Abstract
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key transcriptional regulator for the protection of cells against oxidative and xenobiotic stresses. Recent studies have demonstrated that high constitutive expression of Nrf2 is observed in many types of cancer cells showing resistance to anti-cancer drugs, suggesting that the suppression of overexpressed Nrf2 could be an attractive therapeutic strategy to overcome cancer drug resistance. In the present study, we aimed to find small molecule compounds that enhance the sensitivity of tumor cells to cisplatin induced cytotoxicity by suppressing Nrf2-mediated defense mechanism. A549 lung cancer cells were shown to be more resistant to the anti-cancer drug cisplatin than HEK293 cells, with higher Nrf2 signaling activity; constitutively high amounts of Nrf2-downstream target proteins were observed in A549 cells. Among the three chalcone derivatives 4-methoxy-chalcone (4-MC), hesperidin methylchalcone, and neohesperidin dihydrochalcone, 4-MC was found to suppress transcriptional activity of Nrf2 in A549 cells but to activate it in HEK293 cells. 4-MC was also shown to down-regulate expression of Nrf2 and the downstream phase II detoxifying enzyme NQO1 in A549 cells. The PI3K/Akt pathway was found to be involved in the 4-MC-induced inhibition of Nrf2/ARE activity in A549 cells. This inhibition of Nrf2 signaling results in the accelerated generation of reactive oxygen species and exacerbation of cytotoxicity in cisplatin-treated A549 cells. Taken together, these results suggest that the small molecule compound 4-MC could be used to enhance the sensitivity of tumor cells to the therapeutic effect of cisplatin through the regulation of Nrf2/ARE signaling.
Keywords: 4-methoxychalcone, A549, chemosensitivity, cisplatin, Nrf2
INTRODUCTION
Resistance of cancer cells to chemotherapy is known to be a major obstacle to the treatment. The mechanism of resistance involves mutation or overexpression of target proteins, as well as decreased bioavailability of drugs caused by impaired uptake or enhanced detoxification (Ren et al., 2011). Recent studies have suggested that nuclear factor erythroid 2-related factor 2 (Nrf2), a basic leucine zipper transcription factor, could be an important contributor to chemoresistance (Lau et al., 2008). In fact, mutations in Nrf2 or related signaling molecules are found in many types of cancers, where they cause an increase in Nrf2 activity and are associated with resistance to chemotherapy (Sporn and Liby, 2012).
Nrf2 plays an essential role in regulating cellular redox homeostasis and protects cells from oxidative stress by activating transcription of its target genes (Jaiswal, 2004) and enhancing cellular defense systems such as antioxidant and phase II detoxifying enzymes (Hayes et al., 2010; Kaspar et al., 2009). Because oxidative stress is implicated in the initiation and progression of cancer, activation of Nrf2 signaling has been considered as an useful strategy for chemoprevention (Giudice et al., 2010). However, more recently, high constitutive expression of Nrf2 has been observed in many types of cancer cells showing resistance to anti-cancer drugs (Lau et al., 2008). Many anti-cancer drugs induce apoptosis by producing reactive oxygen species (ROS) in cancer cells; therefore, its antioxidant and cytoprotective potential could be considered the ‘dark side’ of Nrf2 signaling as regards the therapeutic action of these drugs. This perspective is corroborated by reports demonstrating that up-regulation of Nrf2 downstream genes including heme oxygenase-1 (HO-1) and NAD(P)H:quinone oxidoreductase 1 (NQO1) is related to the resistance of cancer cells to chemotherapy (Kweon et al., 2006), and that down-regulation of Nrf2 causes radiosensitization (Singh et al., 2010). Therefore, Nrf2/ARE regulators are needed for a new therapeutic approach against cancer as adjuvant chemotherapeutic drugs.
Chalcone, an open-chain flavonoid that bears two aromatic rings linked by a three-carbon enone moiety (Lee et al., 2012a), is widely synthesized in many plants. Chalcone derivatives have various biological properties including anti-inflammatory, antioxidant, and cytoprotective activities in normal cells (Kachadourian et al., 2012; Park et al., 2009; Szliszka et al., 2010; Vogel et al., 2010). It has also been demonstrated that chalcone derivatives have anti-cancer activity through ROS production in various tumor cells (Kim et al., 2010), as well as potent cytotoxic activity in carcinoma cells (Rao et al., 2010). Although chalcone derivatives have very similar chemical structures, their cellular activities are quite different; they can act as chemopreventors or chemosensitizers depending on their structure and cell type (Yadav et al., 2011).
The aim of this study was to find small molecule compounds that enhance the sensitivity of tumor cells to the cytotoxic action of anti-cancer drugs, and to evaluate the relevant molecular mechanism. To this end, we focused on the effect of 4-methoxychalcone (4-MC) on the sensitivity of human lung epithelial adenocarcinoma A549 cells to the cytotoxicity induced by the anti-cancer drug cisplatin, and on the regulation of Nrf2/ARE signaling.
MATERIALS AND METHODS
Materials
The 4-MC, hesperidine methylchalcone (HMC), neohesperidine dihydrochalcone (NH-DHC), cisplatin, and 2′,7′-dihydrofluorescein-diacetate (DCF-DA) were purchased from Sigma-Aldrich (USA). Antibodies against phospho-Akt (Ser473 and Thr308), Akt and GAPDH were purchased from Cell Signaling Technology (USA), and Lamin B, Nrf2, NQO1 and HO-1 antibodies were from Santa Cruz Biotechnology (USA). Alpha-actin antibody was purchased from Sigma-Aldrich. pGL3-ARE and pRLTK reporter constructs were kindly provided by Prof. Keon Wook Kang (Seoul National University, Korea).
Cell culture
A549 and HEK293 cells were obtained from the American Type Culture Collection (ATCC; USA) and were cultured in RPMI1640 and DMEM media supplemented with 10% FBS and 100 U/ml penicillin/streptomycin at 37°C in 5% CO2 in a humidified atmosphere, respectively.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
A549 and HEK 293 cells were seeded in 48-well plates, incubated for 24 h, then fed with fresh medium for cisplatin treatment. At the end of the treatment, the medium was removed, and MTT (0.5 mg/ml) was added to each well for at least 4 h. The purple formazan precipitate was dissolved with DMSO, and the color intensity was measured at 550 nm with a Versa-max microplate reader (Molecular Devices, USA)(Ma et al., 2012).
Transient transfection and reporter gene assay
Cells were transfected with pGL3-ARE and pRL-TK reporter plasmids using polyethylenimine according to the manufacturer’s protocol (Polysciences, USA). A Dual-Luciferase reporter assay system (Promega, USA) was used to determine ARE-driven promoter activity.
Nuclear fractionation and Western blot analysis
The isolation of the nuclear fraction is described in a previous report (Lee et al., 2011). For immunoblot analysis, cells were washed with phosphate-buffered saline (PBS), harvested and lysed with RIPA buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% DOC and 0.1% SDS). Equal amounts of proteins were separated on a 10% SDS polyacrylamide gel and transblotted onto a PVDF membrane. Membranes were incubated with the appropriate primary antibody and then with horseradish peroxidase-con-jugated secondary antibody. Specific bands were visualized using the enhanced chemiluminescence (ECL) detection kit (Millipore, USA).
Flow cytometry analysis of apoptotic cells by propidium iodide (PI) staining
A549 cells were incubated with 5 μg/ml 4-MC for 24 h. Harvested cells were washed twice with PBS, fixed in 70% ethanol (in PBS) on ice overnight, and then resuspended in PBS containing 10 μg/ml PI, 0.5 mg/ml RNase and 0.1% Triton X-100. After 30 min at 37°C in the dark, the cells were analyzed with a FACS Calibur flow cytometer (Becton Dickinson Immunocytometry System, USA).
Reactive oxygen species (ROS) determination
The intracellular level of ROS was detected using DCF-DA fluorescence analysis. After incubation with the indicated conditions, cells were loaded with 10 μM DCF-DA for 1 h at 37°C, then detached from plates and washed PBS twice. Cells were analyzed by a FACS Calibur Flow Cytometer. For measuring fluorescence intensity, cells were loaded with 10 μM DCF-DA in Krebs-ringer solution for 1 h at 37°C, then measured DCF-fluorescence intensity using fluorescence reader (Infinite M200 Pro, Tecan, Austria) and TE2000U fluorescence microscope (Nikon Corporation, Japan). DCF-DA fluorescence was measured using an excitation of 495 nm and emission of 520 nm.
Statistical analysis
Data were analyzed and expressed as the mean ± S.E.M. Comparisons were made using ANOVA and the Student-Newman-Keuls test. P < 0.05 was considered to be statistically significant.
RESULTS
A549 cells are less susceptible to cisplatin cytotoxicity and show higher Nrf2 signaling activity than HEK293 cells
Because the evidence has shown that A549 cells express high levels of Nrf2 and HO-1 compared to other lung cancer cell lines (Kim et al., 2008), we used these cells in the present study as an in vitro cell model of chemoresistance. To verify the resistance of A549 cells to anti-cancer drug, we first compared the cytotoxicity of cisplatin in A549 and HEK293 cells. Cisplatin, one of the most potent and widely used anti-cancer drugs, leads to cell death via increased generation of ROS (Casares et al., 2012). As shown in Fig. 1A, the viability of both cell lines was decreased by the drug in a dose-dependent manner. However, there were significant differences in cisplatin-induced cytotoxicity between A549 and HEK293 cells; the viability of A549 cells was significantly higher than that of HEK293 cells (59.54 ± 0.79% compared to 39.63 ± 1.35%, respectively). Next, we evaluated whether the endogenous expression of Nrf2 was higher in A549 cells than in HEK293 cells, and found no significant difference between two cell lines (Fig. 1B). However, the nuclear Nrf2 level was higher in A549 cells than in HEK293 cells, suggesting higher Nrf2 signaling activity in A549 cells (Fig. 1C). This finding was corroborated by the fact that constitutively high amounts of the Nrf2-downstream target protein NQO1, an Nrf2-driven phase II detoxifying enzyme, and HO-1, an antioxidant enzyme, were observed in A549 cells but were rarely detected in HEK293 cells (Fig. 1D). Based on these results, our further experiments to find small molecule compounds that enhance the sensitivity of tumor cells to cisplatin-induced cytotoxicity were performed using these two cell lines.
Fig. 1.
A549 cells show high endogenous expression of NQO1 and HO-1, and are resistant to cisplatin toxicity. (A) A549 and HEK293 cells were treated with anti-cancer drug cisplatin at 50 and 100 μM. After 24 h, cell viability was measured by the MTT assay. Data (means ± S.E.M.) were representative of at least three independent experiments and expressed as the fold induction relative to untreated cells (at time zero); **P < 0.01 and *P < 0.05. (B, C) Total (B) and nuclear (C) Nrf2 expression in A549 and HEK293 cells were determined by Western blotting. GAPDH was used as a loading control, Lamin B as a nuclear fraction marker and α-actin as a cytosolic fraction marker. (D) Endogenous NQO1 and HO-1 expression in A549 and HEK293 cells was determined by Western blotting. Alpha-actin was used as a loading control.
The chalcone derivative 4-MC down-regulates Nrf2/ARE signaling in A549 cells but increases it in HEK293 cells
Chalcone compounds are reported to exert both cytotoxic and cytoprotective activities according to their structure and cell type (Yadav et al., 2011). In addition, the exact mechanism by which chalcone compounds affect cellular viability remains unclear. In the present study, we aimed to find candidate compounds that enhance cisplatin-induced cytotoxicity by inhibiting the Nrf2/ARE-mediated defense mechanism in A549 cells. For this purpose, we evaluated the effects of three chalcone derivatives, 4-methoxychalcone (4-MC), hesperidin methylchalcone (HMC), and neohesperidin dihydrochalcone (NH-DHC), on Nrf2/ARE signaling in A549 and HEK293 cells (Fig. 2A). Fig. 2B showed that all three compounds increased the ARE-luciferase activity in HEK293 cells but not in A549 cells. Interestingly, 4-MC significantly decreased ARE-luciferase activity at 20.98 μM (0.64 ± 0.03, 1.19 ± 0.04, and 1.13 ± 0.02-fold induction compared to the control, by 4-MC, HMC, and NH-DHC, respectively). The 4-MC decreased ARE-luciferase activity in a dose-dependent manner in A549 cells, but increased it in HEK293 cells (Fig. 3A). The opposite effects of 4-MC on Nrf2 signaling in A549 and HEK293 cells were also verified by the results shown in Fig. 3B. The total expression levels of Nrf2 and NQO1 were decreased by 4-MC in A549 cells but were increased in HEK293 cells. These results suggest that 4-MC could act as an inhibitor of the Nrf2/ARE signaling pathway, and, therefore, could enhance chemosensitivity to cisplatin in A549 cells. In addition, in non-cancer cells, it could act as a cytoprotective molecule by enhancing Nrf2/ARE signaling.
Fig. 2.
The chalcone derivative 4-MC inhibits Nrf2/ARE transcriptional activity in A549 cells. (A) Structure of chalcone derivatives, 4-methoxychalcone (4-MC), hesperidin methylchalcone (HMC), neohesperidin dihydrochalcone (NH-DHC). (B) A549 and HEK293 cells were treated with 5 μg/ml of each chalcone derivative (4-MC, HMC and NH-DHC). After 6 h, ARE-dependent transcriptional activity was measured by reporter gene assay. Data (means ± S.E.M.) are expressed as the fold induction relative to untreated cells (at time zero); ***P < 0.001, **P < 0.01 and *P < 0.05.
Fig. 3.
The 4-MC inhibits the Nrf2/ARE signaling pathway in A549 cells, but activates it in HEK293 cells. (A) A549 and HEK293 cells were treated with a variety of concentrations of 4-MC. After 30 min, ARE-dependent transcriptional activity was measured by reporter gene assay. (B) A549 and HEK293 cells were treated with 5 μg/ml 4-MC for the indicated time. Nrf2 and Nrf2-driven NQO1 protein levels were analyzed by Western blot. Alpha-actin was used as a loading control. Data (means ± S.E.M.) are expressed as the fold induction relative to untreated cells (at time zero); ***P < 0.001, **P < 0.01 and *P < 0.05.
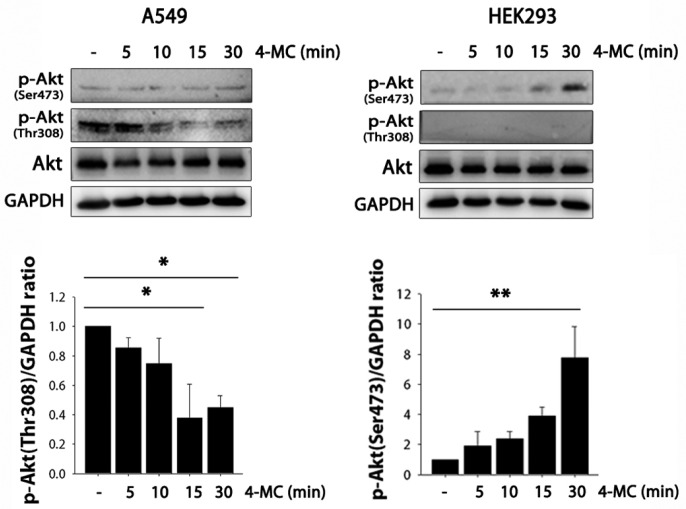
The PI3K/Akt pathway is involved in 4-MC-induced inhibition of Nrf2/ARE activity in A549 cells
Because many studies have reported the contribution of PI3K/Akt to the activation of Nrf2 signaling (Feng et al., 2011; Lim et al., 2008), we evaluated whether this pathway is involved in 4-MC-induced inhibition of Nrf2/ARE activity in A549 cells. Activation of this pathway is dependent on the phosphorylation status of both Thr308 and Ser473 residues (Alessi et al., 1996), and is known to facilitate both the release of Nrf2 from Keap1 and its subsequent translocation into the nucleus as well as ARE-promoter activation (Papaiahgari et al., 2006). As shown in Fig. 4, the amount of phospho-Akt (Ser473) was increased in a time-dependent manner by 4-MC in HEK293 cells, suggesting activation of the PI3K/Akt pathway. However, in A549 cells, there were no significant changes in the level of phospho-Akt (Ser473). In addition, the amount of phospho-Akt (Thr308) was decreased by treatment with 4-MC in A549 cells. 4-MC thus inhibited the PI3K/Akt pathway in A549 cells but not in HEK293 cells; this difference in PI3K/Akt regulation could be a relevant signaling pathway that contributes to the opposite effect of 4-MC in A549 and HEK293 cells.
Fig. 4.
The PI3K/AKT pathway is involved in 4-MC-induced inhibition of Nrf2-ARE signaling in A549 cells. A549 and HEK293 cells were treated with 5 μg/ml 4-MC for the indicated time (5, 10, 15, 30 min). Phospho-Akt (Ser473 and Thr308) and Akt protein levels were analyzed by Western blot. GAPDH was used as a loading control. Data (means ± S.E.M.) are expressed as the fold induction relative to untreated cells (at time zero); **P < 0.01 and *P < 0.05.
4-MC stimulates the generation of oxidative stress and exacerbates cytotoxicity in cisplatin-treated A549 cells
Considering that, in A549 cells, constitutively high levels of nuclear Nrf2 as well as Nrf2-driven phase 2 detoxifying enzyme and anti-oxidant enzyme expression were observed (Fig. 1) and 4-MC inhibited Nrf2/ARE activity (Fig. 2), we next evaluated whether 4-MC enhances cisplatin-induced cytotoxicity in these cells. As shown in Fig. 5A, cisplatin-induced cytotoxicity was increased in A549 cells in the presence of 4-MC compared to cells treated with cisplatin alone. In contrast, 4-MC was cyto-protective against cisplatin cytotoxicity in HEK293 cells. These results were supported by the FACS data from Fig. 5B, which showed that cisplatin-induced apoptosis was significantly exacerbated by treatment with 4-MC in A549 cells. In addition, 4-MC accelerated the generation of ROS in cisplatin-treated A549 cells, which was evidenced by higher DCF-DA fluorescence in cells treated with both 4-MC and cisplatin (Figs. 5C, 5D, and 5E). However, as shown in Figs. 5D and 5E, cisplatin-induced ROS generation in HEK293 cells were decreased by co-treatment of 4-MC. The observed enhancement of cisplatin-induced oxidative stress and cytotoxicity by 4-MC in A549 cells suggests that this compound could make these cells susceptible to chemotherapy.
Fig. 5.
The 4-MC exacerbates cisplatin-induced ROS generation and apoptosis in A549 cells. (A) A549 and HEK293 cells were pre-treated with 4-MC for 30 min and exposed to 100 μM cisplatin for an additional 24 h. Cell viability was determined by MTT assay. Data (means ± S.E.M.) were representative of at least three independent experiments and were expressed as the fold induction relative to untreated cells (at time zero); *** P < 0.001. (B) After treatment with 100 μM cisplatin alone or a combination of 100 μM cisplatin and 5 μg/ml 4-MC for 24 h, A549 cells were stained with 10 μg/ml PI, and cells with a sub-G0/G1 DNA content were identified by flow cytometry. (C–E) After treatment with 100 μM cisplatin alone, or a combination of 100 μM cisplatin and 5 μg/ml 4-MC for 24 h, A549 and HEK293 cells were loaded with 10 μM DCF-DA for 1 h, and measured fluorescence intensity using flow cytometer (C) and fluorescence reader (D), and obtained fluorescence images by fluorescence microscope (E).
DISCUSSION
Enhanced Nrf2-driven gene expression has been suggested not only to protect cells from oxidative stress and contribute to chemoprevention but also to cause resistance to chemotherapy and promote cancer cell growth (Wang et al., 2008; Zhang, 2010). Because of these potential negative roles of Nrf2 signaling in chemotherapy, suppression of Nrf2/ARE activity during cancer treatment could be clinically beneficial (Kensler and Wakabayashi, 2010). The A549 cells used in the present study are known to have constitutively activated Nrf2 signaling (Homma et al., 2009). We also demonstrated higher Nrf2 signaling activity in these cells than in HEK293 cells (Fig. 1). Due to their constitutive activation of the Nrf2 pathway, the Nrf2/ARE signaling activator tert-butylhydroxyquinone failed to increase the additive activation of Nrf2 signaling in A549 cells (data not shown).
We show here that 4-MC sensitizes A549 cells to cisplatin toxicity (Fig. 5). The 4-MC-mediated sensitization of cisplatin-induced cell death relies on its ability to inhibit the Nrf2/ARE signaling activity in A549 cells (Figs. 2 and 3). Interestingly, 4-MC down-regulates Nrf2/ARE signaling in A549 cells, but increases it in HEK293 cells. The signaling mechanism leading to these differences in Nrf2 signaling and cisplatin-induced cell death between the two cell lines seems to be related to the different regulation of PI3K-Akt activity by 4-MC according to the cell type or endogenous Nrf2 signaling activity (Fig. 4). The 4-MC activates Akt phosphorylation (Ser473) in HEK293 cells but not in A549 cells. Moreover, 4-MC induces a significant decrease in the level of phospho-Akt (Thr308) in A549 cells but not in HEK293 cells. Because the PI3K/Akt signaling pathway plays a crucial role in the regulation of Nrf2 activity, this difference in PI3K/Akt regulation could be a relevant signaling pathway that contributes to the opposite effects of 4-MC in A549 and HEK293 cells.
We did not evaluate in the present study why 4-MC exerted different effects on Nrf2 signaling activity in A549 cells compared to the other two chalcone derivatives HMC and NH-DHC, but our study clearly demonstrates that 4-MC could be an attractive adjuvant chemotherapeutic drug to combat chemoresistance while it protects normal cells at the same time (Fig. 5). Cisplatin is known to induce nephrotoxicity, hepatotoxicity, and oxidative stress even in normal tissues (dos Santos et al., 2012; Liao et al., 2008), so one molecule that exacerbates oxidative stress and cytotoxicity in cancer cells but not in normal cells could be clinically attractive in chemotherapy. Ginsenoside Rg3 has been reported to enhance the chemosensitivity of colon cancer cells to cisplatin and prevent normal tissue damage by a similar mechanism to that of 4-MC (Lee et al., 2012b).
This is the first demonstration that 4-MC, a chalcone derivatives that potentiates cisplatin-induced toxicity by blocking Nrf2-mediated antioxidant responses, could be a useful chemosensitizing agent in the treatment of lung cancer. Its opposing regulatory effects on the Nrf2-mediated defense mechanism have the double benefit of promoting cell death in cancer cells and protecting other normal cells from anti-cancer drug-induced toxicity. Further biological and toxicological investigations are required to establish 4-MC as a new drug candidate. Collectively, our data demonstrate that 4-MC acts as a chemopreventor in cells via activation of Nrf2/ARE signaling, but in cancer cells showing chemoresistance, 4-MC acts as chemosensitizer through the reduction of Nrf2/ARE signaling, leading to a decrease in the activity of the cellular defense system.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (331-2008-1-E00456, 2009-0068220, 2012R1A1A3011420) to H.J. Choi.
REFERENCES
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Casares C, Ramirez-Camacho R, Trinidad A, Roldan A, Jorge E, Garcia-Berrocal JR. Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. Eur Arch Otorhinolaryngol. 2012;269:2455–2459. doi: 10.1007/s00405-012-2029-0. [DOI] [PubMed] [Google Scholar]
- dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012;86:1233–1250. doi: 10.1007/s00204-012-0821-7. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhang P, Chen X, He G. PI3K and ERK/Nrf2 pathways are involved in oleanolic acid-induced heme oxygenase-1 expression in rat vascular smooth muscle cells. J Cell Biochem. 2011;112:1524–1531. doi: 10.1002/jcb.23065. [DOI] [PubMed] [Google Scholar]
- Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N, et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–3432. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Kachadourian R, Day BJ, Pugazhenti S, Franklin CC, Genoux-Bastide E, Mahaffey G, Gauthier C, Di Pietro A, Boumendjel A. A synthetic chalcone as a potent inducer of glutathione biosynthesis. J Med Chem. 2012;55:1382–1388. doi: 10.1021/jm2016073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Kim S, Kim EJ, Park JH, Yang SH, Jeong ET, Park C, Youn MJ, So HS, Park R. Suppression of Nrf2-driven heme oxygenase-1 enhances the chemo-sensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer. 2008;60:47–56. doi: 10.1016/j.lungcan.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Kim TH, Seo WD, Ryu HW, Seo HR, Jin YB, Lee M, Ji YH, Park KH, Lee YS. Anti-tumor effects by a synthetic chalcone compound is mediated by c-Myc-mediated reactive oxygen species production. Chem Biol Interact. 2010;188:111–118. doi: 10.1016/j.cbi.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Kweon MH, Adhami VM, Lee JS, Mukhtar H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J Biol Chem. 2006;281:33761–33772. doi: 10.1074/jbc.M604748200. [DOI] [PubMed] [Google Scholar]
- Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IS, Lim J, Gal J, Kang JC, Kim HJ, Kang BY, Choi HJ. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem Int. 2011;58:153–160. doi: 10.1016/j.neuint.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Lee YH, Jeon SH, Kim SH, Kim C, Lee SJ, Koh D, Lim Y, Ha K, Shin SY. A new synthetic chalcone derivative, 2-hydroxy-3′,5,5′-trimethoxychalcone (DK-139), suppresses the Toll-like receptor 4-mediated inflammatory response through inhibition of the Akt/NF-kappaB pathway in BV2 microglial cells. Exp Mol Med. 2012a;44:369–377. doi: 10.3858/emm.2012.44.6.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Park KK, Chung AS, Chung WY. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food Chem Toxicol. 2012b;50:2565–2574. doi: 10.1016/j.fct.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Liao Y, Lu X, Lu C, Li G, Jin Y, Tang H. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol Res. 2008;57:125–131. doi: 10.1016/j.phrs.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Lim JH, Kim KM, Kim SW, Hwang O, Choi HJ. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: novel cytoprotective mechanism against oxidative damage. Pharmacol Res. 2008;57:325–331. doi: 10.1016/j.phrs.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wu X, Li X, Fu J, Shen J, Wang H. Corticosterone regulates the expression of neuropeptide Y and reelin in MLO-Y4 cells. Mol Cells. 2012;33:611–616. doi: 10.1007/s10059-012-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY, Reddy SP. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal. 2006;8:43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- Park SJ, Song HY, Youn HS. Suppression of the TRIF-dependent signaling pathway of toll-like receptors by isoliquiritigenin in RAW264.7 macrophages. Mol Cells. 2009;28:365–368. doi: 10.1007/s10059-009-0130-z. [DOI] [PubMed] [Google Scholar]
- Rao YK, Kao TY, Ko JL, Tzeng YM. Chalcone HTMC causes in vitro selective cytotoxicity, cell-cycle G1 phase arrest through p53-dependent pathway in human lung adenocarcinoma A549 cells, and in vivo tumor growth suppression Bioorg. Med Chem Lett. 2010;20:6508–6512. doi: 10.1016/j.bmcl.2010.09.056. [DOI] [PubMed] [Google Scholar]
- Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci USA. 2011;108:1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid Redox Signal. 2010;13:1627–1637. doi: 10.1089/ars.2010.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szliszka E, Czuba ZP, Mazur B, Paradysz A, Krol W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules. 2010;15:5336–5353. doi: 10.3390/molecules15085336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Barbic M, Jurgenliemk G, Heilmann J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur J Med Chem. 2010;45:2206–2213. doi: 10.1016/j.ejmech.2010.01.060. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VR, Prasad S, Sung B, Aggarwal BB. The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int Immunopharmacol. 2011;11:295–309. doi: 10.1016/j.intimp.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD. The Nrf2-Keap1-ARE signaling pathway: the regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13:1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]