XIAP and phosphorylation activation of the pro-apoptotic protein Bid. Midazolam inhibited growth of HT29 tumors in xenograft mice. Collectively our results demonstrate that midazolam caused growth inhibition of cancer cells via activation of the mitochondrial intrinsic pathway of apoptosis and inhibited HT29 tumor growth in xenograft mice. The mechanism underlying these effects of midazolam might be suppression of ROS production leading to modulation of apoptosis and growth regulatory proteins. These findings present possible clinical implications of midazolam as an anesthetic to relieve pain during in vivo anticancer drug delivery and to enhance anticancer efficacy through its ROS-scavenging and pro-apoptotic properties.
Keywords: anesthesia, anticancer, midazolam, apoptosis induction, ROS scavenging
INTRODUCTION
Midazolam, a γ-aminobutyric acid A (GABAA) receptor agonist, is a widely used anesthetic of the benzodiazepine class. Midazolam and other anesthetic agents have shown neuronal cytotoxicity and apoptosis-inducing activity in hematogenic, ectodermal, mesenchymal and neuronal cells (Jevtovic-Todorovic et al., 2003; Sinner et al., 2011; Stevens et al., 2011; Young et al., 2005). Systemic application of midazolam together with other general anesthetics has shown apoptosis inducing activity in murine Leydig cells in vitro and in neonatal rodent neurons in vivo (So et al., 2010). Midazolam has been shown to induce apoptosis in neuroblastoma cells at low concentrations and necrosis at higher concentrations. The pro-apoptotic effects of midazolam in neuronal cells largely depend on signaling though GABAA receptor and peripheral-type benzodiazepine receptors (PBRs) (Sinner et al., 2011; So et al., 2010; Stevens et al., 2011). PBRs have been reported to regulate various functions including cellular proliferation, oxidative processes, and programmed cell death (Casellas et al., 2002; Olkkola and Ahonen, 2008). Recently midazolam has been shown to induce apoptosis in human Jurkat T-lymphoma cells that lack GABAA receptor (Stevens et al., 2011). In addition, animal studies using midazolam have shown rather contradictory neurotoxicity and apoptosis responses in sheep, rabbits, and rodents (Erdine et al., 1999; Johansen et al., 2004; Malinovsky et al., 1991; Schoeffler et al., 1991; Svensson et al., 1995; Yon et al., 2005; Young et al., 2005). Apoptosis induction is achieved through two main pathways, death receptor-dependent extrinsic or mitochondrial intrinsic. Midazolam has been found to induce apoptosis through both pathways in neuronal cells (Stevens et al., 2011; Yon et al., 2005) in addition to calcium channel blockade in Jurkat cells (Conrad et al., 2010).
In addition to its anesthetic and pro-apoptotic properties, midazolam has been reported to interfere with reactive oxygen species (ROS) production. Midazolam showed ROS scavenging activity by inhibiting the ability of human neutrophils to produce ROS (Nishina et al., 1998). Midazolam also interrupts the synthesis and release of nitric oxide and tumor necrosis factor-α by activated immune cells (Kang et al., 1998). Since the high amount of ROS generated in cancer cells favors their cellular proliferation, the antioxidant properties of midazolam might be the mechanism responsible for suppressing cancer cell proliferation. The apoptosis inducing and ROS scavenging activities of midazolam have drawn considerable attention in cancer biology. Although there is an abundance of reports on GABAA receptor-dependent neurocytotoxic functions of midazolam and its proapoptotic activity relating to cell death in neuronal cells, limited information exists on the roles of midazolam in GABAA receptor-independent cellular proliferation conditions such as cancer cells. We postulated that an anesthetic such as midazolam with antioxidant and apoptosis-inducing properties could be a promising combination therapeutic for cancer-related clinical procedures.
The aim of this study was to test whether midazolam induced apoptosis in human cancer cells in vitro and in vivo. We investigated the effect of midazolam on proliferation of human leukemia and colon cancer cells in culture and in tumor xenograft mice models. We also investigated the effect of midazolam on ROS production in these cells and attempted to elucidate the mechanism of cell death.
MATERIALS AND METHODS
Animals, cell culture and treatment
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC). Female 5-week-old BALB/c-nu mice were purchased from SLC (Hamahatsu, Japan). The K562 human leukemia and HT29 colon cancer cell lines were purchased from American Type Culture Collection (ATCC) and maintained in RPMI 1640 medium (Gibco Invitrogen, USA) supplemented with 10% fetal bovine serum (Hyclone, UT), 2 mM l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (Gibco Invitrogen) at 37°C in humidified atmosphere of 5% CO2. Midazolam solution (1 mg/ml in normal saline) was obtained from the National Cancer Center of Korea.
Cell viability assays
K562 and HT29 cells (4,000 cell/well) in 96-well plates were treated with midazolam for 48 h to observe maximal cytotoxicity. In K562 cells, WST assay for cell viability was performed using EzCytox WST assay kit (Daeil lab, Korea) according to the manufacturer’s instructions. In HT29 cells, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrasolium bromide] (Biosesang Inc., Korea) assay was performed, as described previously (Kang et al., 2011).
Flow cytometric analysis of apoptosis
K562 cells (2 × 106 cell/ml) were treated with midazolam for 18 h. Cells were double stained with annexin V/7-amino-actinomycin (7-AAD) using Annexin V-FITC Apoptosis Detection Kit II (BD Bioscience, USA) according to the manufacturer’s instructions and as described earlier (Kang et al., 2011). Stained cells were subjected to flow cytometry analysis for apoptosis using FACSCalibur (BD Bioscience, USA).
Cell cycle analysis
K562 cells (2 × 106 cells/ml) were treated with midazolam for 18 h. Cells were harvested and total 1 × 106 cells were resuspended in 1 ml of Krishan’s buffer containing 50 μg/ml propidium iodide (PI). Cells were analyzed by flow cytometry using FACSCalibur and the percentage of cells in G1/G0, S, and G2/M phases were determined by the Modfit LT program (Verity Software House, USA).
Measurement of mitochondrial membrane potential (Δψm)
K562 cells (2 × 106 cells/ml) were treated with midazolam for 18 h and then stained with using Cell Meter JC-10 Mitochondria membrane potential assay kit (AAT Bioquest, USA), as per manufacturer’s instructions. The fluorescence intensities for both J-aggregates and monomeric forms were measured at Excitation/Emission: 490/525 nm and 490/590 nm with micro-plate reader.
DNA fragmentation based apoptosis analysis
DNA fragmentation was assessed by electrophoresis of genomic DNA extracted from K562 cells as described previously (Muller et al., 1996). Briefly, K562 cells (2 × 106 cells/ml) were treated with midazolam for 18 h. Cells were homogenized with 50 μl of lysis buffer and then treated with 5 μg RNase A and 1% SDS for RNA digestion and then with 2.5 μg/μl Proteinase K for protein digestion. DNA was precipitated, dissolved in gel loading buffer, and electrophoresed on 1% agarose gel with ethidium bromide staining.
Western blot analysis
K562 and HT29 cells (2 × 106 cells/ml) were treated with midazolam for 24 h. Preparation of whole-cell lysates, protein quantification, gel electrophoresis and Western blotting were performed as described previously (Kang et al., 2011). Bands were detected using ECL Western blotting detection reagents (Ab-Frontier, Korea).
Intracellular superoxide generation assay
K562 cells (2 × 106 cells/ml) were treated with midazolam for 18 h and then stimulated with 0.3 μM phorbol-12-myristate 13-acetate (PMA) to induce generation of ROS. Five min later, cells were harvested and incubated with 10 mM of redoxsensitive dye 2’,7’-dichlorodihydrofluorescein diacetate (DCFHDA) (Invitrogen Molecular probe) for 30 min in dark at 37°C. DCFHA fluorescence was measured by flow cytometry using FACSCalibur.
In vivo mouse xenograft
In vivo antitumor effect of midazolam was observed on female BALB/c-nu mice, subcutaneously inoculated with K562 or HT29 cells (3 × 107/ml). Body weight and tumor appearance was observed daily. When tumor volumes reached 98–130 mm3, mice were randomly distributed in two groups: vehicle control (n = 5) and midazolam (n = 5). Mice were treated daily with either vehicle (normal saline) or midazolam (0.83 mg/kg body weight, i.v.) for 12 days. Tumor growth was externally measured using a digital caliper and tumor volumes were calculated using the formula (width2 × length)/2, where the width represents the smaller tumor diameter. Tumors were expatriated from mice at the end of the study and tumor weight was recorded.
Statistical analyses
All values are expressed as mean with standard deviations (SD). Statistical significance was calculated by Student’s t-test and one way analysis of variance (ANOVA) for multiple comparisons. P values < 0.05 were considered statistically significant
RESULTS
Midazolam suppressed the growth of human cancer cells
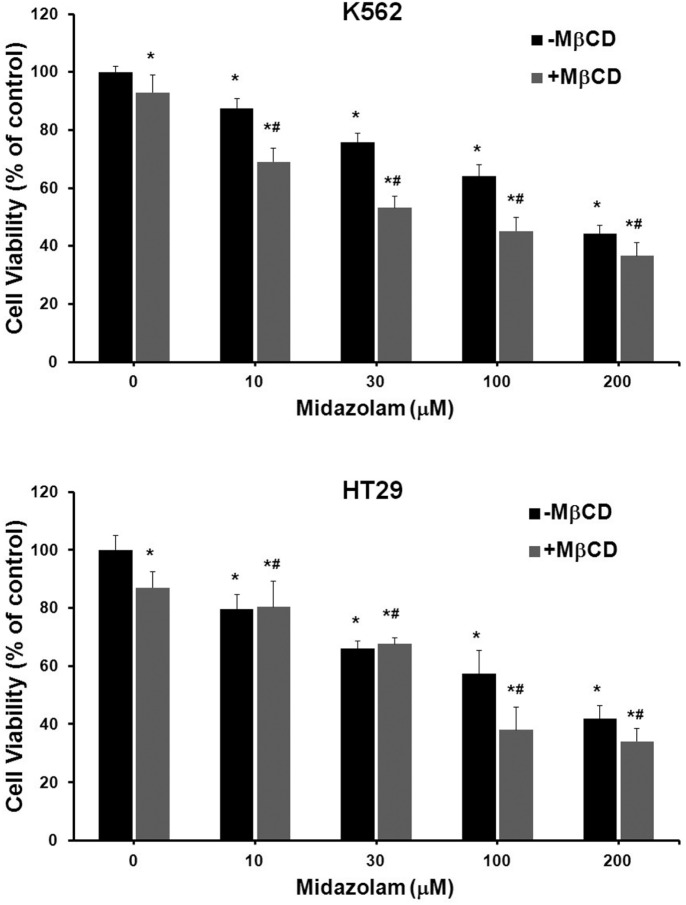
We investigated the effect of midazolam on proliferation of human cancer cell lines. Midazolam suppressed the proliferation of each cell line in a dose-dependent manner (Supplementary Table 1). The K562 human leukemia cell line and HT29 colon cancer cell line were screened for further experimental procedures. Midazolam dramatically suppressed the proliferation of K562 and HT29 cells at 100 and 200 μM (Figs. 1A and 1B). Growth-inhibitory concentrations (GI50) of midazolam were 171.5 μM and 148.5 μM for K562 and HT29 cells, respectively (Supplementary Table 1). Next, we analyzed the possible mechanisms behind the cytotoxicity of midazolam. Methyl-β-cyclodextrin (MβCD) is a cyclic oligosaccharide that depletes cholesterol from lipid raft regions of the plasma membrane. It acts by dissociating proteins from membrane lipid rafts leading to cell death (Simons and Toomre, 2000). In K562 cells, a combination of MβCD and midazolam (100 and 200 μM) suppressed 18% (P = 0.0008) and 8% (P = 0.031) of cell viability more than that of midazolam alone (Fig. 1A). In HT29 cells, this combination suppressed 20% (P = 0.013) and 11% (P = 0.11) of cell viability more than that of midazolam alone (Fig. 1B). These results showing inconsistent combined effects of MβCD with midazolam do not support an additive or synergistic effect. MβCD is reported to inhibit the growth of cells via destabilizing membrane lipid raft. The MβCD-mediated disorganization of lipid raft leads to disfunctional extrinsic apoptosis components. Since MβCD could not reverse the effects of midazolam, we assume that midazolam-induced apoptosis may not be mediated through extrinsic pathway. Consequently, these results raise in the possibility that midazolam-induced cell death may be mediated via the mitochondrial intrinsic pathway of apoptosis.
Fig. 1.
Effect of midazolam on growth of K562 and HT29 cells. K562 and HT29 cells were treated with indicated concentrations of midazolam for 48 h and cell viability was assayed as described in the Materials and Methods. In an independent set, cells were exposed to MβCD (2.5 mg/ml) in combination with midazaolam for 48 h and cell viability was estimated. Viable cells were denoted as the percentage of vehicle controls (VH). The data are expressed as means ± SD of OD values of 6-wells from 96-well plates and whole experiment was repeated three times. *P vs. VH; #P vs. VH + MβCD.
Midazolam induced apoptosis and cell cycle arrest in K562 cells
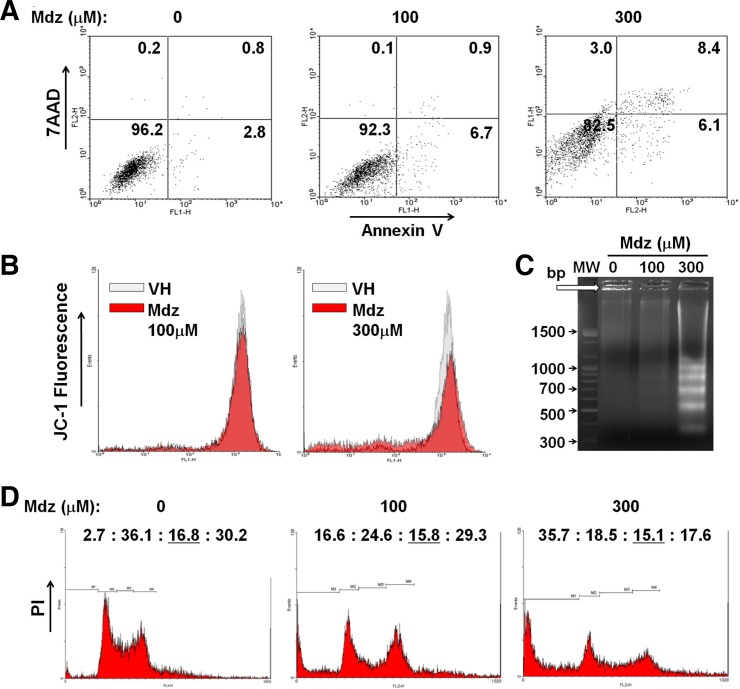
To determine whether the cytotoxic activity of midazolam involves apoptosis, K562 cells were double-stained with annexin V/7AAD and analyzed by FACS. The proportion of positively stained cells (regarded as apoptotic) was increased by treatment with midazolam in a dose-dependent manner (Fig. 2A). At 100 and 300 μM midazolam, 6–7% of cells transited to early apoptosis phase. At 300 μM midazolam, 8.4% of cells were in late apoptosis phase and a small population (3%) of cells was in necrosis phase (Fig. 2A). These results indicate that midazolam induced early apoptosis at a lower concentration (100 μM) and late apoptosis at a higher concentration (300 μM). To confirm the pro-apoptotic activity of midazolam, we measure the mitochondrial membrane potential in K562 cells. Midazolam caused depolarization of the mitochondrial membrane as reflected by the decreased percentage of JC-1 positive (high red fluorescent) staining. Midazolam lowered Δψm by approximately 20% and 40% at 100 and 300 μM, respectively (Fig. 2B). Apoptotic activity in K562 cells was also confirmed by analyzing an early apoptosis marker, DNA strand fragmentation. The band pattern on gel indicated a remarkable increase in internucleosomal DNA fragmentation (∼200 bp) at 300 μM midazolam (Fig. 2C). Furthermore, we observed the effect of midazolam on cell cycle progression in K562 cells. PI staining of midazolam-treated K562 cells revealed increased accumulation of cells in the subG1 peak (indicating apoptotic cells) in a dose-dependent manner (Fig. 2B). Analysis of the distribution of live cells in different phases of the cell cycle showed a reduced percentage of cells in G1 phase with midazolam treatment [37.1% (vehicle control), 29.5% (100 μM), and 28.8% (300 μM)] and increased accumulation of live cells in S phase [17.3% (vehicle control), 19.0% (100 μM), and 23.5% (300 μM)] (Fig. 2B). It appears that midazolam causes S phase cell cycle arrest in K562 cells. Collectively, these observations demonstrate that midazolam induced mitochondrial apoptotic cell death and caused growth arrest at S phase in K562 cells.
Fig. 2.
Effect of midazolam on apoptosis and cell cycle progression in K562 cells. (A) K562 cells were exposed to various concentrations of midazolam for 24 h and stained with annexin-V/7AAD. Detection of apoptotic cells was performed by flow cytometry. (B) K562 cells were treated with midazolam for 18 h and then exposed to JC-10 fluorescence dye. The fluorescence intensities were measured at Excitation/ Emission: 490/525 nm and 490/590 nm. Red colored areas in the image represent the ratio of Δψm fluorescence intensity. (C) DNA fragmentation in K562 cells was measured by agarose gel electrophoresis. Midazolam aberrantly increased ∼200 bp DNA strand laddering at 300 μM. Vehicle- and 100 μM midazolam-treated cells showed distinct DNA band (marked with arrow). (D) K562 cells were treated with midazolam for 24 h and then stained with PI for cell-cycle analysis by FACS. Values on top of each box represent the percentage of cells distributed in difference phases of cell cycle (subG1 : G1 : S : G2/M). Mdz, midazolam.
Midazolam activated caspases in K562 and HT29 cells
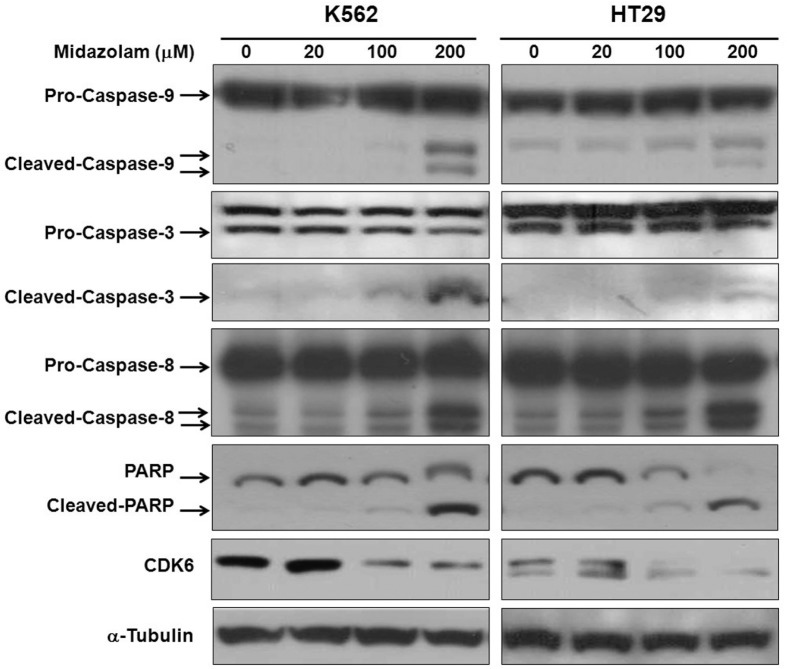
To evaluate the pro-apoptotic response of midazolam, we measure the levels of caspases by Western blotting. As shown in Fig. 3, midazolam caused activation of caspase-9 in both cell lines, as observed by a reduction in pro-caspase-9 and accumulation of cleaved caspase-9 in a dose-dependent manner. Midazolam further activated caspase-3, as evident by reduced pro-caspase-3 and enriched cleaved-caspase-3. Caspase-3 activation by midazolam was followed by cleavage of poly (ADP-ribose) polymerase (PARP), with a marked increase in cleaved PAPR in both cell lines. PARP is a family of proteins that are nuclear substrates of caspase-3 and are involved in a number of cellular processes mainly DNA repair and programmed cell death. Activation of caspase-9 (Fig. 3) and lowering of mitochondrial membrane potential (Fig. 2B) suggests involvement of the mitochondrial intrinsic pathway of apoptosis as a mechanism responsible for the pro-apoptotic effects of midazolam. Although midazolam showed activation of caspase-8 especially at higher concentration (200 μM), this could be non-specific activation because caspase-8 is primarily activated in the extrinsic pathway of apoptosis but often partly activated in the intrinsic apoptosis pathway. We also observed a remarkable reduction in the protein levels of CDK6 following midazolam treatment (Fig. 3). Inhibition of CDK6 activity corresponds to S phase cell cycle arrest in K562 cells (Fig. 2D). These results collectively indicate that midazolam induced the mitochondrial intrinsic pathway of apoptosis in both cell lines.
Fig. 3.
Effect of midazolam on activation of caspases. K562 and HT29 cellls were treated with midazolam for 24 h and then protein extraction and Western blotting was performed as described in the “Materials and Methods”. Caspase-9, -3, -8, PARP, and CDK6 were analyzed and detection of α-tubulin was used as a loading control.
Midazolam suppressed the intracellular ROS generation in K562 cells
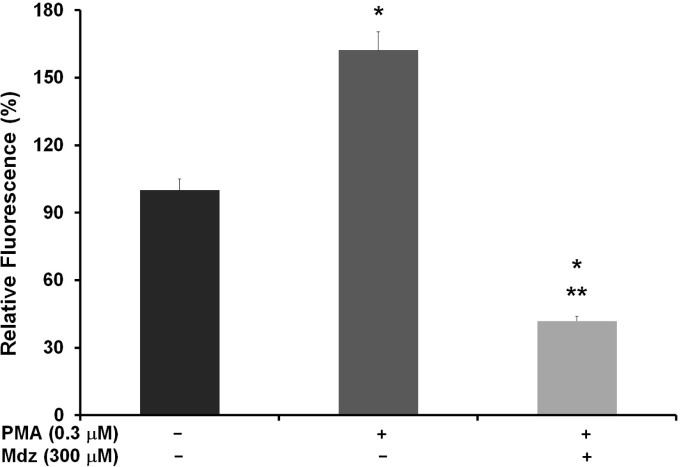
Midazolam has shown ROS scavenging activity associated with its cytotoxic effects (Kang et al., 1998; Nishina et al., 1998). To elucidate the mechanism responsible for apoptosis induction by midazolam, we investigated the effect of midazolam on intracellular ROS production. ROS generation in cancer cells is observed also through non-mitochondrial sources such as membrane bound NADPH oxidases (Bae et al., 2011; Leto et al., 2009). Nox2 and Nox4 are the membrane bound nonmitochondrial sources of intracellular ROS generation that has been also been associated with the growth promotion in cancer cells (Jeon et al., 2010; Leto et al., 2009; Rao Malla et al., 2010). We utilized DCFH-DA assay to estimate the superoxide generation in K562 cells sensitized by phorbol 12-myristate 13-acetate (PMA). PMA is a diester of phorbol, which functions as tumor promoter via activation of protein kinase C signaling. PMA is a known and specific activator of Nox2, which stimulates cells for ROS generation through phosphorylation of p47phox sites of Nox2 (Chanock et al., 1992; Faust et al., 1995). PMA is reported to activate the phosphorylation of cytosolic tail of Nox2 in a PKC-dependent mechanism (Raad et al., 2009). Thus the detection of PMA-induced ROS generation by DCFH-DA assay is specific for the detection of Nox2 activity in terms of superoxide generation. As shown in Fig. 4, PMA-treated cells showed high levels of ROS (162%) compared with vehicle control (100%). Treatment with midazolam significantly inhibited the generation of superoxide, as demonstrated by decreased fluorescence levels (42%) compared with those of PMA- and vehicle- treated cells. Each treatment groups were statistically significant vs control- as well PMA- treated cells (P < 0.001). This assay demonstrates that inhibition of ROS generation by cedrol is possibly mediated by the inhibition of Nox2 activity. Since cancer cells have high ROS levels due to their higher metabolic activity, the ROS scavenging effect of midazolam might help in growth inhibition of cancer cells via suppression of pro-survival signaling.
Fig. 4.
Effect of midazolam on intracellular ROS generation in K562 cells. Activity of Nox2 subunit was detected by means of detecting PMA-induced ROS generation. K562 cells were treated without or with midazolam (300 μM) for 18 h and then exposed to PMA (0.3 μM) for 5 min. Cells were then incubated with redox-sensitive fluorescence dye DCFH-DA (10 mM) and fluorescence of product DCFHA was measured by flow cytometry. Data are expressed as the means ± SD of precent relative fluorescnece units of three independent experiments. *P vs. VH; **P vs. VH + PMA. PMA, phorbol-12-myristate 13-acetate; VH, vehicle control; Mdz, midazolam.
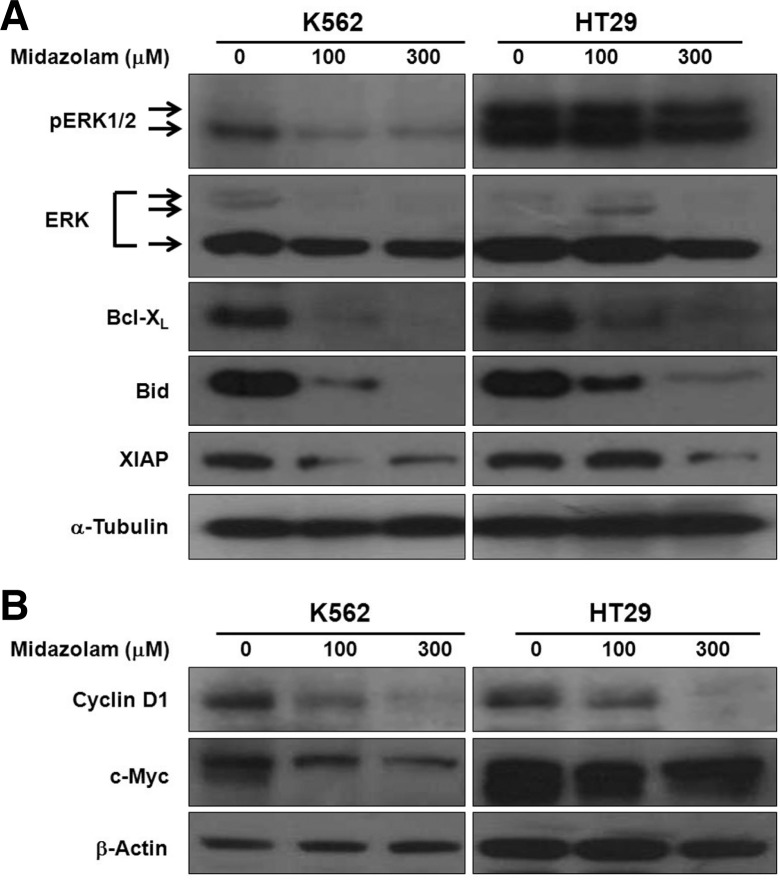
Midazolam modulated the levels of apoptosis regulatory proteins
The ROS scavenging activity of midazolam indicated a probable interaction with cell survival signals and thus with anti- and pro-apoptosis proteins. Therefore, we measured the protein levels of pERK, ERK, Bid, Bcl-XL, and XIAP by western blotting. As shown in Fig. 5, midazolam inhibited the levels of pERK1/2 (42/44 kDa) in K562 and HT29 cell lines in a dose-dependent manner. The effect of midazolam on K562 was more drastic that its effect on HT29. Total ERK level was also reduced to a little extent only especially at 300 μM concentration. Midazolam inhibited the levels of pERK in a dose-dependent manner in both cell lines. Next, we investigated the effect of midazolam on the B-cell lymphoma 2 (Bcl-2) family of apoptosis regulatory proteins. Midazolam treatment led to inhibition of the major antiapoptosis protein Bcl-XL and phosphorylation activation of major pro-apoptosis protein Bid (Fig. 5). Bid is known to initiate the intrinsic apoptosis cascade through translocation in to mitochondria (Breckenridge and Xue, 2004). Midazolam also inhibited the levels of anti-apoptotic protein XIAP (Fig. 5). XIAP, also known as inhibitor of apoptosis protein 3 and baculoviral IAP repeat-containing protein 4, is a protein that stops apoptotic cell death and is encoded by the XIAP gene located on the X chromosome (Breckenridge and Xue, 2004). In addition to apoptosis regulatory proteins, midazolam also inhibited the levels of two major cell proliferation regulatory proteins, cyclin D1 and c-Myc (Fig. 5). Cyclin D1 is an essential cell cycle regulatory protein and its overexpression is frequently associated with tumorigenesis, angiogenesis, and tumor growth (Fu et al., 2004). Suppression of cyclin D1 by midazolam corresponds to the S phase cell c ycle a rrest in K562 cells (Fig. 2D). These observations support the notion that the growth inhibitory effects of midazolam in cancer cells are mediated by induction of cellular apoptosis via downregulation of pERK depending on inhibition of ROS production.
Fig. 5.
Effect of midazolam on apoptosis and growth regulatory proteins. K562 and HT29 cellls were treated with midazolam for 24 h and protein extraction and western blotting was performed as described in the “Materials and Methods”.
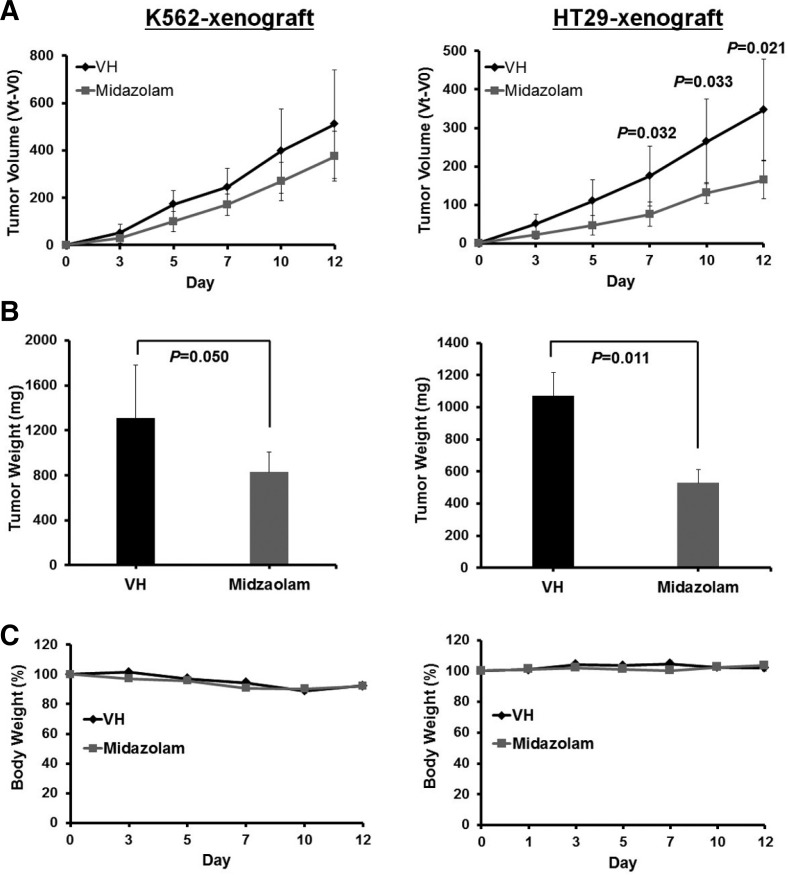
Midazolam suppressed tumor growth in human tumor xenograft mice
The in vivo effect of midazolam was determined in K562 and HT29 human tumor xenograft mice models. As shown in Fig. 6, a daily regimen of midazolam injection (0.83 mg/kg) suppressed tumor growth in both xenograft mice models but more effectively in HT29 xenograft mice. Midazolam reduced tumor volume by 26.6% in K562 xenograft mice at day 12 but this was not statistically significant (Fig. 6A). Midazolam reduced total tumor weight by 37% in K562 xenograft mice with nominal statistical significance (P = 0.050) (Fig. 6B). Midazolam dramatically reduced the tumor volume (52.4%) in HT29 xenograft mice at day 12 (P = 0.021) (Fig. 6A). Total tumor weight in HT29 xenograft mice was also significantly reduced (50.7%, P = 0.011) by midazolam (Fig. 6B). There was no significant difference in the body weight of xenograft mice treated with or without midazolam (Fig. 6C). Physical observation of mice showed that midazolam treatment did not cause signs of sedation or dizziness. In addition, the mice showed normal food intake behavior and normal locomotive activity. The dose of midazolam (0.83 mg/kg) used in this study was low compared with other studies that used higher concentrations of benzodiaze-pines such as midazolam for different experimental procedures related to neuronal toxicity and apoptosis, anesthesia, and other applications (Bittigau et al., 2002; Ikonomidou et al., 2000; Jevtovic-Todorovic et al., 2003; Loepke et al., 2009; Young et al., 2005). These results indicate promising implication of midazolam, apart from anesthesia, to enhance anticancer efficacy when applied in combination with other anticancer agents.
Fig. 6.
Effect of midazolam on tumor growth in human tumor xenograft mice. K562 and HT29 cells were subcutaneously inoculated into female BALB/c-nu mice. Mice were treated daily with either vehicle or midazolam (0.83 mg/kg body weight, i.v.) for 12 days. (A) Tumor volume was routinely measured and calculated using the formula described in “Materials and Methods”. (B) Tumor weight was recorded at the end of the study by expatriating tumors from mice. (C) Body weight was observed routinely and presented as percent change from day 0. All values are expressed as mean ± SD for five mice. Statistical significances are indicated at respective time points.
DISCUSSION
This study demonstrates the growth inhibitory effects of midazolam in human cancer cells in vitro and in vivo. Midazolam induced apoptotic cell death in the K562 human leukemia and HT29 colon cancer cell lines. Midazolam also inhibited the growth of tumor in HT29 bearing human tumors in xenograft mice bearing HT29 human tumors. Midazolam showed potent ROS scavenging activity through inhibition of Nox2 activity. Suppression of ROS by midazolam led to the downregulation of pERK followed by alterations in the levels of pro- and antiapoptotic genes. These effects of midazolam expose its potential to inhibit cancer cell growth in vitro and in vivo. Our findings have interesting clinical implications for the use of midazolam as a sedative for relieving pain caused during anticancer drug delivery as well as a pro-apoptotic agent that enhances anticancer efficacy when used in combination treatment.
Midazolam has shown apoptosis-inducing activity in a GABAA receptor-dependent manner in neuronal cells (Jevtovic-Todorovic et al., 2003; Stevens et al., 2011; Young et al., 2005). It has also been shown to induce apoptosis in human Jurkat Tlymphoma cells that are devoid of GABAA receptor (Stevens et al., 2011). In this study, we found that midazolam suppresses the growth of human cancer cells by inducing apoptosis and S phase cell cycle arrest (Figs. 1 and 2), as well as it inhibited the tumor growth in K562 and HT29-xenograft mice (Fig. 6). In K562 cells, midazolam showed an early apoptotic response at a lower concentration (100 μM) that became a late apoptosis response at higher concentration (300 μM; Fig. 2A). A similar phenomenon has been reported in B35 neuroblastoma cells in which midazolam induced early apoptosis at a lower concentration and late apoptosis or necrosis at higher concentration (400 μM) (Stevens et al., 2011). Midazolam-induced mitochondrial apoptosis was evident based on two key cellular apoptosis markers, lowered mitochondrial membrane potential (Fig. 2B) and enhanced apoptotic DNA fragmentation (Fig. 2C). Midazolam was found to induce the caspase-9 dependent mitochondrial intrinsic pathway of apoptosis in K562 and HT29 cells (Fig. 3). Apoptosis induction is a key regulator of cell proliferation that has been considered as a target for anticancer drug development. Apoptosis can be executed through two different biochemical pathways, intrinsic and extrinsic. Both pathways lead to activation of effector caspases, mainly caspase-3. A activated caspase-3 ultimately executes cell death by cleaving a number of cytoplasmic and nuclear substrates such as PARP (Hengartner, 2000; Meier et al., 2000). Studies report that midazolam induces apoptosis through several mechanisms in different cellular conditions such as extrinsic apoptosis in cortical neuronal cells in vitro (Stevens et al., 2011), mitochondrial intrinsic and extrinsic pathways in neuronal cells in vivo (Nishina et al., 1998), caspase-8-independent calcium channel blockade in Jurkat cells (Conrad et al., 2010), and neuronal apoptosis by suppressing Ca2+-oscillations (Sinner et al., 2011). Our results are consistent with reports of Stevens et al. (2011) and Conrad et al. (2010), which demonstrate that midazolam-induced mitochondrial intrinsic apoptosis is probably unrelated to the GABAA receptor signaling in Jurkat cells. Furthermore, midazolam inhibited the growth of tumors in K562 and HT29 human tumor xenograft mice models (Fig. 6). Interestingly, midazolam-treated mice showed no signs of sedation or dizziness and all mice showed normal physical activity. Midazolam is known to cause occasional side effects such as respiratory depression and hypotension, particularly when rapidly administered or when combined with other anesthetics (Riss et al., 2008). A limitation of this study appears to be the high dose used for treatment (Reves et al., 1985). However, lower dose of midazolam appear to be effective in dose-dependent manner. Collectively, our results expose interesting clinical implications of midazolam: 1) midazolam sedation may lower the pain response caused by rapid delivery of anticancer drugs; 2) the apoptosis-inducing and ROS scavenging properties of midazolam may enhance the efficacy of anticancer therapy.
To elucidate the molecular mechanisms underlying the growth inhibitory effects of midazolam, we analyzed signaling events dependent on the ROS status of cancer cells. Cancer cells are saliently characterized by persistent intrinsic oxidative stress, which promotes cell survival signaling and suppresses cell death pathways (Burdon, 1995; Toyokuni et al., 1995). Thus oxidative stress has emerged as an important pathogenic factor in the development of cancer. Mitochondria are the main source of ROS generation; however, non-mitochondrial production of superoxide anion via the NADPH oxidase pathway has also been reported in cancer cells (Leto et al., 2009). ROS generation in cancer cells is observed also through non-mitochondrial sources involving membrane bound NADPH oxidases (Bae et al., 2011; Leto et al., 2009). Nox2 and Nox4, located in lipid raft regions of cell membrane, have been associated with the ROS generation and growth promotion in cancer cells (Jeon et al., 2010; Leto et al., 2009; Rao Malla et al., 2010). Intrinsic oxidative stress in cancer cells maintains the cell survival (Burdon, 1995; Toyokuni et al., 1995) by activating a variety of redoxsensitive transcription factors such as NF-κB, Nrf2, HIF, and p53 (Trachootham et al., 2008). The NF-κB pathway augments pro-survival signaling by activating PI3K/AKT/ERK1/2 pathways and ultimately leads to the phosphorylation inactivation of proapoptotic proteins such as Bid and Bax and upregulation of anti-apoptotic genes such as Bcl-2 and Bcl-XL (McCubrey et al., 2007). The Nox-dependent superoxide generation has been found to be abnormally enhanced in several chronic diseases including cancer (Davalos et al., 2009). We found that midazolam inhibits the enzyme activity of Nox2 subunits in K562 cells (Fig. 4). A recent report demonstrated the role of Nox-dependent ROS generation in breast cancer cells and showed that ROS inhibition led to the growth arrest of cells (Rao Malla et al., 2010). A role of Nox in pancreatic cell survival and tumor growth promotion has been also reported, in which K-ras (usually tethered to cell membranes) mediates Nox-dependent superoxide production (Teoh et al., 2007). Midazolam has also shown to suppress ROS generation in neuronal and neuroblastoma cells (Chong et al., 2012; Schoeffler et al., 1991; Svensson et al., 1995). We found that midazolam inhibited Nox2-dependent ROS generation in K562 cells which might be associated with the suppression of pERK signaling (Fig. 5A). furthermore, inhibition of pERK possibly led to the transcriptional downregulation of the anti-apoptotic protein Bcl-XL and upregulated phosphorylation of the pro-apoptotic protein Bid. Another anti-apoptosis protein, XIAP. was also found to be inhibited by midazola (Fig. 5A). In addition, midazolam treatment caused inhibition of the cell growth regulatory proteins cyclin D1 and c-Myc (Fig. 5B). Overall, midazolam-mediated suppression of ROS production led to the modulation of apoptosis and growth regulatory proteins, which ultimately caused growth inhibition of cancer cells. Taking these findings in to consideration, either midazolammediated inhibition Nox2 enzyme or scavenging the superoxides produced by this enzyme are plausible mechanisms for regulation of the growth of cancer cells. The ROS scavenging/ antioxidant properties of midazolam appear to be more important because detoxifying ROS is believed to enhance the therapeutic efficacy of chemotherapy and reduce its side effects.
In conclusion, to the best of our knowledge, the present study is the first demonstration of the growth inhibitory effects of midazolam in human leukemia and colon cancer cells, in vitro and in vivo. Midazolam suppressed proliferation of K562 and HT29 cells by inducing the mitochondrial intrinsic pathway of apoptosis and S phase cell cycle arrest. Midazolam also inhibited tumor growth in HT29-bearing xenograft mice. The mechanism underlying the growth inhibitory effects of midazolam appears to be mediated by suppression of Nox2-dependent ROS production. This later causes a reduction in pERK signaling, leading to modulation of apoptosis and growth regulatory proteins. K562 and HT29 cell lines are naturally devoid of GABAA receptor which suggests that the effect of midazolam in these cell lines is not dependent on GABAA receptor signaling. However, further investigation is warranted to identify the exact receptor/ signaling mechanisms involved in the effects of midazolam in cancerous conditions. Translating our results to clinical applications, we propose that, in addition to its role in anesthesia, midazolam may enhance anticancer therapeutic efficacy by virtue of its ROS scavenging and pro-apoptotic properties.
Supplementary Material
Acknowledgments
This work was supported by Gachon Institute of Pharmaceutical Sciences Research Fund 2012.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Bae Y, Oh H, Rhee S, Yoo Y. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl. Acad. Sci. USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge DG, Xue D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol. 2004;16:647–652. doi: 10.1016/j.ceb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int. 2002;40:475–486. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chanock SJ, Faust LR, Barrett D, Bizal C, Maly FE, Newburger PE, Ruedi JM, Smith RM, Babior BM. O2- production by B lymphocytes lacking the respiratory burst oxidase subunit p47phox after transfection with an expression vector containing a p47phox cDNA. Proc. Natl. Acad. Sci. USA. 1992;89:10174–10177. doi: 10.1073/pnas.89.21.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong WS, Hyun CL, Park MK, Park JM, Song HO, Park T, Lim YS, Cho CK, Kang PS, Kwon HU. Midazolam protects B35 neuroblastoma cells through Akt-phosphorylation in reactive oxygen species derived cellular injury. Korean J Anesthesiol. 2012;62:166–171. doi: 10.4097/kjae.2012.62.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad DM, Furlong SJ, Doucette CD, West KA, Hoskin DW. The Ca(2+) channel blocker flunarizine induces caspase-10-dependent apoptosis in Jurkat T-leukemia cells. Apoptosis. 2010;15:597–607. doi: 10.1007/s10495-010-0454-3. [DOI] [PubMed] [Google Scholar]
- Davalos A, de la Pena G, Sanchez-Martin CC, Teresa Guerra M, Bartolome B, Lasuncion MA. Effects of red grape juice polyphenols in NADPH oxidase subunit expression in human neutrophils and mononuclear blood cells. Br J Nutr. 2009;102:1125–1135. doi: 10.1017/S0007114509382148. [DOI] [PubMed] [Google Scholar]
- Erdine S, Yucel A, Ozyalcin S, Ozyuvaci E, Talu GK, Ahiskali B, Apak H, Savci N. Neurotoxicity of midazolam in the rabbit. Pain. 1999;80:419–423. doi: 10.1016/s0304-3959(98)00240-1. [DOI] [PubMed] [Google Scholar]
- Faust LR, el Benna J, Babior BM, Chanock SJ. The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J Clin Invest. 1995;96:1499–1505. doi: 10.1172/JCI118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jeon SM, Lee S-J, Kwon TK, Kim K-J, Bae Y-S. NADPH oxidase is involved in protein kinase CKII down-regulation-mediated senescence through elevation of the level of reactive oxygen species in human colon cancer cells. FEBS Lett. 2010;584:3137–3142. doi: 10.1016/j.febslet.2010.05.054. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen MJ, Gradert TL, Satterfield WC, Baze WB, Hildebrand K, Trissel L, Hassenbusch SJ. Safety of continuous intrathecal midazolam infusion in the sheep model. Anesth Analg. 2004;98:1528–1535. doi: 10.1213/01.ANE.0000120086.35289.9D. [DOI] [PubMed] [Google Scholar]
- Kang MY, Tsuchiya M, Packer L, Manabe M. In vitro study on antioxidant potential of various drugs used in the perioperative period. Acta Anaesthesiol Scand. 1998;42:4–12. doi: 10.1111/j.1399-6576.1998.tb05073.x. [DOI] [PubMed] [Google Scholar]
- Kang JH, Song KH, Jeong KC, Kim S, Choi C, Lee CH, Oh SH. Involvement of Cox-2 in the metastatic potential of chemotherapy-resistant breast cancer cells. BMC Cancer. 2011;11:334. doi: 10.1186/1471-2407-11-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, et al. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- Malinovsky JM, Cozian A, Lepage JY, Mussini JM, Pinaud M, Souron R. Ketamine and midazolam neurotoxicity in the rabbit. Anesthesiology. 1991;75:91–97. doi: 10.1097/00000542-199107000-00015. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- Muller A, Hacker J, Brand BC. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, Niwa Y. The inhibitory effects of thiopental, midazolam, and ketamine on human neutrophil functions. Anesth Analg. 1998;86:159–165. doi: 10.1097/00000539-199801000-00032. [DOI] [PubMed] [Google Scholar]
- Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Handb Exp Pharmacol. 2008;182:335–360. doi: 10.1007/978-3-540-74806-9_16. [DOI] [PubMed] [Google Scholar]
- Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB J. 2009;23:1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Malla R, Raghu H, Rao JS. Regulation of NADPH oxidase (Nox2) by lipid rafts in breast carcinoma cells. Int J Oncol. 2010;37:1483–1493. doi: 10.3892/ijo_00000801. [DOI] [PubMed] [Google Scholar]
- Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310–324. [PubMed] [Google Scholar]
- Riss J, Cloyd J, Gates J, Collins S. Benzodiaze-pines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118:69–86. doi: 10.1111/j.1600-0404.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- Schoeffler P, Auroy P, Bazin JE, Taxi J, Woda A. Subarachnoid midazolam: histologic study in rats and report of its effect on chronic pain in humans. Reg Anesth. 1991;16:329–332. [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Sinner B, Friedrich O, Zausig Y, Bein T, Graf BM. Toxic effects of midazolam on differentiating neurons in vitro as a consequence of suppressed neuronal Ca2+-oscillations. Toxicology. 2011;290:96–101. doi: 10.1016/j.tox.2011.08.022. [DOI] [PubMed] [Google Scholar]
- So EC, Chang YT, Hsing CH, Poon PW, Leu SF, Huang BM. The effect of midazolam on mouse Leydig cell steroidogenesis and apoptosis. Toxicol Lett. 2010;192:169–178. doi: 10.1016/j.toxlet.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Stevens MF, Werdehausen R, Gaza N, Hermanns H, Kremer D, Bauer I, Kury P, Hollmann MW, Braun S. Midazolam activates the intrinsic pathway of apoptosis independent of benzodiazepine and death receptor signaling. Reg Anesth Pain Med. 2011;36:343–349. doi: 10.1097/AAP.0b013e318217a6c7. [DOI] [PubMed] [Google Scholar]
- Svensson BA, Welin M, Gordh T, Jr, Westman J. Chronic subarachnoid midazolam (Dormicum) in the rat Morphologic evidence of spinal cord neurotoxicity. Reg Anesth. 1995;20:426–434. [PubMed] [Google Scholar]
- Teoh ML, Sun W, Smith BJ, Oberley LW, Cullen JJ. Modulation of reactive oxygen species in pancreatic cancer. Clin Cancer Res. 2007;13:7441–7450. doi: 10.1158/1078-0432.CCR-07-0851. [DOI] [PubMed] [Google Scholar]
- Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.