Abstract
Interleukin-21 (IL-21)+CD4+ T cells are involved in the immune response against hepatitis B virus (HBV) by secreting IL-21. However, the role of IL-21+CD4+ T cells in the immune response against chronic hepatitis C (CHC) virus infection is poorly understood. This study aimed to investigate the role of IL-21+CD4+ T cells in CHC patients and the potential mechanisms. The study subjects included nineteen CHC patients who were grouped by viral load (low, < 106 RNA copies/ml, n = 8; high, > 106 RNA copies/ml, n = 11). The peripheral frequency of HCV-specific IL-21+CD4+ T cells was higher in the low viral load group and was negatively correlated with the serum HCV RNA viral load in all CHC patients. Meanwhile, IL-21+ cells accumulated in the liver in the low viral load group. In vitro, IL-21 treatment increased the expression of proliferation markers and cytolytic molecules on HCV-specific CD8+ T cells. In summary, these findings suggest that HCV-specific IL-21+CD4+ T cells might contribute to HCV control by rescuing HCV-specific CD8+ T cells in CHC patients.
Keywords: chronic hepatitis C, HCV-specific CD8+ T cells, HCV-specific IL-21+CD4+ T cells, interleukin-21, viral control
INTRODUCTION
HCV infection is prone to chronicity and is a major global health problem, as it currently affects 160 million people worldwide (Lavanchy, 2011; Shepard et al., 2005; Yang et al., 2002). Approximately 50–90% of HCV infections are not spontaneously eradicated. Meanwhile, individual differences play an important role in viral clearance, as the pre-treatment HCV viral load varies greatly among individuals. This difference may derive from the immunity of CD4+ and CD8+ T cells, which are the main mechanisms for viral elimination. CD8+ T cells are considered the major effector cells in viral control, while CD4+ T cells are considered the major helper cells in viral elimination.
During the phases of chronic viral infection, including chronic HCV infection, CD8+ T cell responses are induced, but they fail to induce or are unable to elaborate key effector functions (Gallimore et al., 1998; Neumann-Haefelin and Thimme, 2013). As a result, antiviral CD8+ T cells become non-responsive and persist in a non-functional “exhausted” state (Elsaesser et al., 2009). Conversely, CD4+ T cells become hypoactive, which further contributes to viral persistence because in the absence of CD4+ T cell help, CD8+ T cell responses are compromised (Ou et al., 2001). IL-21, a member of the common-γ chain family of cytokines, could serve as a rescue mechanism by facilitating crosstalk between CD4+ T cells and CD8+ T cells. Produced primarily by CD4+ T cells, IL-21 is critical for promoting B cell and antibody responses as well as inducing the development of the Th17 and T follicular helper lineages (Fazilleau et al., 2009; Korn et al., 2007; Ozaki et al., 2002). IL-21 specifically maintains CD8+ T cell effector activity through the help of CD4+ T cells during chronic viral infection, as has been observed in lymphocytic choriomeningitis virus infection (Elsaesser et al., 2009). Nonetheless, little is known about HCV-specific IL-21+CD4+ T cell responses in patients with chronic hepatitis C, particularly in treatment-naive patients.
In the present study, we investigated individual differences in HCV-specific IL-21+CD4+ T cells as well as their supporting role in CD8+ T cell responses against HCV infection in CHC treatment-naïve patients. We first characterized the peripheral frequency of HCV-specific IL-21+CD4+ T cells and its correlation with HCV RNA, and we then evaluated IL-21 expression in the livers of patients grouped by viral load. Finally, we investigated the impact of IL-21 on the expression of proliferation markers and cytolytic molecules in HCV-specific CD8+ T cells. The results of this study further the understanding of the immunological state of CHC patients.
MATERIALS AND METHODS
Patients
Nineteen treatment-naïve genotype 1b CHC patients were enrolled in the study and were grouped based on their viral load (low, < 106 RNA copies/ml, n = 8; high, > 106 RNA copies/ml, n = 11). No evidence of concurrent hepatitis B virus or human immunodeficiency virus (HIV) infection, autoimmune disease or alcoholic liver disease was found for any enrolled individual. HCV RNA was measured using the quantitative Agilent Strata-Gene Mx3000P polymerase chain reaction assay with a lower limit of detection of 100 IU/ml. In addition, liver biopsy samples from 12 CHC patients and 1 healthy control were immunohistochemically analyzed. The study protocol was approved by the Ethics Committee of our unit at Beijing 302 Hospital, and informed consent was obtained from each subject. The clinical data at baseline are shown in Table 1.
Table 1.
Clinical parameters of enrolled subjects
| Group | CHC
|
|
|---|---|---|
| high viral load | low viral load | |
| Case | 11 | 8 |
| Sex (male/female) | 5/6 | 4/4 |
| Age (years) | 46.15 ± 11.28 | 42.77 ± 10.18 |
| ALT (IU/L) | 63.09 ± 25.43 | 52.25 ± 27.19 |
| HCV RNA (Log10 IU/ml) | 6.84 ± 6.79 | 5.23 ± 5.21 |
| HCV genotype (1b) | 11 | 8 |
Abbreviations: CHC, chronic hepatitis C; ALT, alanine aminotransferase
Flow cytometric analysis
All antibodies were purchased from BD Biosciences (USA), except for the fluorescein isothiocyanate (FITC)-conjugated anti-CD107a, anti-GrA, anti-GrB, anti-perforin and anti-ki67 antibodies, which were purchased from BD PharMingen (USA).
For intracellular staining, thawed peripheral blood mononuclear cells (PBMCs) were incubated with 1 μg/ml anti-CD28 (eBioscience, USA) and 1 μg/ml core peptide pool (a total of 21 peptides covering the entire HCV core protein; SBS Genetech, China) with or without IL-21 (50 ng/ml; eBioscience, USA) in 96-well round bottom plates at 5 × 105 cells/well at 37°C in 5% CO2. After 1 h of stimulation, Golgi-plug (BD PharMingen, USA) was added, and the cells were incubated for 15 h. The cells were then collected, stained with surface antibodies, permeabilized and intracellularly stained with the following: FITC-conjugated anti-GrA, anti-GrB, anti-perforin and anti-ki67 and phycoerythrin (PE)-conjugated anti-IL-21 and anti-IFN-γ. The cells were then fixed and analyzed using a FACSCalibur flow cytometer and FlowJo software (TreeStar, USA).
Detection of CD8+ T Cell degranulation
CD107a degranulation is now widely used to assess CD8+ T cell cytotoxic potential. Briefly, thawed PBMCs were seeded into 96-well round bottom plates at 5 × 105 cells/well and directly stimulated with 1 μg/ml anti-CD28 and 1 μg/ml core peptide pool with or without 50 ng/ml IL-21 for 16 h at 37°C in 5% CO2. Before incubation, FITC-conjugated anti-CD107a was first directly added to the medium. After incubation, the cells were collected and stained with surface antibodies.
Analysis of IFN-γ-producing T cells by ELISPOT
Thawed PBMCs were plated (5 × 104 cells/well) and cocultured with core 1 μg/ml peptide pool with or without 50 ng/ml IL-21 for 36 h. PBMCs incubated in medium with or without anti-CD3 were used as positive and negative controls, respectively. A 96-well ELISPOT assay (Mabtech, Sweden) for IFN-γ production was performed and analyzed according to the manufacturer’s instructions. The number of spot-forming units was determined with computer-assisted image analysis software (ChampSpot, China). The results were expressed as spot-forming units per 104 PBMCs.
Immunohistochemistry
Endogenous peroxidase activity was blocked with 0.3% H2O2 in paraffin-embedded, formalin-fixed liver tissue sections (5 μm). The sections were then incubated with rabbit anti-human IL-21 polyclonal antibody (AbD Serotec, UK) overnight at 4°C. 3-amino-9-ethyl-carbazole (red color) was used as the substrate, followed by counterstaining with hematoxylin for single staining. Positively stained cells were counted in high-powered fields (x 200).
Statistical analysis
All of the data were summarized and presented as the mean ± standard deviation. The data were analyzed using SPSS software 17.0 (SPSS Inc., USA). The Mann-Whitney U test was performed for comparisons between groups. The Spearman coefficient was calculated to evaluate correlations between variables. The Wilcoxon matched-pairs t-test was used to compare data from the same individual. For all tests, P < 0.05 was considered to be significant.
RESULTS
HCV-specific IL-21+CD4+ T cell responses were negatively correlated with HCV RNA viral load
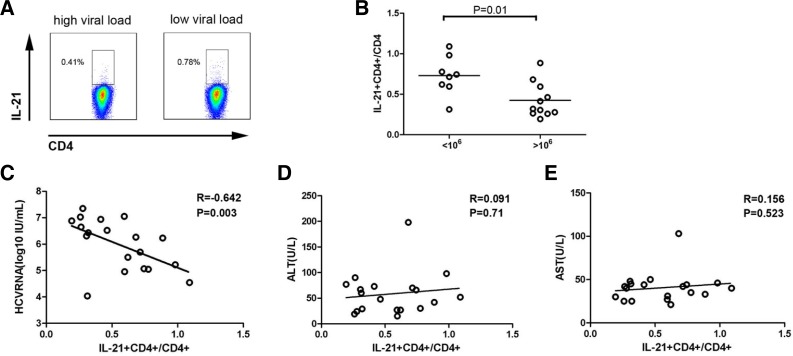
Peripheral IL-21+CD4+ T cells in the low (< 106 copies/ml, n = 8) and high (> 106 copies/ml, n = 8) viral load groups were measured in vitro following core peptide pool stimulation. The frequency of HCV-specific IL-21+CD4+ T cells in the low viral load group was higher than that in the high viral load group (Figs. 1A and 1B, 0.73 ± 0.24, n = 8; 0.42 ± 0.21, n = 11; P = 0.01). Accordingly, Spearman analysis showed a significantly negative correlation between HCV-specific IL-21+CD4+ T cells and serum HCV RNA viral load (Fig. 1C, r = −0.642, P = 0.003), whereas no correlation was observed between HCV-specific IL-21+CD4+ T cells and serum ALT or AST (Figs. 1D and 1E). The results suggest that increased HCV-specific IL-21+CD4+ T cells are associated with a positive immune response that led to viral control in CHC patients.
Fig. 1.
Peripheral HCV-specific IL-21+CD4+ T cell responses were negatively correlated with the HCV RNA viral load. (A) Representative dot plots of HCV-specific IL-21+CD4+ T cells from low viral load and high viral load groups of CHC patients following core peptide pool stimulation. (B) Pooled data indicate the percentages of HCV-specific IL-21+CD4+ T cells in low viral load and high viral load groups of CHC patients (n = 19). Horizontal bars represent the median HCV-specific IL-21+CD4+ T cell numbers. One dot represents one individual. Correlations between HCV-specific IL-21+CD4+ T cell frequency and plasma HCV RNA (C), serum ALT (D) and AST (E) in CHC patients. One dot represents one individual. P-values are shown.
IL-21+ cells were increased in the liver of low viral load patients with CHC
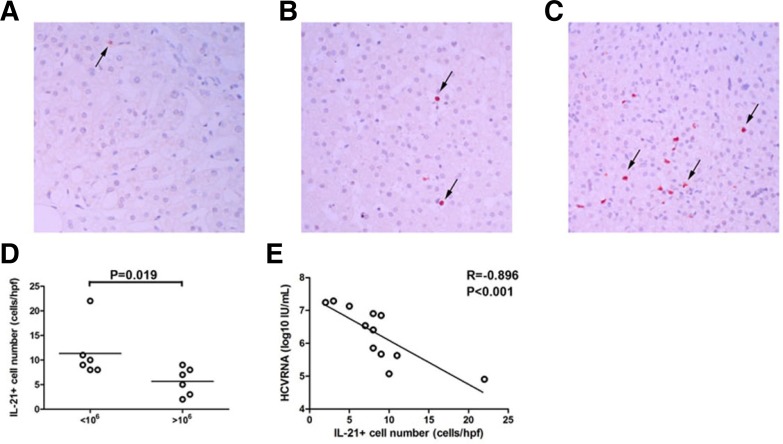
HCV-specific IL-21+CD4+ T cells primarily function by secreting IL-21. To further investigate the role of IL-21, the distribution of IL-21+ cells was compared in the liver of low viral load and high viral load CHC patients. More IL-21 staining was present in liver tissue from low viral load patients (Fig. 2C) when compared with high viral load patients (Fig. 2B). Quantitative analysis of intrahepatic IL-21+ cells also revealed more IL-21+ cell infiltration in livers from CHC patients with a low viral load (Fig. 2D, P = 0.019). There was a significantly negative correlation between HCV-specific IL-21+CD4+ T cells and serum HCV RNA viral load (Fig. 2E, R = −0.896, P < 0.001).
Fig. 2.
IL-21+ cells accumulated in the livers of CHC patients with a low viral load. Immunohistochemical staining for IL-21+ cells in situ in the liver of (A) healthy control (n = 1) (magnification, 200×) and CHC patients with (B) a high viral load (n = 6) and (C) a low viral load (n = 6) (magnification, 200×). Positive cells stained red, as indicated by the arrows. (D) Numbers of IL-21+ cells in CHC patients with a low viral load and a high viral load. Horizontal bars represent the median numbers of IL-21+ cells. One dot represents one individual. (E) Correlations between IL-21+ cells and plasma HCV RNA in CHC patients. One dot represents one individual. P-values are shown.
IL-21 increased the proliferation of HCV-specific CD8+ T cells in CHC patients
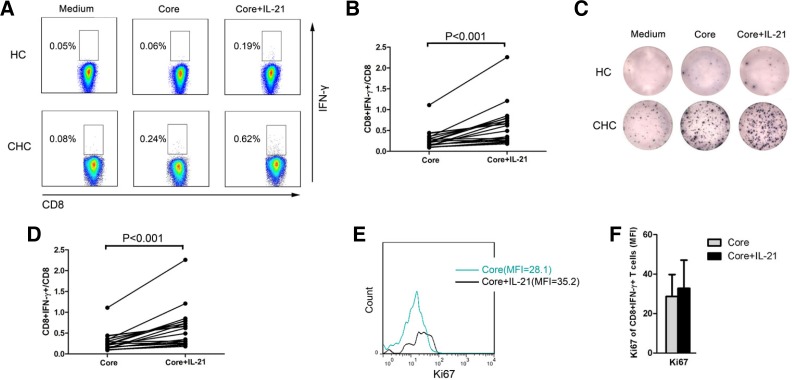
To determine how HCV-specific IL-21+CD4+ T cell responses affect HCV control, we measured whether IL-21 could increase IL-21R-expressing HCV-specific CD8+ T cells, which play a vital role in viral control. To detect virus-specific CD8+ T cells, we measured IFN-γ responses using flow cytometry after co-culturing the cells with antigenic peptides in vitro (Betts et al., 2000). We detected the frequency of HCV-specific CD8+ T cells from CHC patients in vitro following core peptide pool stimulation for 16 h by intracellular staining. Stimulation with IL-21 significantly increased the frequency of HCV-specific CD8+ T cells among PBMCs from CHC patients (Figs. 3A and 3B, n = 19; P < 0.001). To further validate these results, an IFN-γ ELISPOT assay was performed. Following coculture with the core peptide pool for 36 h, stimulation with IL-21 significantly increased the number of IFN-γ spot-forming units (Figs. 3C and 3D, n = 19; P < 0.001), which reflected HCV-specific T-cell responses. In addition, the proliferation marker Ki67 was detected. Stimulation with IL-21 significantly increased the level of Ki67 (Figs. 3E and 3F, n = 10; P < 0.05) in HCV-specific CD8+ T cells, indicating that IL-21 may enhance the proliferation of HCV-specific CD8+ T cells in CHC patients.
Fig. 3.
IL-21 increased the proliferation of HCV-specific CD8+ T cells in CHC patients in vitro. (A) Representative dot plots and (B) pooled data for the intracellular staining of HCV-specific CD8+ T cells from CHC patients (n = 19) and healthy controls (n = 2) following core peptide pool and IL-21 stimulation. One dot represents one individual. (C) Representative plots and (D) pooled data for ELISPOT assays of IFN-γ-producing T cells from CHC patients (n = 19) and healthy controls (n = 2) following core peptide pool and IL-21 stimulation. One dot represents one individual. P-values are shown. (E) Representative histograms and (F) pooled data for intracellular staining for Ki67 expression in HCV-specific CD8+ T cells from CHC patients following core peptide pool and IL-21 stimulation (n = 10). Bars represent means with standard deviations. *Compared with medium group, P < 0.05.
IL-21 increased cytolytic molecule expression on HCV-specific CD8+ T cells in CHC patients
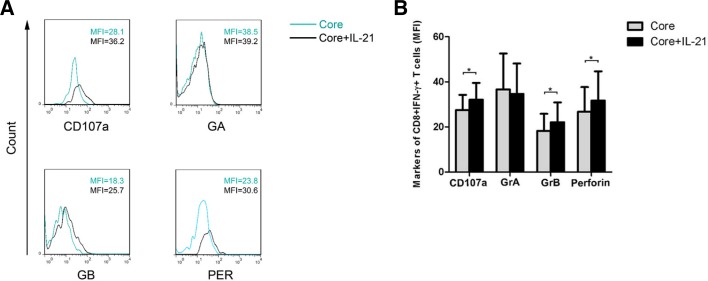
GrA, GrB, perforin and CD107a are cytolytic molecules that mediate CD8+ T cell effector functions. We analyzed the expression of GrA, GrB, perforin and CD107a in PBMCs from 10 CHC patients using flow cytometry. Stimulation with IL-21 significantly up-regulated the expression of GrB, perforin and CD107a but not GrA (Figs. 4A and 4B, n = 10; P < 0.05) in HCV-specific CD8+ T cells. These findings suggest that IL-21 promotes the cytolytic function of HCV-specific CD8+ T cells in CHC patients.
Fig. 4.
IL-21 increased cytolytic molecule expression on HCV-specific CD8+ T cells from CHC patients in vitro. (A) Representative histograms and (B) pooled data for intracellular staining of CD107a, GrA, GrB and perforin expression on HCV-specific CD8+ T cells from CHC patients (n = 10) following core peptide pool and IL-21 stimulation. Bars represent means with standard deviations. *Compared with medium group, P < 0.05.
DISCUSSION
This study evaluated the role of HCV-specific IL-21+CD4+ T cell responses in viral control in CHC patients infected with HCV genotype 1. We demonstrated that HCV-specific IL-21+CD4+ T cell responses were negatively correlated with the HCV RNA viral load. Moreover, greater IL-21 staining was observed in liver tissue from low viral load CHC patients when compared with high viral load patients. Finally, stimulation with IL-21 increased the expression of HCV-specific CD8+ T cells as well as the expression of Ki67, GrB, perforin and CD107a in HCV-specific CD8+ T cells isolated from CHC patients.
The immunoregulatory functions of CD4+ T cells are primarily realized through the secretion of cytokines that can support cytotoxic T lymphocyte (CTL) functions (Mosmann et al., 1996) and mediate long-lived antiviral CD8+ T cell functions in viral infection (Sun et al., 2003; 2004). Interestingly, IL-21, a recently discovered multifunctional and pleiotropic cytokine that is produced primarily by CD4+ T cells (Spolski et al., 2008), exerts a potent immunoregulatory function. Additionally, previous studies have revealed that IL-21 is involved in the pathogenic mechanisms of HIV infection (Iannello et al., 2010) and in a mouse model of human HBV infection (Publicover et al., 2011). IL-21 is also required for viral control in chronic viral infection (Elsaesser et al., 2009). In addition, some studies have shown CD4+ T cell responses to core protein in CHC patients (Leroux-Roels et al., 1996). Therefore, we analyzed the relationship between HCV core peptide pool-specific IL-21+CD4+ T cells and HCV RNA viral load. We found that HCV-specific IL-21+CD4+ T cell responses were negatively correlated with HCV RNA viral load and that the frequency of HCV-specific IL-21+CD4+ T cells was higher in the low viral load group when compared with the high viral load group. These findings were accompanied by the observation of greater IL-21 staining in the liver tissue of low viral load CHC patients compared with high viral load CHC patients. These results indicate that HCV-specific IL-21+CD4+ T cells are associated with HCV control.
Virus-specific CD8+ T cells can eliminate virus-infected hepatocytes through cytolytic and non-cytolytic pathways. Previous studies have shown that HCV-specific CD8+ T cells from CHC patients exhibit reduced abilities to proliferate and secrete anti-viral cytokines (Gruener et al., 2001; Wedemeyer et al., 2002). The potential contributors to such cellular dysfunction include the inhibitory signals mediated by PD-1 and Tim-3 (Golden-Mason et al., 2009; McMahan et al., 2010), immunoregulatory cytokines and regulatory T cells. Moreover, blocking a single pathway, such as the PD-1 pathway, might be insufficient for immune reconstitution (Blackburn et al., 2009). Therefore, a new method of restoring the function of HCV-specific CD8+ T cells is necessary. IL-2, as a well-known T cell immunoregulatory cytokine, can rescue exhausted HCV-specific CD8+ T cells, which are prone to apoptosis (Radziewicz et al., 2008). Unfortunately, in vivo, high doses of IL-2 are toxic. Interestingly, IL-21, as a member of the common-γ chain family of cytokines, which also includes IL-2 (Mehta et al., 2004), can regulate the proliferation and the effector function of CD8+ T cells (Spolski et al., 2008), and it has shown no evidence of toxic effects even at high doses (Wang et al., 2003). In our study, we demonstrated that stimulation with IL-21 promoted the proliferation of HCV-specific CD8+ T cells and up-regulated Ki67 expression in HCV-specific CD8+ T cells from CHC patients. Thus, IL-21 might be a potential therapy for rescuing exhausted HCV-specific CD8+ T cells in chronic HCV infection.
To better characterize these HCV-specific CD8+ T cells, several functional markers were analyzed, including CD107a, GrA, GrB and perforin. CD107a was a surrogate marker for cytolytic activity. Likewise, GrA, GrB and perforin are granule components of CD8+ T cells, and they mediate CD8+ T cell cytolytic activity on virally infected cells (Fu et al., 2007). We found that adding IL-21 significantly increased the expression of GrB, perforin and CD107a but not GrA in HCV-specific CD8+ T cells, which suggests that IL-21 enhances the cytolytic activity of HCV-specific CD8+ T cells.
In summary, due to their secretion of IL-21, HCV-specific IL-21+CD4+ T cells appear to be favorable immunomodulators for increasing the proliferation and cytolytic activity of HCV-specific CD8+ T cells in CHC patients. The findings from this study suggest a new immunotherapeutic strategy that may partially restore the function of HCV-specific CD8+ T cells in CHC patients, which may be associated with HCV control.
Supplementary Material
Acknowledgments
We thank all the patients enrolled in this study for their kindly understanding and supporting. This work was supported by grants from the National Grand Program on Key Infectious Disease (No. 2012ZX10002007), Beijing Nova Program of China (No. Z12110702512071), the National Natural Science Foundation of China (No. 81271848 and No. 81302593) and the National Key Basic Research Program of China (No. 2009CB522507).
REFERENCES
- Betts MR, Casazza JP, Patterson BA, Waldrop S, Trigona W, Fu TM, Kern F, Picker LJ, Koup RA. Putative immunodominant human immunodeficiency virus-specific CD8(+) T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J Virol. 2000;74:9144–9151. doi: 10.1128/jvi.74.19.9144-9151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, Routy JP, Ahmad A. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol. 2010;184:114–126. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- Leroux-Roels G, Esquivel CA, DeLeys R, Stuyver L, Elewaut A, Philippe J, Desombere I, Paradijs J, Maertens G. Lymphoproliferative responses to hepatitis C virus core, E1, E2, and NS3 in patients with chronic hepatitis C infection treated with interferon alfa. Hepatology. 1996;23:8–16. doi: 10.1002/hep.510230102. [DOI] [PubMed] [Google Scholar]
- McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta DS, Wurster AL, Grusby MJ. Biology of IL-21 and the IL-21 receptor. Immunol Rev. 2004;202:84–95. doi: 10.1111/j.0105-2896.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Thimme R. Adaptive immune responses in hepatitis C virus infection. Curr Top Microbiol Immunol. 2013;369:243–262. doi: 10.1007/978-3-642-27340-7_10. [DOI] [PubMed] [Google Scholar]
- Ou R, Zhou S, Huang L, Moskophidis D. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J Virol. 2001;75:8407–8423. doi: 10.1128/JVI.75.18.8407-8423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HR, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- Publicover J, Goodsell A, Nishimura S, Vilarinho S, Wang ZE, Avanesyan L, Spolski R, Leonard WJ, Cooper S, Baron JL. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest. 2011;121:1154–1162. doi: 10.1172/JCI44198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziewicz H, Ibegbu CC, Hon H, Osborn MK, Obideen K, Wehbi M, Freeman GJ, Lennox JL, Workowski KA, Hanson HL, et al. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J Virol. 2008;82:9808–9822. doi: 10.1128/JVI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, Kim G, Leonard WJ, Hwu P. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–9022. [PubMed] [Google Scholar]
- Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- Yang SH, Lee CG, Lee CW, Choi EJ, Yoon SK, Ahn KS, Sung YC. Hepatitis C virus core inhibits the Fas-mediated p38 mitogen activated kinase signaling pathway in hepatocytes. Mol Cells. 2002;13:452–462. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.