Abstract
S-adenosyl methionine (SAM) is a key intermediate in the metabolism of sulfur amino acids and is a major methyl donor in the cell. Although the low plasma level of SAM has been associated with atherosclerosis, the effect of SAM administration on atherosclerosis is not known. Endothelial dysfunction is an early prerequisite for atherosclerosis. This study was undertaken to investigate the possible preventive effect of SAM on endothelial dysfunction and the molecular mechanism of its action. SAM treatment prevented endothelial dysfunction in high fat diet (HFD)-fed rats. In cultured human aortic endothelial cells, linoleic acid (LA) increased and SAM decreased cell apoptosis and endoplasmic reticulum stress. Both LA and SAM increased heme oxygenase-1 (HO-1) expression in an NF-E2-related factor 2-dependent manner. However, knockdown of HO-1 reversed only the SAM-induced preventive effect of cell apoptosis. The LA-induced HO-1 expression was dependent on PPARα, whereas SAM induced HO-1 in a PPAR-independent manner. These data demonstrate that SAM treatment prevents endothelial dysfunction in HFD-fed animals by inducing HO-1 in vascular endothelial cells. In cultured endothelial cells, SAM-induced HO-1 was responsible for the observed prevention of cell apoptosis. We propose that SAM treatment may represent a new therapeutic strategy for atherosclerosis.
Keywords: endoplasmic reticulum stress, endothelial dysfunction, heme oxygenase-1, S-adenosyl methionine, vascular endothelial cell
INTRODUCTION
S-adenosyl methionine (SAM) is a key intermediate in the metabolism of sulfur amino acids and a major methyl donor in the cell. SAM has antioxidant and cytoprotective effects (Erdmann et al., 2008; Wu and Cederbaum, 2006), and administration of SAM has been shown to have therapeutic benefits in various human diseases, including chronic liver disease (Mato and Lu, 2007), osteoarthritis (Soeken et al., 2002), Alzheimer’s disease (Tchantchou et al., 2008), depression (Miller, 2008), and cancer (Poschl et al., 2004).
SAM donates its methyl group to a methyl acceptor, thereby forming a methylated product and S-adenosylhomocysteine (SAH). SAH is then converted into homocysteine. Hyperhomocysteinemia (HHcy) is regarded as an important cardiovascular risk factor. One of the hypotheses explaining the association between HHcy and atherosclerosis is that HHcy may be a marker for altered methylation of cellular substrates that utilize SAM as a methyl donor (Dayal et al., 2001). Therefore, it has been suggested that the association between HHcy and cardiovascular disease may be explained by low SAM levels or a low SAM/SAH ratio (Spijkerman et al., 2005; Wagner and Koury, 2007). A recent study reported that SAM administration prevents neointimal formation after balloon injury in obese diabetic rats (Lim et al., 2011). Restenosis after vascular injury and atherosclerosis share common pathogenic mechanisms. However, the effect of SAM administration on atherosclerosis has not been established.
The endothelium is important in the regulation of smooth muscle cell growth, migration, and proliferation, and endothelial cell apoptosis is an important early event in the pathogenesis of atherosclerosis (Lee et al., 2005). Recent studies have emphasized the role of endoplasmic reticulum (ER) stress in the pathogenesis of atherosclerosis (Han et al., 2006; Tabas, 2010) and in endothelial apoptosis (Austin et al., 2004). The ER is an organelle responsible for the folding and assembly of membrane and secreted proteins, synthesis of lipids and sterols, and calcium storage (Kaufman, 2002). When misfolded or unfolded proteins accumulate in the ER lumen, the cells activate a group of signal transduction pathways collectively termed unfolded protein response (UPR). ER stress is chronically increased in atherosclerotic lesional macrophages and endothelial cells, and contributes to apoptosis and inflammatory responses in macrophages (Han et al., 2006). In the liver, an alcohol-induced decrease in the SAM to SAH ratio is thought to induce ER stress (Ji, 2012).
Heme oxygenase-1 (HO-1) catalyzes the oxidative degradation of heme to free iron, carbon monoxide (CO), and biliverdin. In the vascular system, HO-1 and heme degradation products perform important physiological functions, which are ultimately linked to the protection of vascular cells (Wang and Chau, 2010; Wu et al., 2011). A previous study showed that SAM increases HO-1 expression in cultured endothelial cells (Erdmann et al., 2008). In this study, we found that SAM prevents endothelial apoptosis by increasing HO-1 expression.
MATERIALS AND METHODS
Animals and experimental protocol
Eight-week-old male Sprague-Dawley (SD) rats (Orient, Korea) weighing 250 to 300 g were given normal rat chow or a high fat diet (HFD) that provided 60% of calories as fat (D12492, Research Diets, USA) with or without 30 mg/kg/day of SAM (Dalim Biotech, Korea; Lim et al., 2011). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences.
Endothelium-dependent and -independent vascular relaxation
After 8 weeks of HFD feeding, 20 mg/kg xylazin and 125 mg/kg ketamine were injected intraperitoneally in SD rats for induction of narcosis. The thoracic aorta was excised and cleaned by removing fat and adhering tissue. The vessel was cut into several individual ring segments 2–3 mm in width and suspended in a tissue bath. Ring segments were washed in Krebs-Henseleit buffer (118 mM NaCl, 4.6 mM KCl, 27.2 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.75 mM CaCl2, 0.03 mM Na2 EDTA, and 11.1 mM glucose), maintained at 37°C, and aerated with 95% O2−5% CO2. The vascular tension was measured with an isotonic force displacement transducer (Hugo Sachs Elektronik KG D-7806, Germany) and recorded using a polygraph (Graphtec Linerecorder mark 8 WR3500, Hugo Sachs Electronic). After exposure to 4 μg/ml lysophosphatidylcholine (LPC, Sigma-Aldrich, USA), sub-maximal contraction of the aortic ring was induced by treatment with 300 μmol/L phenylephrine (Research Biochemicals International, USA). When the vascular tension reached a plateau, acetylcholine (from 10−9 to 10−5 mol/L) was added serially to the bath to induce endothelium-dependent vasorelaxation. In a separate set of experiments, endothelium-independent vasorelaxation was determined by the serial addition of sodium nitroprusside (from 10−11 to 10−7 mol/L). Vascular relaxation data were calculated as the percentage of the maximal vasorelaxation, and the dose-response profile for each experiment was analyzed (Won et al., 2010).
Measurement of metabolic parameters
Serum total cholesterol, triglyceride (TG), high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, and free fatty acid (FFA) measurements were performed with an auto-analyzer Hitachi 7180 biochemical analyzers (Hitachi, Japan).
Determination of plasma homocysteine
The blood samples were placed on ice immediately after collection and centrifuged at 4°C, and serum was collected and stored at −20°C. Fasting plasma homocysteine level was determined by using ELISA kits (A/C diagnostics, USA), according to the manufacturer’s instruction.
Cell culture
Human aortic endothelial cells (HAECs, BioWhittaker, USA) were cultured in endothelial growth medium-2 (EGM-2, BioWhittaker) supplemented with specific growth factors and 2% fetal bovine serum (FBS). Before experiments, the growth medium was replaced with M199 (BioWhittaker) without growth factors and with 1% FBS. HAECs were incubated for 1 h with or without SAM, and then linoleic acid (LA) was added to the cells for the indicated times.
Apoptosis assays
TUNEL assay
Apoptosis was detected by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) using an in situ cell death detection kit (Roche, USA). Briefly, transfected cells were washed with PBS, fixed with 4% paraformaldehyde in PBS for 1 h, and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 5 minutes on ice. The fixed cells were incubated with TUNEL reaction mixture containing the terminal deoxynucleotidyl transferase at 37°C for 1 h. Double-stranded DNA in nuclei was counterstained after TUNEL staining with 4′, 6-diamidino-2-phenylindole (DAPI) (5 μg/ml). The cells were air-dried, and coverslips were placed on a drop of anti-fade solution and sealed on the slide with mounting solution. Images of nuclear fluorescence were obtained by fluorescence microscopy.
ELISA assay
The levels of cytosolic histone-bound DNA fragments were measured using a cell death ELISA kit (Roche).
Western blot analysis
Protein expression in cells and tissues was measured by Western blot analysis as previously described (Won et al., 2010). The following primary antibodies were used: anti-HO-1 (Calbiochem, USA); anti-cleaved-caspase 3, anti-cleaved-Poly ADP-ribose polymerase (PARP), anti-uncoupling protein 2 (UCP2; Novus biological, USA); anti-NADH quinone oxidoreductase (NQO1), anti-nuclear factor erythroid 2-related factor 2 (Nrf2) and anti-C/EBP homologous protein (CHOP; Santa Cruz Biotechnology, USA); anti-X-box binding protein 1 (XBP-1S; Biolegend, USA); anti-superoxide dismutase (MnSOD; R&D, USA); anti-glutathione peroxidase 1 (Gpx1); anti-inositol-requiring enzyme 1 (IRE1) and anti-translation initiation factor 2α (eIF2α; cell signaling, USA); and anti-β-actin (Sigma). Secondary antibodies were species-appropriate horseradish peroxidase-labeled antibodies (Vector Laboratories, USA). Bands densities were quantified with a densitometer (3000 VersaDoc Imaging System: Bio-Rad Laboratories, USA). Results were normalized to β-actin to correct for variations in sample loading and are expressed as percentages of control signals (% control) in each blot to correct for variations between blots. Only protein levels of peIF2α and pIRE1 were normalized relative to expression of each total eIF2α or total IRE1.
Real-time PCR analysis
Total RNA was isolated using Trizol (Invitrogen, USA). For quantitative RT-PCR analysis, 2 μg of total RNA was reverse-transcribed with oligo(dt) using ReverseAid M-MuLV Reverse Transcriptase (Roche). Target cDNA levels were quantified by real-time PCR using the ABI PRISM 7000 sequence detection system (Applied Biosystems, USA) utilizing SYBR green. The gene-specific primers for the measurement of HO-1 mRNA were as follows: forward primer, 5′-AGCCGTGACCACTGA CAACG-3′; reverse primer, 5′- GCTGCATGGTTCTGAGTGC -3′ (NM 008904). The primers for the measurement of mouse 18S rRNA, the control gene, were as follows: forward primer, 5′-GGGAGCCTGAGAAACGGC-3′; reverse primer, 5′-GGGTCG GGAGTGGGTAATTT-3′ (NR 003278).
Transfection of siRNAs
We designed siRNA targeted against human HO-1 and Nrf2 using the design algorithm developed by GenScript. The sequences for human HO-1 siRNA are as follows: sense, 5′-CUG CGU UCC UGC UCA ACA U-3′, and antisense, 5′-AUG UUG AGC AGG AAC GCA G-3′ (Bioneer, Korea). The sense and antisense strands of the nonspecific siRNA duplex are as follows: sense, 5′-CCU ACG CCA CCA AUU UCG U-3′, and antisense, 5′-ACG AAA UUG GUG GCG UAG G-3′. The sequences for human Nrf2 siRNA are as follows: sense, 5′-CUG CGU UCC UGC UCA ACA U-3′, and antisense, 5′-AUG UUG AGC AGG AAC GCA G-3′ (Bioneer). The sense and antisense strands of the nonspecific siRNA duplex are as follows: sense, 5′-CCU ACG CCA CCA AUU UCG U-3′, and antisense, 5′-ACG AAA UUG GUG GCG UAG G-3′. Cells were transfected with double-stranded siRNAs (100 pmol/ml) for 3 h using the Lipofectamine method according to the protocol of the manufacturer (Invitrogen) and were recovered in fresh media containing 10% FBS for 48 h.
Measurement of ROS levels
Intracellular ROS generation was measured by flow cytometry using DCFH2-DA (Molecular Probes, USA). For measurement of intracellular ROS levels, cells were incubated for 15 min with 2.5 μmol/ml DCFH2-DA at 37°C for 30 min. The increase in DCFH2-DA oxidation was measured by a flow cytometry (FACSCalibur, USA). Fluorescence was measured at an excitation wavelength of 488 nm and an emission wavelength of 530 nm.
Immunofluorescence microscopy
Cultured cells grown on coverslips were fixed with 4% paraformaldehyde for 5 minutes followed by permeabilization with 0.5% Triton X-100 in PBS for 5 min at room temperature (RT). Cells were probed with mouse monoclonal antibodies against Nrf2 (1:200) for 30 min. This was followed by incubation with fluorescein isothiocyanate (FITC)-conjugated IgG secondary antibody (Zymed, USA) for 1 h at RT. For nuclear counterstaining, 300 μl of diluted DAPI was added to each well and incubated for 2-5 min at RT. Cells were examined by confocal microscopy using a Zeiss LSM 510 META system.
Statistical analysis
All data are shown as the means ± SEM. Comparisons between two groups were done using unpaired Student’s t-tests and among multiple groups by ANOVA. Differences were classified as significant at P < 0.05.
RESULTS
SAM improves endothelial function in HFD-fed rats
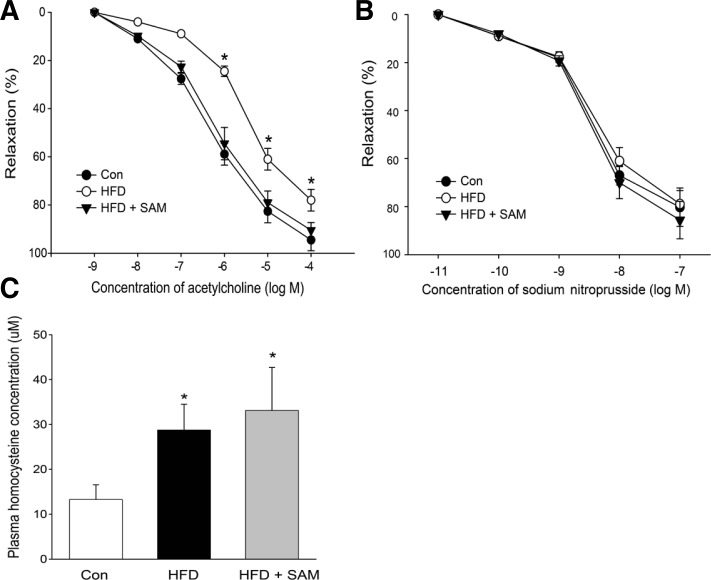
Impaired endothelium-dependent vascular relaxation (endothelial dysfunction) is an early prerequisite for atherosclerosis (Won et al., 2010). Concentration-dependent relaxation of aortic rings in response to acetylcholine was significantly impaired in the rats fed a HFD for 8 weeks compared with rats fed a control diet. SAM treatment significantly improved these changes in HFD-fed rats (Fig. 1A). On the other hand, endothelium-independent (nitroprusside-induced) relaxation was not affected by SAM treatment (Fig. 1B).
Fig. 1.
Dietary supplementation with SAM ameliorates vascular dysfunction in HFD-fed rats. Eight-week-old SD rats were fed a HFD with or without SAM supplementation for 8 weeks as described in “Materials and Methods”. Endothelium-dependent (A) and endothelium-independent vasorelaxation (B) in response to acetylcholine and sodium nitroprusside, respectively, were assessed in rat aortas ex vivo. (C) Plasma homocysteine levels measured by ELISA kits. Data are presented as the mean ± SEM (n = 5). *P < 0.05 vs control.
The body weight of rats fed a HFD and rats fed a HFD with SAM treatment did not differ. In addition, SAM treated rats did not exhibit differences in serum total cholesterol, LDL cholesterol, HDL cholesterol, and FFA compared to HFD-fed rats (Table 1). As expected and in accordance with previous studies (Yun et al., 2013), administration of HFD significantly increased plasma homocysteine concentration (Fig. 1C). Plasma homocysteine level was not decreased by SAM treatment (Fig. 1C), suggesting that the change in homocysteine level is not responsible for the improvement of vascular function by SAM treatment.
Table 1.
Metabolic parameters in the SD rat
| Con | HFD | HFD + SAM | |
|---|---|---|---|
| Body weight (g) | 464.7 ± 53.8 | 583.3 ± 67.1* | 568.8 ± 45.1* |
| Serum cholesterol (mg/dl) | 46.1 ± 5.6 | 55.6 ± 8.9 | 43.9 ± 7.8 |
| Serum TG (mg/dl) | 52.5 ± 10.9 | 92.0 ± 21.0* | 55.0 ± 10.1# |
| Serum HDL cholesterol (mg/dl) | 21.1 ± 6.7 | 24.4 ± 5.6 | 20.1 ± 4.5 |
| Serum LDL cholesterol (mg/dl) | 4.0 ± 0.9 | 4.3 ± 0.8 | 4.3 ± 0.8 |
| Serum FFA (μE/l) | 498.5 ± 67.4 | 625.7 ± 56.7* | 610.8 ± 70.1* |
Values are the mean ± SEM (n = 5 each).
P < 0.05 compared with control mice,
P < 0.05 compared with HFD-fed mice.
SAM protects cultured endothelial cells from LA-induced apoptosis and ER stress
Endothelial apoptosis is considered an early prerequisite for atherosclerosis (Lee et al., 2005). We therefore examined the mechanism of SAM-dependent prevention of atherosclerosis in cultured endothelial cells by examining endothelial apoptosis.
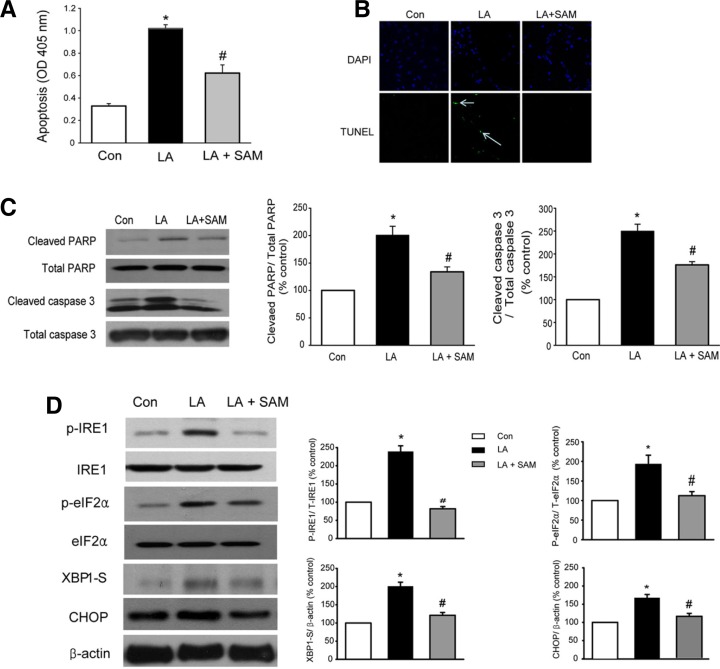
LA is an omega-6 (n-6) essential fatty acid and is the most abundant fatty acid in the plasma of subjects living in western countries (Simopoulos, 2008). Incubation of HAECs with LA significantly increased apoptosis (Figs. 2A–2C), whereas SAM treatment reversed the LA-induced increase in apoptosis (Figs. 2A–2C). LA treatment increased the levels of various markers of ER stress, as reported previously (Ou et al., 2008). On the other hand, SAM significantly decreased LA-induced changes in ER stress markers (Fig. 2D).
Fig. 2.
SAM prevents LA-induced cell apoptosis and ER stress. (A–C) SAM inhibits LA-induced cell apoptosis. HAECs were cultured with EBM-2 media. HAECs were pretreated for 1 h in M199 containing SAM (200 μM), and then LA (300 μM) was added to the cells for 6 h. (A) Cytosolic histone-bound DNA fragments were quantified using a cell death ELISA kit. (B) Apoptotic nuclei of HAECs were detected by TUNEL staining. Original magnification, 400x. Green fluorescence, TUNEL-positive nuclei; blue fluorescence, all nuclei. (C) Representative Western blots for cleaved PARP and caspase 3. The optical density of each individual protein band was normalized to those of total PARP and caspase 3. (D) SAM suppresses the LA-induced ER stress response. Western blot analysis of ER stress markers. HAECs were pretreated for 1 h with SAM (200 μM) and then LA (300 μM) was added for 3 h. CHOP protein expression was examined only after 6 h of LA treatment. Data in (A), (C), and (D) are presented as the mean ± SEM (n = 5). *P < 0.05 vs untreated cell, #P < 0.05 vs LA-treated cells.
SAM induces HO-1 expression to prevent cell apoptosis
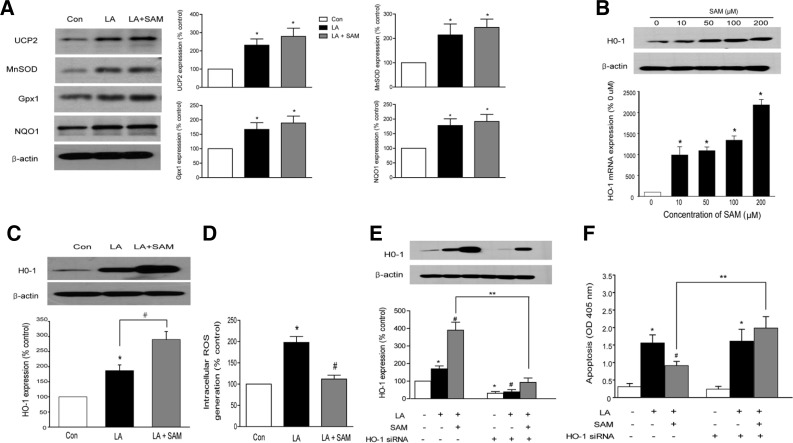
Previous studies have shown that LA increases the expression of antioxidant enzymes (Won et al., 2010). Similarly, we found that LA increased the protein expression of UCP2, MnSOD, Gpx1 and NQO-1 (Fig. 3A). These findings are consistent with the notion that oxidative stress induces cellular antioxidant responses (Bianchi et al., 2002).
Fig. 3.
SAM inhibits endothelial apoptosis by inducing HO-1. (A) Representative Western blots of UCP2, MnSOD, Gpx1 and NQO1 under the experimental conditions described in Fig. 2A. (B) Concentration-dependent increase in HO-1 mRNA and protein expression by SAM. (C) Effect of SAM and LA on HO-1 expression. HAECs were pretreated for 1 h with SAM (200 μM) and then exposed to LA (300 μM) for 6 h. (D) Effect of SAM on intracellular ROS levels. Intracellular ROS production was determined by measuring the intensity of DCF. (E) Representative Western blot and quantification showing suppression of HO-1 protein expression by HO-1 siRNA. (F) HO-1 knockdown with siRNA opposes the effects of SAM to prevent cell apoptosis. HAECs were transfected with control siRNA or siRNA targeting HO-1, pretreated with SAM (200 uM) for 1 h and then exposed to LA (300 μM) for 6 h. Cell apoptosis was measured by a cell death ELISA kit. Data are presented as the mean ± SEM (n = 5). *P < 0.05 vs untreated cell, #P < 0.05 vs LA-treated cells, **P < 0.05 vs control siRNA-transfected cells.
SAM is well known to have antioxidant actions (Erdmann et al., 2008). We therefore asked whether SAM increases the expression levels of various antioxidant enzymes. However, SAM did not potentiate a LA-induced increase in the expression of these enzymes (Fig. 3A).
In agreement with a previous study that SAM increases HO-1 expression in cultured endothelial cells (Erdmann et al., 2008), SAM increased HO-1 mRNA and protein expression in HAECs in a dose-dependent manner (Fig. 3B). LA also increased HO-1 expression (Wright et al., 2009) (Fig. 3C).
Previous studies established that fatty acids increase ROS generation and cell apoptosis in endothelial cells (Lee et al., 2005) In accordance with this study, incubation of HAECs with LA significantly increased intracellular ROS generation. SAM prevented LA-induced increases in ROS generation (Fig. 3D). We next examined the effect of HO-1 inhibition on cell apoptosis. HO-1 siRNA (Fig. 3E) did not affect cell apoptosis in cells treated with LA alone (Fig. 3F). On the other hand, HO-1 siRNA abrogated the SAM-dependent inhibition of cell apoptosis (Fig. 3F).
Mechanism of HO-1 induction by LA and SAM
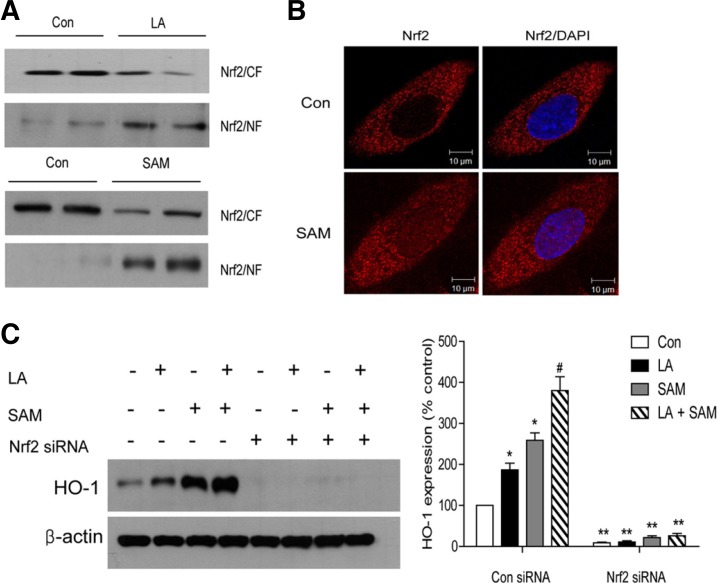
HO-1 expression is regulated by various transcriptional factors and signaling pathways (Alam and Cook, 2007), among which the best known is Nrf-2. Western blot analysis showed that SAM treatment for 4 h increased Nrf2 protein expression in the nucleus (Fig. 4A). Immunofluorescent staining also showed that SAM treatment for 4 h increased Nrf2 translocation to the nucleus (Fig. 4B). To determine whether Nrf2 activation is necessary for HO-1 induction, we examined the effect of Nrf2 siRNA on HO-1 expression. Transfection of Nrf2 siRNA led to a significant reduction in HO-1 protein induction by both LA and SAM (Fig. 4C), showing that HO-1 induction by LA or SAM is Nrf2-dependent.
Fig. 4.
SAM and LA increase HO-1 expression by activating Nrf2. (A–C) Nuclear translocation of Nrf2 by SAM and LA. (A) Nuclear (NF) and cytoplasmic fractions (CF) were subjected to immunoblot analysis with anti-Nrf2. HAECs were treated with SAM or LA for 4 h. (B) Immunofluorescent staining showing nuclear translocation of Nrf2 (red) at 4 h after SAM treatment. The nucleus was stained with DAPI (blue). (C) Effect of siRNA against Nrf2 on HO-1 expression. HAECs were transfected with control siRNA or Nrf2 siRNA, pretreated with SAM (200 uM) for 1 h and then exposed to LA (300 μM) for 4 h. Data in (C) are presented as the mean ± SEM (n = 5). *P < 0.05 vs untreated cell, #P < 0.05 vs LA-treated cells, **P < 0.05 vs control siRNA-transfected cells.
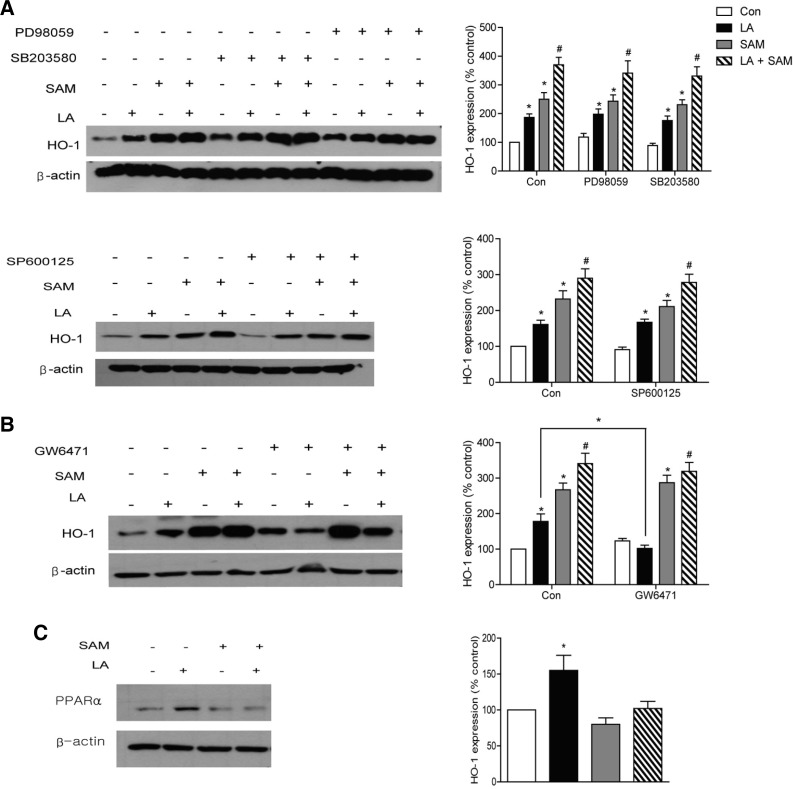
We also examined other signaling pathways that are known to be involved in HO-1 regulation (Alam and Cook, 2007; Chen et al., 2010; Kietzmann et al., 2003). Incubation of cells with the Erk-, p38 MAPK-, and JNK-specific inhibitors PD98059, SB203580 and SP600125, respectively, did not change LA-and SAM-induced HO-1 induction (Fig. 5A). However, treatment with GW6471, a PPARα inhibitor (Xu et al., 2002), decreased LA-induced HO-1 induction but did not change SAM-induced changes (Fig. 5B). LA, but not SAM, increased PPARα expression (Fig. 5C). These findings suggest that LA induces HO-1 induction through the PPARα pathway.
Fig. 5.
Different mechanism of HO-1 induction by LA and SAM. (A) Effect of inhibition of p38 MAPK, ERK and JNK on HO-1 induction by LA or SAM. HAECs were preincubated in M199 media containing 10 μM PD98059, SB203580 or SP600125 for 30 min, and then incubated with 200 μM SAM for 1 h. LA was then added to the media for 4 h. (B) Effect of inhibition of PPARα on HO-1 induction by LA or SAM. HAECs were preincubated with 50 μM GW6471 for 30 min. (C) Western blot analysis of PPARα expression under the same conditions as described in Fig. 2A. Data are presented as the mean ± SEM (n = 5). *P < 0.05 vs untreated cell, #P < 0.05 vs LA-treated cells.
DISCUSSION
In the present study, we found that SAM treatment prevented vascular dysfunction in HFD-fed animals. Previous studies have suggested that low plasma levels of SAM or a decreased SAM/SAH ratio are associated with an increased risk of atherosclerosis (Spijkerman et al., 2005; Wagner and Koury, 2007). A recent study also showed that SAM administration prevents neointimal formation after balloon injury (Lim et al., 2011). However, our study is the first to show that SAM administration prevents endothelial dysfunction in HFD-fed animals.
The antioxidant and cytoprotective effects of SAM are well established. Proposed mechanisms for these protective effects include increased synthesis of glutathione and activation of endothelial nitric oxide synthase (Caballero et al., 2010; Vázquez-Chantada et al., 2009). In this study, we revealed a new additional mechanism, specifically the SAM-mediated increase in the expression of HO-1.
Recent studies have emphasized the role of ER stress in atherosclerosis. Prolonged activation of IRE1 and CHOP was shown to trigger cell apoptosis (Szegezdi et al., 2006; Tabas, 2010). In our study, LA increased and SAM decreased the expression of CHOP and other ER stress markers in cultured HAECs. Although previous studies have shown that SAM attenuates ER stress in hepatocytes (Esfandiari et al., 2007; Ji, 2012), the mechanism by which SAM decreases the ER stress response remains to be established.
Of particular interest in our study is the finding that both LA and SAM increased HO-1 expression. Many previous studies have shown that Nrf2 is involved in the transcriptional regulation of the HO-1 gene (Surh et al., 2009). If a cell experiences oxidative stress, Nrf2, the transcriptional factor for antioxidant responsive element (ARE), is liberated from Keap1 with subsequent translocation of Nrf2 to the nucleus. In the nucleus, Nrf2 binds to the ARE in the promoter of cytoprotective genes, including HO-1, and up-regulates their expression. In our study, both LA and SAM increased Nrf2 translocation to the nucleus, and Nrf2 siRNA significantly reduced HO-1 induction by LA or SAM, showing that this process is Nrf2-dependent. However, LA increased ROS generation whereas SAM decreased LA-induced increase in ROS generation. These data suggest that LA activates Nrf2 in an oxidative stress-dependent mechanism, but that SAM activates Nrf2 by a currently unidentified mechanism.
In this study, we also showed that the mechanisms by which LA and SAM induce HO-1 expression are different. We showed that LA-induced HO-1 expression was dependent on PPARα, whereas SAM induced HO-1 in a PPAR-independent manner. PPARα is a transcription factor responsible for fatty acid oxidation, and the PPARα/HO-1 signaling pathways constitute a protective mechanism against various cellular insults (Cheng et al., 2012; Yu et al., 2010). The precise mechanism responsible for the difference observed in HO-1 induction by LA and SAM awaits future studies.
Another interesting finding of our study is the effect of HO-1 siRNA on cell apoptosis. As we expected, HO-1 siRNA abrogated SAM-dependent inhibition of cell apoptosis. On the other hand, HO-1 siRNA did not affect cell apoptosis in LA-treated cells, suggesting that LA-induced increase in HO-1 may not participate in or may not be a major mechanism of cell protection. In addition to HO-1, the expression of various antioxidant enzymes was increased by LA. LA also increased various markers of UPR. UPR is a highly specific signaling pathway, which protects against ER stress, even though prolonged activation of UPR is deleterious to cells (Rao et al., 2004). In fact, LA increased the expression of CHOP, a transcription factor that induces apoptosis during periods of prolonged ER stress (Rao et al., 2004). Our data suggest that, even though HO-1 induction may be a compensatory response to LA-induced cellular stress, this is not adequate enough to protect the cells from cellular stress. On the other hand, HO-1 induction by SAM contributes to the protection of endothelial cells from apoptosis. Finally, we examined whether SAM treatment affects vascular function in the rat aorta. As reported previously (Won et al., 2010), HFD impaired endothelium-dependent but not endothelium-independent vascular relaxation. Defective endothelium-dependent vasodilation is an important early event in the development of atherosclerosis (Won et al., 2010). SAM partially reversed the endothelium-dependent vascular dysfunction induced by HFD. Hyperhomocysteinemia is regarded as an important cardiovascular risk factor. However, the mechanisms responsible for this association are incompletely understood. A leading hypothesis is that homocysteine increases oxidative stress and impairs endothelial function (Bao et al., 2010). Our data showed that HFD significantly increased plasma homocysteine concentration. This is in agreement with previous studies showing that HFD increases plasma homocysteine concentration (Yun et al., 2013). Interestingly, SAM improves vascular dysfunction without decreasing homocysteine concentration. These findings suggest a possible clinical use for SAM treatment in the prevention of atherosclerosis.
In this study, we did not examine the precise mechanism by which SAM and SAM-mediated induction of HO-1 prevent endothelial apoptosis. However, HO-1 and CO, a heme degradation product, are well known to play a protective role in the vasculature (Leffler et al., 2011). In addition, previous studies have shown that SAM increases mitochondrial biogenesis (Piantadosi et al., 2008), and the synthesis of glutathione, an important intracellular antioxidant (Rahman, 1999).
In conclusion, our study showed that SAM prevents endothelial dysfunction by reducing apoptosis and ER stress in vascular endothelial cells. This preventive effect of SAM could partly be attributed to the induction of HO-1. Based on our findings, we propose that SAM represents a potential new therapeutic drug for the prevention and/or treatment of atherosclerosis.
Acknowledgments
This work was supported by the Korea Health Industry Development Institute (A084335).
REFERENCES
- Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11:S56–S64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- Bao XM, Wu CF, Lu GP. Atorvastatin inhibits homocysteine-induced dysfunction and apoptosis in endothelial progenitor cells. Acta Pharmacol Sin. 2010;31:476–484. doi: 10.1038/aps.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A, Becuwe P, Franck P, Dauca M. Induction of MnSOD gene by arachidonic acid is mediated by reactive oxygen species and p38 MAPK signaling pathway in human HepG2 hepatoma cells. Free Radic Biol Med. 2002;32:1132–1142. doi: 10.1016/s0891-5849(02)00834-1. [DOI] [PubMed] [Google Scholar]
- Caballero F, Fernandez A, Matias N, Martinez L, Fucho R, Elena M, Caballeria J, Morales A, Fernandez-Checa JC, Garcia-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem. 2010;285:18528–18536. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Chen TW, Lin H. Pravastatin attenuates carboplatin-induced nephrotoxicity in rodents via peroxisome proliferator-activated receptor alpha-regulated heme oxygenase-1. Mol Pharmacol. 2010;78:36–45. doi: 10.1124/mol.109.061101. [DOI] [PubMed] [Google Scholar]
- Cheng CF, Lian WS, Chen SH, Lai PF, Li HF, Lan YF, Cheng WT, Lin H. Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPARalpha-heme oxygenase-1 signaling pathway. J Cell Physiol. 2012;227:239–249. doi: 10.1002/jcp.22726. [DOI] [PubMed] [Google Scholar]
- Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, Heistad DD, Faraci FM, Lentz SR. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res. 2001;88:1203–1209. doi: 10.1161/hh1101.092180. [DOI] [PubMed] [Google Scholar]
- Erdmann K, Cheung BW, Immenschuh S, Schroder H. Heme oxygenase-1 is a novel target and antioxidant mediator of S-adenosylmethionine. Biochem Biophys Res Commun. 2008;368:937–941. doi: 10.1016/j.bbrc.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Esfandiari F, You M, Villanueva JA, Wong DH, French SW, Halsted CH. S-adenosylmethionine attenuates hepatic lipid synthesis in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res. 2007;31:1231–1239. doi: 10.1111/j.1530-0277.2007.00407.x. [DOI] [PubMed] [Google Scholar]
- Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Ji C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int. 2012;2012:216450. doi: 10.1155/2012/216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzmann T, Samoylenko A, Immenschuh S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. J Biol Chem. 2003;278:17927–17936. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- Lee KU, Lee IK, Han J, Song DK, Kim YM, Song HS, Kim HS, Lee WJ, Koh EH, Song KH, et al. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res. 2005;96:1200–1207. doi: 10.1161/01.RES.0000170075.73039.5b. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol. 2011;301:H1–H11. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Moon MK, Shin H, Kim TH, Cho BJ, Kim M, Park HS, Choi SH, Ko SH, Chung MH, et al. Effect of S-adenosylmethionine on neointimal formation after balloon injury in obese diabetic rats. Cardiovasc Res. 2011;90:383–393. doi: 10.1093/cvr/cvr009. [DOI] [PubMed] [Google Scholar]
- Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13:216–226. [PubMed] [Google Scholar]
- Ou L, Wu Y, Ip C, Meng X, Hsu YC, Ip MM. Apoptosis induced by t10,c12-conjugated linoleic acid is mediated by an atypical endoplasmic reticulum stress response. J Lipid Res. 2008;49:985–994. doi: 10.1194/jlr.M700465-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl G, Stickel F, Wang XD, Seitz HK. Alcohol and cancer: genetic and nutritional aspects. Proc Nutr Soc. 2004;63:65–71. doi: 10.1079/PNS2003323. [DOI] [PubMed] [Google Scholar]
- Rahman I. Inflammation and the regulation of glutathione level in lung epithelial cells. Antioxid Redox Signal. 1999;1:425–447. doi: 10.1089/ars.1999.1.4-425. [DOI] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- Soeken KL, Lee WL, Bausell RB, Agelli M, Berman BM. Safety and efficacy of S-adenosylmethionine (SAMe) for osteoarthritis. J Fam Pract. 2002;51:425–430. [PubMed] [Google Scholar]
- Spijkerman AM, Smulders YM, Kostense PJ, Henry RM, Becker A, Teerlink T, Jakobs C, Dekker JM, Nijpels G, Heine RJ, et al. S-adenosylmethionine and 5-methyltetrahydrofolate are associated with endothelial function after controlling for confounding by homocysteine: the Hoorn Study. Arterioscler Thromb Vasc Biol. 2005;25:778–784. doi: 10.1161/01.ATV.0000157981.57694.d2. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Li MH, Na HK, Cha YN. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch Pharm Res. 2009;32:1163–1176. doi: 10.1007/s12272-009-1807-8. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchantchou F, Graves M, Falcone D, Shea TB. S-adenosylmethionine mediates glutathione efficacy by increasing glutathione S-transferase activity: implications for S-adenosyl methionine as a neuroprotective dietary supplement. J Alzheimers Dis. 2008;14:323–328. doi: 10.3233/jad-2008-14306. [DOI] [PubMed] [Google Scholar]
- Vázquez-Chantada M, Ariz U, Varela-Rey M, Embade N, Martínez-Lopez N, Fernández-Ramos D, Gómez-Santos L, Lamas S, Lu SC, Martínez-Chantar ML, et al. Evidence for LKB1/AMP-activated protein kinase/endothelial nitric oxide synthase cascade regulated by hepatocyte growth factor, S-adenosylmethionine, and nitric oxide in hepatocyte proliferation. Hepatology. 2009;49:608–617. doi: 10.1002/hep.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Koury MJ. S-Adenosylhomocysteine a better indicator of vascular disease than homocysteine? Am J Clin Nutr. 2007;86:1581–1585. doi: 10.1093/ajcn/86.5.1581. [DOI] [PubMed] [Google Scholar]
- Wang CY, Chau LY. Heme oxygenase-1 in cardiovascular diseases: molecular mechanisms and clinical perspectives. Chang Gung Med J. 2010;33:13–24. [PubMed] [Google Scholar]
- Won JC, Park JY, Kim YM, Koh EH, Seol S, Jeon BH, Han J, Kim JR, Park TS, Choi CS, et al. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha overexpression prevents endothelial apoptosis by increasing ATP/ADP translocase activity. Arterioscler Thromb Vasc Biol. 2010;30:290–297. doi: 10.1161/ATVBAHA.109.198721. [DOI] [PubMed] [Google Scholar]
- Wright MM, Kim J, Hock TD, Leitinger N, Freeman BA, Agarwal A. Human haem oxygenase-1 induction by nitro-linoleic acid is mediated by cAMP, AP-1 and E-box response element interactions. Biochem J. 2009;422:353–361. doi: 10.1042/BJ20090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Opposite action of S-adenosyl methionine and its metabolites on CYP2E1-mediated toxicity in pyrazole-induced rat hepatocytes and HepG2 E47 cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G674–G684. doi: 10.1152/ajpgi.00406.2005. [DOI] [PubMed] [Google Scholar]
- Wu ML, Ho YC, Yet SF. A central role of heme oxygenase-1 in cardiovascular protection. Antioxid Redox Signal. 2011;15:1835–1846. doi: 10.1089/ars.2010.3726. [DOI] [PubMed] [Google Scholar]
- Xu HE, Stanley TB, Montana VG, Lambert MH, Shearer BG, Cobb JE, McKee DD, Galardi CM, Plunket KD, Nolte RT, et al. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature. 2002;14:813–817. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- Yu J, Chu ES, Wang R, Wang S, Wu CW, Wong VW, Chan HL, Farrell GC, Sung JJ. Heme oxygenase-1 protects against steatohepatitis in both cultured hepatocytes and mice. Gastroenterology. 2010;138:694–704. doi: 10.1053/j.gastro.2009.09.058. [DOI] [PubMed] [Google Scholar]
- Yun KU, Ryu CS, Oh JM, Kim CH, Lee KS, Lee CH, Lee HS, Kim BH, Kim SK. Plasma homocysteine level and hepatic sulfur amino acid metabolism in mice fed a high-fat diet. Eur J Nutr. 2013;52:127–134. doi: 10.1007/s00394-011-0294-0. [DOI] [PubMed] [Google Scholar]