Abstract
Apigenin, a member of the flavone subclass of flavonoids, has long been considered to have various biological activities. Its glucosides, in particular, have been reported to have higher water solubility, increased chemical stability, and enhanced biological activities. Here, the synthesis of apigenin glucosides by the in vitro glucosylation reaction was successfully performed using a UDP-glucosyltransferase YjiC, from Bacillus licheniformis DSM 13. The glucosylation has been confirmed at the phenolic groups of C-4′ and C-7 positions ensuing apigenin 4′-O-glucoside, apigenin 7-O-glucoside and apigenin 4′,7-O-diglucoside as the products leaving the C-5 position unglucosylated. The position of glucosylation and the chemical structures of glucosides were elucidated by liquid chromatography/mass spectroscopy and nuclear magnetic resonance spectroscopy. The parameters such as pH, UDP glucose concentration and time of incubation were also analyzed during this study.
Keywords: apigenin, enzymatic synthesis, glucosides, UDP-glucosyltransferase, YjiC
INTRODUCTION
Flavonoids are polyphenolic compounds that are ubiquitous in nature and are categorized according to chemical structure into flavonols, flavones, flavanones, isoflavones, catechins, anthocyanidins, and chalcones (Rice-Evans et al., 1996; Ross and Kasum, 2002). Over 9,000 flavonoids have been identified, many of which occur in fruits, vegetables, and beverages (tea, coffee, beer, wine and fruit drinks) (Middleton, 1998). Flavonoids have aroused considerable interest recently because of their potential beneficial effects on human health, as they have antiviral, anti-allergic, antiplatelet, anti-inflammatory, antitumor, and antioxidant activities (Havsteen, 1983; Middleton, 1998; Ielpo et al., 2000; Toker et al., 2004; Wang et al., 1998). Regardless of their diverse pharmacological and physiological activities, their use as drugs and food additives has been restricted because of their water-insolubility and low absorbability (Desmet et al., 2012). Such compounds convert to water soluble and stable compounds when glycosylated and show improved bioavailability and pharmacological properties (Jones and Vogt, 2001; Makino et al., 2009; Shimoda et al., 2007; Weymouth-Wilson, 1997).
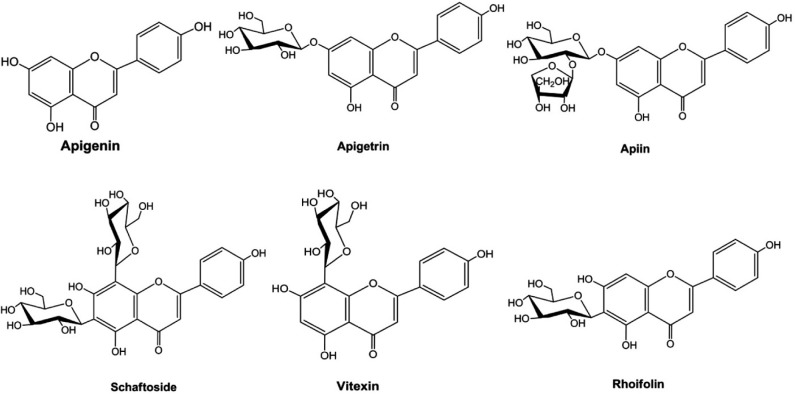
Apigenin (4′, 5, 7 - trihydroxyflavone) (Fig. 1) is a member of the flavone subclass of flavonoids present in fruits and vegetables such as onions, oranges, parsley (Shukla and Gupta, 2010), and chamomile (Manach et al., 2004; Surh, 2003). It is also an active ingredient in the memory herb Gingko biloba (Patel et al., 2007). Apigenin has long been considered to have various biological activities such as antioxidant (Singh et al., 2004), anti-inflammatory (Ha et al., 2008), anti-mutagenic (Myhrstad et al., 2002), and anti-tumorigenic (Wei et al., 1990) properties in various cell types. Current research trials indicate that apigenin may reduce DNA oxidative damage, inhibit the growth of human leukemia cells and induce these cells to differentiate. Furthermore, this compound inhibits cancer cell signal transduction and induce apoptosis, as well as acts as an anti-inflammatory, anti-spasmodic or spasmolytic agent (Gupta et al., 2002; Kobayashi et al., 2002; Lin et al., 1997; Segaert et al., 2000). Apigenin-rich plants such as passion flower have been used to treat intransigent insomnia, as an anti-spasmodic in Parkinson’s disease and asthma, to reduce nerve pain in neuralgia, and to treat shingles. Apigenin and apigenin 7-O-β-glucoside penetrate deep into skin layers when applied topically as a antiphlogistic agent to treat inflammation in deep tissues (Merfort et al., 1994). Some of the naturally occurring apigenin glucosides (Fig. 1) have been isolated from various plant sources and their activities have been proven (Akingbala, 1991; Choo et al., 2012; Essokne et al., 2012; Hattori and Matsuda, 1952; Meyer et al., 2006; Nakazaki et al., 2013; Tsolmon et al., 2011; Zhang et al., 2008). Apigenin is the subject of intensive research for its biological properties. The most widespread research has been for its potential to fight cancer (Patel et al., 2007).
Fig. 1.
Structures of apigenin and some of its naturally occurring glycosides isolated from various plant sources.
The UDP-glucosyltransferases (UGTs) are a group of enzymes that transfer a sugar residue from an activated donor to an acceptor molecule (Richman et al., 2005). There are more than 87,000 glucosyltransferases (GTs) in the protein data bank, which have been classified into 94 families according to their sequences, signature, motifs, stereochemistry of the glycoside linkage, and known target specificity (CAZy database; http://www.cazy.org/) (Breton et al., 2012; Campbell et al., 1997; Cantarel et al., 2009; Erb et al., 2009). The family 1 GTs, which include UGTs common in plants, animals, fungi, and bacteria, predominantly recognize low molecular weight small molecules such as flavonoids, alkaloids, and antibiotics as sugar acceptors with high regioselectivity and UDP-activated sugar moieties as donor molecules (Mackenzie et al., 1997). Attaching sugars to flavonoids using microbial GTs enhances their solubility, which makes the compounds more readily absorbed in the small intestine (Ko et al., 2006). In our present work, YjiC, a UGT from Bacillus licheniformis DSM 13 which is a member of the GT1 family, has been used to synthesize apigenin glucosides by an in vitro method. Recent studies with this UGT have reported successful glucosylation of geldanamycin analogs (Wu et al., 2012) and phloretin (Pandey et al., 2013). Both the aromatic and aliphatic hydroxyl groups in these substrates have been glucosylated. Besides, UGTs from other Bacillus species which are homologous to YjiC have been found to have more flexibility (Ko et al., 2006).
MATERIALS AND METHODS
Chemicals and reagents
Most of the chemicals used in this study were purchased from Sigma-Aldrich Chemical Co. (USA). High performance liquid chromatography (HPLC) grade trifluoroacetic acid (TFA) and acetonitrile were purchased from Mallinckrodt Baker (USA). UDP-d-glucose (UDPGlc) was obtained from GeneChem (Korea). The restriction enzymes were from Takara (Japan). All other chemicals were high-grade products obtained from commercially available sources.
Bacterial strains, vectors, media, and growth conditions
B. licheniformis (DSM 13) was used to isolate genomic DNA, and the Escherichia coli BL21 (DE3) host (Stratagene, USA) was used to express the protein. All E. coli strains were grown at 37°C in Luria Bertani (LB) liquid or plates supplemented with ampicillin (100 μg ml−1) when required. E. coli BL21 (DE3) was grown at 20°C after induction with isopropylthio-β-d-galactosidase (IPTG) to express the recombinant protein. The pET302/NT-His vector (Invitrogen, USA) was used as the expression vector.
Cloning of glucosyltransferase
The yjiC was amplified from genomic DNA of B. licheniformis by using primer pairs: YjiC-F; 5′-GAACTCGAGATGGGACAT AAACATATCGCG-3′ (XhoI) and YjiC-R; 5′-CCGGATCCTTA TTTTACTCCTGCGGGTG-3′ (BamHI), using 2U Taq polymerase (Solgent) and 1× Taq DNA polymerase buffer (MgCl2). The PCR was performed in a total volume of 20 μl with 100 ng genomic DNA, 0.2 μM primer, 200 nM dNTP under the following conditions: denaturation at 94°C for 3 min, 25 cycles of denaturation at 94°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 1 min. The PCR product of yjiC (1,191 bp) was purified and cloned separately into pGEM-T easy vector for sequencing to confirm that no mutation had occurred during PCR amplification. The XhoI/BamHI fragment of yjiC was excised from pGEM-T vector and cloned into the same sites of pET302/NT-His to generate recombinant expression vector pET302-YjiC.
Expression and purification of UDP-glucosyltransferase (YjiC)
For expression of yjiC, pET302-YjiC was transformed into E. coli BL21 (DE3) by heat shock transformation method. A single transformant of pET302-YjiC was inoculated in 4 ml LB medium supplemented with 100 μg ml−1 ampicillin and grown at 37°C overnight. A 50 μl seed culture was inoculated into 50 ml LB medium with 100 μg ml−1 of ampicillin and was grown at 37°C until the absorbance at 600 nm (OD600) was 0.5–0.7. Then, expression was induced by adding 0.5 mM IPTG followed by continued growth at 20°C with shaking for 12 h. The cells were harvested by centrifugation at 3,000 rpm for 15 min, washed, and resuspended in 50 mM Tris-HCl buffer, pH 7.4, followed by sonication. The lysate was cleared by centrifugation at 12,000 rpm for 30 min at 4°C. The lysate was mixed with 1 ml Ninitrilotriacetic acid agarose (Qiagen, USA), shaken gently for 30 min in ice, and then packed into a His-TALON Gravity Column (Clontech, USA). The resin-bound protein was washed stepwise with increasing concentrations of imidazole using gravity flow (10, 50, 100, and 200 mM in buffer containing 300 mM NaCl and 50 mM Tris-HCl, pH 7.4). The protein was eluted in buffer (300 mM NaCl and 50 mM Tris-HCl, pH 7.4) supplemented with 200 mM imidazole, and 1 ml fractions were collected. Fractions containing purified protein were analyzed via 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, concentrated using an Amicon Ultra-15 (Millipore, USA; 10K NMWL device), and then dialyzed overnight at 4°C against buffer containing 50 mM Tris-HCl (pH 7.4) and 10% (v/v) glycerol. The concentration of protein was calculated by Bradford protein assay. The purified protein was stored in buffer containing 50 mM Tris-HCl, pH 7.4, and 10% glycerol until use.
Glucosylation of apigenin
The glucosylation was carried out using purified glucosyltransferase, YjiC. In a total volume of 1 ml reaction mixture, the final concentration of 100 mM Tris-Cl (pH 8.8), 1 mM MgCl2 · 6H2O, 3 mM apigenin, and 3 mM UDPGlc was maintained along with 35 μg/ml of appropriately diluted enzyme (GT). The reaction mixture without GT served as a control. The reaction mixture was incubated at 30°C for 3 h and then quenched by heating in boiling water for 5 min. Then, the mixture was centrifuged to remove protein. However, the concentration of UDPGlc and the incubation time for different reactions were varied according to our interest for analyzing different parameters. To elucidate the structure of the products, the reaction was performed at 30°C in a 10 ml volume containing 3 mM acceptor substrate, 6 mM UDPGlc, 35 μg/ml of appropriately diluted purified enzyme, and other components in the proportion as mentioned above for the 1 ml reaction. The reaction was quenched and centrifuged as stated before. The products were further purified by preparative HPLC, dried in a rotary evaporator, and reduced in vacuo with a freeze dryer. Thus purified products were analyzed by nuclear magnetic resonance spectroscopy.
Analyses of glucosylation products
The reaction products were analyzed by HPLC (SCL-10 AVP; Shimadzu, Japan) using a reverse-phase C18 column (Mightysil RP- 18 GP, 150 × 4.6 mm, Kanto Chemical, Japan) at 320 nm. The isocratic mobile phases were composed of solvent A [0.05% TFA in water, (v/v)] and solvent B (100% acetonitrile). Flow rate was 1.0 ml min−1. Detection was carried out with a UV detector (Shimadzu, SPD-20A, UV/VIS Detector) at 320 nm. The masses of the in vitro glucosylation reaction products were confirmed by high resolution liquid chromatography-electro-spray ionization-quadrupole-time-of-flight-mass spectrometry (HRLC-ESI-Q-TOF-MS/MS) analysis using the ACQUITY UPLC® (Waters Corp., USA) coupled with SYNAPT G2-S (Waters Corp., USA). The products were purified by prep-HPLC with a C18 column [YMC-Pack ODS-AQ (150 × 20 mm I. D., 10 μm)] connected to a UV detector (320 nm). Nuclear magnetic resonance (NMR) analyses were performed on Varian Unity Inova 300 MHz spectrometer (Varian, USA) or by 800 and 900 MHz Bruker Biospin nuclear magnetic resonance (NMR) (USA).
RESULTS AND DISCUSSION
In vitro glucosylation of apigenin
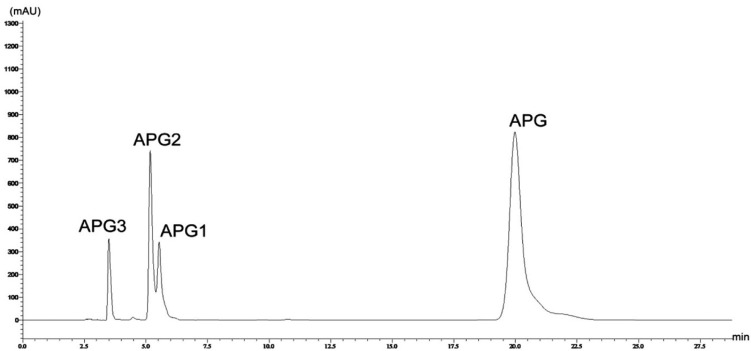
The in vitro glucosylation reaction was carried, out and the reaction products were analyzed by HPLC, which suggested the formation of three products (Fig. 2). The substrate molecule, apigenin, has three phenolic hydroxyl groups at the C-5, C-7, and C-4′ positions. Therefore, glucosylation at these three positions is possible. Mono-, di- and tri-glucosylated derivatives of apigenin have been previously reported in various plants as well as synthesized enzymatically (Moussaoui et al., 2010; Rajbhandari and Roberts, 1983; Švehlíková et al., 2004; Terasaka et al., 2012).
Fig. 2.
HPLC analysis of in-vitro glucosylation reaction mixture, monitored at 320 nm. APG, apigenin; APG1, apigenin 4′-O-glucoside; APG2, apigenin 7-O-glucoside; APG3, apigenin 4′,7-O-diglucoside.
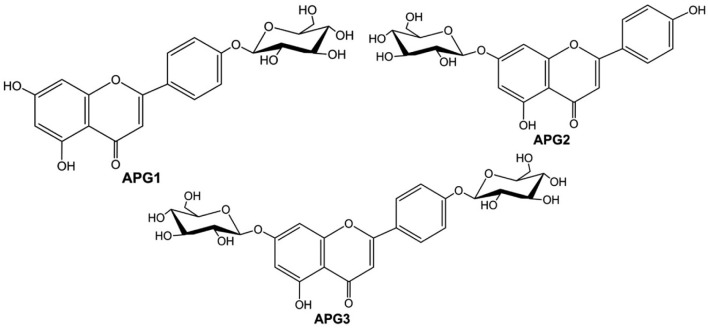
When the reaction products were analyzed by HRLC-ESI-QTOF-MS/MS, the first peak (APG3, retention time of 3.49 min) was confirmed to be a diglucoside with a molecular ion [M + H]+ at m/z 595.1677. Similarly, the remaining two peaks (APG2 and APG1, retention times of 5.17 and 5.53 min respectively) produced molecular ion [M + H]+ peaks at m/z of 433.1092 and 433.1112 respectively, which confirmed their identity as monoglucosides of apigenin, [M + H]+, m/z 271.0613 (refer to Supplementary Figs. S2a–S2c). Although the substrate molecule contained three phenolic hydroxyl groups, triglucoside was not detected. Furthermore, the chemical structures of APG1-APG3 (Fig. 3) were confirmed by comparison of the 1H and 13C NMR spectra with those reported previously. When the 1H NMR of APG1 was analyzed, the presence of signals at δH 12.91 (1H, s, 5-OH) and δH 10.91 (1H, s, 7-OH) confirmed the glucosylation at C-4′ position. The anomeric proton signal at δH 5.04 (1H, d, J = 6.8 Hz) was consistent with the presence of a β-glucoside unit in APG1. The structure of APG1 was, therefore, determined to be apigenin 4′-O-glucoside (Lin et al., 2000; She et al., 2009). The signals obtained in the 1H-NMR and 13C-NMR for APG2 were compared with previously published results (Ersoz et al., 2002; Moussaoui et al., 2010; Yassa et al., 2007) which confirmed the product to be apigenin 7-O-glucoside. Similarly, the 1H-NMR and 13C-NMR of APG3 confirmed its chemical structure as apigenin 4′, 7-O-diglucoside when the spectra were compared (Agrawal and Bansal, 1989; Markham and Geiger, 1993; Terasaka et al., 2012). The NMR spectra of these products have been presented in the Supplementary Figs. S3–S6.
Fig. 3.
Chemical structures of glucosylation products. APG1, apigenin 4′-O-glucoside; APG2, apigenin 7-O-glucoside; APG3, apigenin 4′,7-O-diglucoside.
Effect of incubation time on glucosylation
Commercially available UDPGlc was used for apigenin glucosylation. The concentration of apigenin and UDPGlc was 3 mM. However, the incubation time was varied (1, 2, 3 and 10 h) for the four sets of reactions but maintaining the temperature (30°C) and other parameters constant. The HPLC analyses of the reaction mixture showed that the concentration of APG1 was the least at 1 h incubation time and increased gradually with increasing time of incubation. Even the two other products, APG2 and APG3 followed the same trend until 3 h of incubation. The concentration of all three products was the highest at 3 h time of incubation which decreased when the time of incubation was extended to 10 h. The product APG2 at 10 h was even lesser than that at 1 h (Fig. 4A). One reason for the decrease in product concentration when the incubation period was extended might be because of the deglucosylation as reported by Pandey et al. (2013). Time course experiments indicated that approximately 90% of the substrate was converted into the glucoside product after 24 h of incubation (data not shown).
Fig. 4.
(A) Effect of incubation time on glucosylation of apigenin. (B) Effect of UDPGlc concentration on the glucosylation of apigenin. (C) Effect of pH of Tris-HCl buffer used in the glucosylation of apigenin. The bar diagrams are marked with error bars with percentage (5% value).
Effect of UDP-D-glucose concentration on apigenin glucosylation
In a set of three reactions, 3 mM apigenin was used but the concentration of UDPGlc was varied. All three reactions were incubated for 3 h at 30°C. HPLC analysis of the reaction products showed that APG1 favored low concentration of UDPGlc. The formation of APG1 was highest at a 3 mM concentration of UDPGlc. In contrast, the formation of APG2 was highest with a 6 mM concentration of UDPGlc, but it decreased slightly at 9 mM concentration. Similarly, APG3 formation was highest with 9 mM UDPGlc and lowest with 3 mM UDPGlc. Not much difference was observed in the amount of APG3 between the two reactions with 6 mM and 9 mM UDPGlc (Fig. 4B). These results show that the choice of suitable UDPGlc concentration is important for synthesizing a particular glucoside.
Effect of buffer pH on apigenin glucosylation
Buffer of different pHs were prepared at 30°C to study the effect of pH of Tris-Cl buffer on glucosylation, and the reactions were carried out under similar conditions using buffer of different pHs. When the reaction products were analyzed by HPLC, the Tris-Cl buffer of pH 8.8 provided a better result, as the proportion of glucosides formed was almost same in this case. Therefore, Tris-Cl buffer of pH 8.8 was selected to carry out all reactions during this study. But, the formation of APG2 was higher in more alkaline conditions, whereas APG3 preferred a pH range of 7.5–8.8 (Fig. 4C). The results showed that the buffer of lower pHs is less favored by this enzyme for glucosylation of apigenin.
DISCUSSION
Flavonoids are present in food and medicinal plants and are thus consumed by humans. Much attention has been received by flavonoids over the past decade and a variety of potential beneficial effects have been revealed. They are found in plants mainly as glycoside derivatives. Flavonoids undergo deglycosylation either by lactase phloridzin hydrolase or cytosolic β-glucosidase before oral absorption. The absorbed aglycone is then conjugated by methylation, sulfanation, or glucuronidation (Jäger and Saaby, 2011). Besides, several natural products used as antibiotics have glucose or other sugar moieties attached, and these sugars play a vital role in the water solubility and biological activity of those antibiotics (Harle and Bechthold, 2009). Thus, apigenin was glucosylated to synthesize its glucosides, which might increase water solubility and enhance biological activity of this compound since the glucosyl conjugation of such low molecular weight compounds help to enhance water solubility, improve stability which will ultimately help in increasing bioavailability and modify bioactivity (Lim and Bowles, 2004; Shimoda et al., 2007; Weymouth-Wilson, 1997).
The UDP-glucosyltransferase YjiC has been successfully used to glucosylate apigenin. Though apigenin consists of phenolic hydroxyl groups at C-5, C-7 and C-4′ positons, glucosylation at C-5 position was not observed in this study. Most probably the hydroxyl group at this position undergoes intramolecular hydrogen bond formation with the carbonyl oxygen at the C-4 position and is not available under normal conditions. This may be one reason for making 5-O-glucosides of flavonoids rare in nature. However, the triglucoside of apigenin was reported previously (Terakasa et al., 2012). Although three different glucosides were reported during this study, each favored different sugar concentrations, pHs, and incubation times. When the glucosylation reactions were performed for different incubation times, 3 h of incubation time produced three products in almost equal proportion. The concentration of products were 74.3, 80.2, and 72.5 mg/L respectively for APG1, APG2 and APG3. However, the extended incubation time reduced the amount of product (Fig. 4A). This reduction may be attributed to deglucosylation reaction by the enzyme (Pandey et al., 2013).
Similarly, when 6 mM UDPGlc was used in the reaction. The concentration of both APG1 and APG2 decreased in the reaction with 9 mM UDPGlc but the concentration of APG3 was maximum (136.1 mg/L) in the same (Fig. 4B). This showed that formation of diglucoside favored higher concentration of donor sugar in the reaction whereas the monoglucosides favored moderate to low concentration. Therefore, these parameters of the reaction can be varied to synthesize the glucoside of interest.
This presents an attractive alternative, as a GT that can catalyze the attachment of sugars to an aglycone. The GT can transfer various UDP-sugars to both aromatic and aliphatic nucleophiles (Pandey et al., 2013; Wu et al., 2012) with broad substrate specificity. In the case of the plant GTs, more than one glycosylated product has been reported for flavonoids when several glycosylation positions are available. Our results indicate that even the bacterial GTs can glycosylate at multiple positions when such positions are available. Such GTs with substrate flexibility and less regioselectivity can be utilized for glucosylation of broad range of pharmacologically and Industrially important compounds.
Acknowledgments
This study was supported by a grant from the Next-Generation BioGreen 21 Program (SSAC, grant#: PJ0094832), Rural Development Administration and by Technology Development Program for Agriculture and Forestry, Ministry of Agriculture and Forestry (20100368), Republic of Korea, 2010.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Agrawal PK, Bansal MC. Flavonoid glycosides. In: Agrawal PK, editor. Carbon-13 NMR of Flavonoids. Amsterdam, Oxford, New York, Tokyo: Elsevier; 1989. pp. 283–364. [Google Scholar]
- Akingbala JO. Effect of processing on flavonoids in Millet (Pennisetum americanum) flour. Cereal Chem. 1991;68:180–183. [Google Scholar]
- Breton C, Fournel-Gigleux S, Palcic MM. Recent structures, evolution and mechanisms of glycosyltransferases. Curr Opin Struct Biol. 2012;22:540–549. doi: 10.1016/j.sbi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Campbell JA, Davies GJ, Bulone V, Henrissat B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem J. 1997;326:929–939. doi: 10.1042/bj3260929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 2009;37:233–238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo CY, Sulong NY, Man F, Wong TW. Vitexin and isovitexin from the leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J Ethnopharmacol. 2012;142:776–781. doi: 10.1016/j.jep.2012.05.062. [DOI] [PubMed] [Google Scholar]
- Desmet T, Soetaert W, Bojarová P, Kren V, Dijkhuizen L, Eastwick-Field V, Schiller A. enzymatic glycosylation of small molecules: challenging substrates require tailored catalysts. Chemistry. 2012;18:10786–10801. doi: 10.1002/chem.201103069. [DOI] [PubMed] [Google Scholar]
- Erb A, Weiss H, Härle J, Bechthold A. A bacterial glycosyltransferase gene toolbox: generation and applications. Phytochemistry. 2009;70:1812–1821. doi: 10.1016/j.phytochem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Ersoz T, Harput US, Saracoglu I, Calis I. Phenolic compounds from Scutellaria pontica. Turk J Chem. 2002;26:581–588. [Google Scholar]
- Essokne RS, Grayer RJ, Porter E, Kite GC, Simmonds MSJ, Jury SL. Flavonoids as chemosystematic markers for the genus Adenocarpus. Biochem Syst Ecol. 2012;42:49–52. [Google Scholar]
- Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor kappa B, Bax and Bcl2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park JH, Lee EH, Ryu JH, Lee KR, Kim SY. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int. 2008;52:878–886. doi: 10.1016/j.neuint.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Harle J, Bechthold A. The power of glycosyltransferases to generate bioactive natural compounds. In: Hopwood DA, editor. Methods Enzymol (Complex Enzymes in Microbial Natural Product Biosynthesis, Part A: Overview Articles and Peptides) Vol. 458. USA, Academic Press; 2009. pp. 309–333. [DOI] [PubMed] [Google Scholar]
- Hattori S, Matsuda H. Rhoifolin, a new flavone glycoside, isolated from the leaves of Rhus succedanea. Arch Biochem Biophys. 1952;37:85–89. [Google Scholar]
- Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Ielpo MT, Basile A, Miranda R, Moscatiello V, Nappo C, Sorbo S, Laghi E, Ricciardi MM, Ricciardi L, Vuotto ML. Immunopharmacological properties of flavonoids. Fitoterapia. 2000;71:S101–S109. doi: 10.1016/s0367-326x(00)00184-2. [DOI] [PubMed] [Google Scholar]
- Jäger AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16:1471–1485. doi: 10.3390/molecules16021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Vogt T. Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta. 2001;213:164–174. doi: 10.1007/s004250000492. [DOI] [PubMed] [Google Scholar]
- Ko JH, Kim BG, Ahn JH. Glycosylation of flavonoids with a glycosyltransferase from Bacillus cereus. FEMS Microbiol Lett. 2006;258:263–268. doi: 10.1111/j.1574-6968.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nakata T, Kusumaki T. Effect of flavonoids on cell cycle progression in prostate cancer cells. Cancer Lett. 2002;176:17–23. doi: 10.1016/s0304-3835(01)00738-8. [DOI] [PubMed] [Google Scholar]
- Lim EK, Bowles DJ. A class of plant glycosyltransferases involved in cellular homeostasis. EMBO J. 2004;23:2915–2922. doi: 10.1038/sj.emboj.7600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JK, Chen YC, Huang YT, Lin-Shiau SY. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J Cell Biochem. 1997;67:39–48. [PubMed] [Google Scholar]
- Lin YL, Kuo YH, Shiao MS. Flavonoid glycosides from Terminalia catappa L. J Chinese Chem Soc. 2000;47:253–256. [Google Scholar]
- Mackenzie PI, Owens IS, Burchell B, Bock KW, Bairoch A, Belanger A, Fournel-Gigleux S, Green M, Hum DW, Iyanagi T, et al. The UDP-glycosyltransferase gene super-family: Recommended nomenclature update based on evolutionary divergence. Pharmacogenetics. 1997;7:255–269. doi: 10.1097/00008571-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Makino T, Shimizu R, Kanemaru M, Suzuki Y, Moriwaki M, Mizukami H. Enzymatically modified isoquercitrin, α-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol Pharm Bull. 2009;32:2034–2040. doi: 10.1248/bpb.32.2034. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Markham KR, Geiger H. 1H nuclear magnetic resonance spectroscopy of flavonoids and their glycosides in hexadeuterodimethylsulfoxide. In: Harborne JB, editor. In the Flavonoids, Advances in Research Since 1986. London, UK: Chapman & Hall; 1993. pp. 441–497. [Google Scholar]
- Merfort I, Heilmann J, Hagedorn-Leweke U, Lippold BC. In vivo skin penetration studies of chamomile flavones. Pharmazie. 1994;49:509–511. [PubMed] [Google Scholar]
- Meyer H, Bolarinwa A, Wolfram G, Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann Nutr Metab. 2006;50:167–172. doi: 10.1159/000090736. [DOI] [PubMed] [Google Scholar]
- Middleton E., Jr Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol. 1998;439:175–182. doi: 10.1007/978-1-4615-5335-9_13. [DOI] [PubMed] [Google Scholar]
- Moussaoui F, Zellagui A, Segueni N, Touil A, Rhouati S. Flavonoid constituents from Algerian Launaea resedifolia (O.K.) and their antimicrobial activity. Rec Nat Prod. 2010;4:91–95. [Google Scholar]
- Myhrstad MC, Carlsen H, Nordstrom O, Blomhoff R, Moskaug JO. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med. 2002;32:386–393. doi: 10.1016/s0891-5849(01)00812-7. [DOI] [PubMed] [Google Scholar]
- Nakazaki E, Soninkhishig T, Han J, Isoda H. Proteomic study of granulocytic differentiation induced by apigenin 7-glucoside in human promyelocytic leukemia HL-60 cells. Eur J Nutr. 2013;52:25–35. doi: 10.1007/s00394-011-0282-4. [DOI] [PubMed] [Google Scholar]
- Pandey RP, Li TF, Kim EH, Yamaguchi T, Park YI, Kim JS, Sohng JK. Enzymatic synthesis of novel phloretin glucosides. Appl Environ Microbiol. 2013;79:3516–3521. doi: 10.1128/AEM.00409-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: Progress, potential and promise. Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- Rajbhandari A, Roberts MF. The flavonoids of Stevia rebaudiana. J Nat Prod. 1983;46:194–195. [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Richman A, Swanson A, Humphrey T, Chapman R, McGarvey B, Pocs R, Brandle J. Functional genomics uncovers three glucosyltransferases involved in the synthesis of the major sweet glucosides of Stevia rebaudiana. Plant J. 2005;41:56–67. doi: 10.1111/j.1365-313X.2004.02275.x. [DOI] [PubMed] [Google Scholar]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailabilty, metabolic effects and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Segaert S, Courtois S, Garmyn M, Degreef H, Bouillon R. The flavonoid apigenin suppresses vitamin D receptor expression and vitamin D responsiveness in normal human keratinocytes. Biochem Biophys Res Commun. 2000;268:237–241. doi: 10.1006/bbrc.2000.2099. [DOI] [PubMed] [Google Scholar]
- She G, Guo Z, Lv H, She D. New flavonoid glycosides from Elsholtzia rugulosa Hemsl. Molecules. 2009;14:4190–4196. doi: 10.3390/molecules14104190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda K, Otsuka T, Morimoto Y, Hamada H, Hamada H. Glycosylation and malonylation of quercitin, epicate-chin, and catechin by cultured plant cells. Chem Lett. 2007;36:1292–1293. [Google Scholar]
- Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JP, Selvendiran K, Banu SM, Padmavathi R, Sakthisekaran D. Protective role of apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine. 2004;11:309–314. doi: 10.1078/0944711041495254. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat. Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Švehlíková V, Bennett RN, Mellon FA, Needs PW, Piacente S, Kroon PA, Bao Y. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert) Phytochemistry. 2004;65:2323–2332. doi: 10.1016/j.phytochem.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Terasaka K, Misutani Y, Nagatsu A, Mizukami H. In situ UDP-glucose regeneration unravels diverse functions of plant secondary product glycosyltransferases. FEBS Lett. 2012;586:4344–4350. doi: 10.1016/j.febslet.2012.10.045. [DOI] [PubMed] [Google Scholar]
- Toker G, Küpeli E, Memisoglu M, Yesilada E. Flavonoids with antinociceptive and anti-inflammatory activities from the leaves of Tilia argentea (silver linden) J Ethnopharmacol. 2004;95:393–397. doi: 10.1016/j.jep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Tsolmon S, Nakazaki E, Han J, Isoda H. Apigetrin induces erythroid differentiation of human leukemia cells K562: proteomics approach. Mol Nutr Food Res. 2011;55:S93–S102. doi: 10.1002/mnfr.201000650. [DOI] [PubMed] [Google Scholar]
- Wang HK, Xia Y, Yang ZY, Natschke SL, Lee KH. Recent advances in the discovery and development of flavonoids and their analogues as antitumor and anti-HIV agents. Adv Exp Med Biol. 1998;439:191–225. doi: 10.1007/978-1-4615-5335-9_15. [DOI] [PubMed] [Google Scholar]
- Wei H, Tye L, Bresnick E, Birt DF. Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990;50:499–502. [PubMed] [Google Scholar]
- Weymouth-Wilson AC. The role of carbohydrates in biologically active natural products. Nat Prod Rep. 1997;14:99–110. doi: 10.1039/np9971400099. [DOI] [PubMed] [Google Scholar]
- Wu CZ, Jang JH, Woo M, Ahn JS, Kim JS, Hong YS. Enzymatic glycosylation of non-benzoquinone geldanamycin analogs via Bacillus UDP-glycosyltransferase. Appl Environ Microbiol. 2012;78:7680–7686. doi: 10.1128/AEM.02004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa N, Saeidnia S, Pirouzi R, Akbaripour M, Shafiee A. Three phenolic glycosides and immunological properties of Achillea millefolium from Iran, population of Golestan. Daru. 2007;15:49–52. [Google Scholar]
- Zhang Y, Jiao J, Liu C, Wu X, Zhang Y. Isolation and purification of four flavone C-glycosides from antioxidant bamboo leaves by macroporous resin column chromatography and preparative high-performance liquid chromatography. Food Chem. 2008;107:1326–1336. [Google Scholar]