Abstract
The aim of the present study was to investigate the therapeutic efficacy of genetically engineered stem cells (GESTECs) expressing bacterial cytosine deaminase (CD) and/or human interferon-beta (IFN-β) gene against HeLa cervical cancer and the migration factors of the GESTECs toward the cancer cells. Anticancer effect of GESTECs was examined in a co-culture with HeLa cells using MTT assay to measure cell viability. A transwell migration assay was performed so as to assess the migration capability of the stem cells to cervical cancer cells. Next, several chemoattractant ligands and their receptors related to a selective migration of the stem cells toward HeLa cells were determined by real-time PCR. The cell viability of HeLa cells was decreased in response to 5-fluorocytosine (5-FC), a prodrug, indicating that 5-fluorouracil (5-FU), a toxic metabolite, was converted from 5-FC by CD gene and it caused the cell death in a co-culture system. When IFN-β was additionally expressed with CD gene by these GESTECs, the anticancer activity was significantly increased. In the migration assay, the GESTECs selectively migrated to HeLa cervical cancer cells. As results of real-time PCR, chemoattractant ligands such as MCP-1, SCF, and VEGF were expressed in HeLa cells, and several receptors such as uPAR, VEGFR2, and c-kit were produced by the GESTECs. These GESTECs transduced with CD gene and IFN-β may provide a potential of a novel gene therapy for anti-cervical cancer treatments via their selective tumor tropism derived from VEGF and VEGFR2 expressions between HeLa cells and the GESTECs.
Keywords: cervical cancer, cytosine deaminase, GESTECs, interferon-β, VEGF
INTRODUCTION
Recently, due to the development of human papilloma virus (HPV) vaccine, many people are able to expect a decrease in occurrence of cervical cancer. However, cervical cancer still remains a significant public health problem for women (Jung et al., 2011). According to the World Health Organization (WHO), the cancer of cervix uteri is the second most common cancer among women worldwide, with an estimated 529,409 new cases and 274,883 deaths in 2008. About 86% of the cases occur in developing countries, occupying 13% of female cancers (Francis and Katz, 2013). Although the hysterectomy is the best preventive method against cervical cancer, this therapy can cause sterility in patients. To reduce this side effect, safer and more efficient treatments for cervical cancer are highly needed.
One of the most prominent treatments for human cancers is the gene therapy which uses DNA that encodes a functional therapeutic gene in order to replace a mutated gene or provide a therapeutic protein (Yi et al., 2012a). Although there have been many therapeutic trials, several obstacles still exist, such as the low efficiency of gene transfer by viral vectors and the inability of these vectors to specifically target cancer cells (Kang et al., 2012a; Yi et al., 2012b). Recently, a gene therapy using stem cells appears to be interest in clinical use of stem cells for cancer treatment (Yi et al., 2013). Genetically engineered stem cells (GESTECs) with tumor-tropism could be therapeutically potential for cell-based gene delivery (Kim et al., 2011), while a suicide gene is a specific gene which is adopted to be delivered for gene therapy and induces cell death itself through apoptosis (Kang et al., 2006).
Previously, several studies have used stem cells that express suicide genes to treat cancers in vivo and in vitro (Kim et al., 2012a; 2012b; Niess et al., 2011). For example, human neural stem cells (hNSCs) are one of the candidate stem cells showing a therapeutic potential via introduction of suicide genes and tumor tropism for the treatment of malignant tumors in the human brain including medulloblastomas and gliomas (Aboody et al., 2000; 2006; Kim et al., 2006). In this study, authors used several kinds of stem cells; HB1.F3, HB1.F3.CD, and HB1.F3. CD.IFN-β cells. CD gene expressed by these stem cells as a suicide gene can convert a non-toxic prodrug, 5-fluorocytosine (5-FC), to the toxic agent, 5-fluorouracil (5-FU). IFN-β is a powerful cytokine with anti-viral and anti-cancer effects. In cervical cancer therapy, IFNs have been used to treat mucosal lesions caused by human papilloma virus (HPV) infection, such as intraepithelial precursor lesions to cancer of the uterine cervix, genital warts or recurrent respiratory papillomatosis, by potentially reducing or eliminating the replication of HPV plasmid genomes (Lace et al., 2009).
The use of non-toxic pro-drugs seems to minimize side effects compared to using active anti-cancer drugs, but there is a difficulty in delivering the gene that converts a non-toxic pro-drug to its active metabolite to the exact tumor site for a selective activity. In this respect, these GESTECs are suitable for delivering the converting enzymes because of tumor tropism of hNSC. Stem cells carrying CD and/or IFN-β migrate to tumor sites and convert pro-drugs to toxic drugs. The tumor tropism of stem cells is known to be caused by a response to several chemoattractants secreted by cancer cells via the action of related receptors produced by them (Kang et al., 2012a; 2012b; 2012c; Kim et al., 2012a; 2012b).
It can be hypothesized that GESTECs may have an anti-cancer effect against HeLa cervical cancer cells by expressing the therapeutic genes such as CD and IFN-β gene and induce a selective cancer cell death by migrating the right tumor site owing to the specific interactions of chemoattractant ligands and their receptors between the stem cells and HeLa cancer cells.
MATERIALS AND METHODS
Cell culture
A human cervical cancer cell line, HeLa, was purchased from the American Tissue Type Culture Collection (ATCC, USA) and cultured in DMEM (Hyclone Laboratories Inc., USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Hyclone Laboratories), 1% HEPES (Invitrogen Life Technologies, USA), 1% penicillin/streptomycin (Cellgro Mediatech, USA), and 0.1% anti-mycoplasmal plasmocin (Invivogen, USA) at 37°C in a humidified atmosphere of 5% CO2-95% air. In addition, hNSCs such as HB1.F3, HB1.F3.CD, and HB1.F3.CD.IFN-β cells were obtained from Chungang University (Korea). HB1.F3 is an immortalized hNSC line derived from human fetal brain at 15 weeks of gestation by an amphotropic and replication-incompetent retroviral vector v-myc. The clonal HB1.F3.CD and HB1. F3.CD.IFN-β cell lines were derived from parental HB1.F3 cell line by transducing E. coli CD and human IFN-β genes. All hNSCs such as HB1.F3, HB1.F3.CD, and HB1.F3.CD.IFN-β cells and human dermal fibroblasts (HDF ; OBM Lab., Korea) were cultured in DMEM supplemented with 10% FBS, 1% penicillin G and streptomycin, 1% HEPES, and 0.1% plasmocin at 37°C in a humidified atmosphere of 5% CO2-95% air. Cells were trypsinized with 0.05% trypsin/0.02% EDTA in Mg2+/Ca2+− free HBSS (Hyclone Laboratories).
Cell viability assay
MTT assay was performed to measure the cytotoxic effect of 5-FC and 5-FU on cervical cancer cells (5,000 cells/well). HeLa cells, a cervical cancer cell line, were seeded in 96-well plates and cultured in 0.1 ml medium with 5% FBS. After pre-incubation of 24 h, 5-FC (Sigma-Aldrich Corp., USA) and 5-FU (Sigma-Aldrich Corp.) were serially diluted with phosphate buffered saline (PBS; final concentration 0.1, 0.3, 0.5, 1, and 10 mmol/L) and added to the tumor cell cultures for 4 days. On the following day, MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was performed to measure cell viability. MTT solution (10 μl of stock at 5 mg/ml) was added to each well in the plates and incubated at 37°C for 3 h and 30 min. Supernatants were removed and 100 μl of dimethyl sulfoxide (DMSO, 99.0%; Junsei Chemical Co. Ltd., Japan) was added to each well to dissolve the resultant formazan crystals. Optical densities were measured at 540 nm using an ELISA reader (VERSA man, USA). MTT assay was carried out in duplicate.
Anticancer effects of GESTECs after 5-FC treatment were examined by co-culture experiments. Hela cells (1,250 cells/ well) and GESTECs (3,750 cells/ well) were suspended in 5% FBS DMEM and were seeded in 96-well plates. After a pre-incubation of 24 h, the mixed cell culture was treated with 5-FC (0.1, 0.3, 0.5, 1, and 5 mmol/L) for 4 days. After treatment with 5-FC, MTT assay was performed to measure cell viability as described above and also carried out in duplicate.
In vitro migration assay
Transwell migration assay was performed so as to assess the migration capability of stem cells to cervical cancer cells. HeLa cells and HDF were seeded in 24-well plates and cultured with DMEM containing 10% FBS at 37°C for 6 h. Then cells were incubated with new DMEM with 1% FBS. Transwell plates (8 μm pore membrane; BD Biosciences, USA) were coated with fibronectin (250 μg/ml; Sigma-Aldrich Corp.) and incubated overnight at 37°C. On the following day, HB1.F3.CD and HB1.F3.CD.IFN-β cells (1 × 105 cells/well) labeled with 2 M of chloromethylbenzamido-1,1′-dioctadecyl-3,3,3′-tetramethyl-indo-carbocyanine perchlorate (CM-DiI; Invitrogen) were plated in the upper chambers of the transwell plates and cultured at 37°C for 16 h. The next day, HeLa cells and HDF were stained by addition of 200 ng/ml 4, 6-diamidino-2-phenylindole solution (DAPI; Invitrogen). Each well was washed with PBS and non migrated GESTECs on the upper face of the transwell were removed with cotton swabs. Cells stained with CM-DiI and DAPI were examined by fluorescence microscopy (IX71 Inverted Microscope, Olympus, Japan). The number of migrated cells from upper chamber of a transwell to lower chamber toward HeLa cells was counted.
Real-time polymerase chain reaction (Real-time PCR)
The presence of chemoattractant ligands and their cognate receptors which are related with tumor tropism of stem cells were detected by real-time PCR. Total RNA was extracted using TriZol reagent (Invitrogen) from cultured HeLa cells. Single-stranded cDNA was synthesized by reverse transcription reaction using random primers from 1 μg of total RNA by MMLV RT (iNtRON Biotechnology, Korea). The cDNA prepared via reverse transcription reaction was used in real-time PCR. Real-time PCR was performed in a 20 μl reaction mixture containing primer, ROX as a reference dye, cDNA, and 2× SYBR green premix (Invitrogen). The real-time PCR condition is 95°C for 5 min 1 cycle, 40 cycles in 95°C for 15 s, 58°C for 20 s, and 72°C for 15 s. The forward and reverse primers and the predicted sizes of the real-time PCR reaction products are shown in Table 1.
Table 1.
The oligonucleotide sequences of the primers used for real-time PCR
| Gene | Oligonucleotide sequences | |
|---|---|---|
| uPA | Reverse | GGCAGGCAGATGGTCTGTAT |
| Forward | TTGCTCACCACAACGACATT | |
| uPAR | Reverse | TCCCCTTGCAGCTGTAACACT |
| Forward | GCCCAATCCTGGAGCTTGA | |
| SDF-1 | Reverse | TCCCATCCCACAGAGAGAAG |
| Forward | GTGTCACTGGCGACACGTAG | |
| CXCR4 | Reverse | GAGGGCCTTGCGCTTCTGGTG |
| Forward | ATCCCTGCCCTCCTGCTGACTATTC | |
| VEGF | Reverse | TCTTTCTTTGGTCTGCATTCACAT |
| Forward | CCAGCACATAGGAGAGATGAGCTT | |
| VEGFR2 | Reverse | AGCATGGAAGAGGATTCTGGACT |
| Forward | CGGCTCTTTCGCTTACTGTTCT | |
| MCP-1 | Reverse | TCTTCGGAGTTTGGGTTTGC |
| Forward | CAAGCAGAAGTGGGTTCAGGA | |
| CCR2 | Reverse | ACATTTACAAGTTGCAGTTTTCAGC |
| Forward | CTACCTTCCAGTTCCTCATTTTT | |
| SCF | Reverse | GCCTTCAGAAATATTTGAAAACTTG |
| Forward | GGCAAATCTTCCAAAAGACTACA | |
| c-kit | Reverse | TCACAGATGGTTGAGAAGAGCCT |
| Forward | CGCCTGGGATTTTCTCTGC | |
| GAPDH | Reverse | ATGTTCGTCATGGGTGTGAACCA |
| Forward | TGGCAGGTTTTTCTAGACGGCAG |
Inhibition of migration of GESTECs
Before a migration assay was performed, the cultured GESTECs were treated with KRN633 (Selleck Chemicals, USA) for 6 h. KRN633 is a selective inhibitor of VEGFR2 tyrosine kinase, which is known to suppress tumor angiogenesis and migration of epithelial cells (Nakamura et al., 2004). KRN633 (170 nM) was diluted in culture medium containing 0.1% DMSO. After that, the in vitro migration assay was performed as previously mentioned in an in vitro migration assay. The number of migrated cells from upper chamber of a transwell to lower chamber toward HeLa cells was counted.
Statistical analysis
The results of all cell viability assays are presented as means ± SD. One-way ANOVA was performed and P < 0.05 was considered statistically significant. In migration assay, the number of migrated cells is presented as means ± SD. The t-test was performed and P < 0.05 was considered statistically significant.
RESULTS
Effects of 5-FC/5-FU with hNSCs or GESTECs on HeLa cells
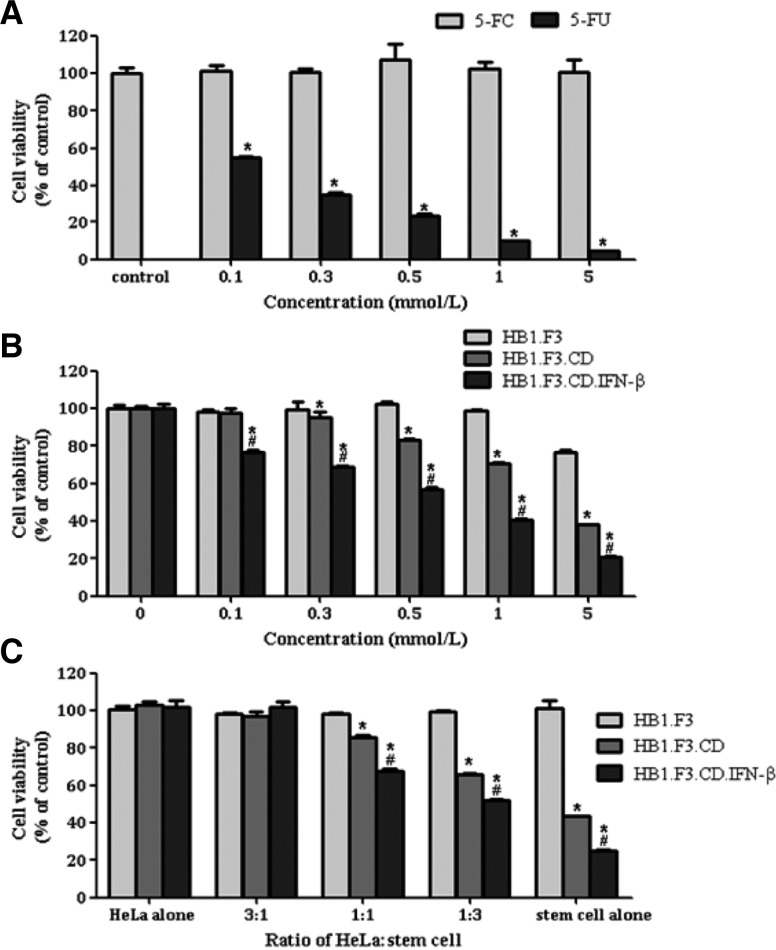
The cytotoxic effect of a prodrug, 5-FC, and its active metabolite, 5-FU, on HeLa cells was measured with MTT assay (Fig. 1A). According to these results, 5-FC did not have any cytotoxic effects on HeLa cells. On the other hand, 5-FU showed a significant anti-cancer effect on HeLa cells, indicating that HeLa cells are highly sensitive to the cytotoxicity of 5-FU, even at low concentration (0.1 mmol/ml) as shown in Fig. 1A. Secondly, to determine the prodrug conversion efficiency of GESTECs, HeLa cells and stem cells were co-cultured in the presence of 5-FC at different concentrations (0.1, 0.3, 0.5, 1, and 5 mmol/ ml) and their cell viability was measured as seen Fig. 1B. In contrast with HB1.F3.CD and HB1.F3.CD.IFN-β, HB1.F3 cells as the non-modified control of hNSCs did not affect cell viability at any concentrations of 5-FC. HB1.F3.CD cells started to reduce the cancer cell viability at 0.3 mmol/L of 5-FC and this cytotoxic effect was increased gradually with the increase of 5-FC concentration. Another difference is that HB1.F3.CD.IFN-β cells showed a greater reduction of cell viability than HB1. F3.CD cells at the same concentration of 5-FC (Fig. 1B).
Fig. 1.
Therapeutic effects of HB1.F3.CD and HB1.F3.CD.IFN-β cells on HeLa cells in the presence of 5-FC. (A) HeLa cells (5,000 cells/well) were seeded in 96-well plates and cultured in 0.1 ml medium with 5% FBS. After pre-incubation of 24 h, 5-FC and 5-FU were serially diluted with phosphate buffered saline (PBS; final concentration 0.1, 0.3, 0.5, 1, and 5 mmol/L) and were added to the tumor cell cultures for 4 days. On the following day, MTT assay was performed to measure cell viability. (B) HeLa cells (1,250 cells /well) and hNSCs (3,750 cells/well) were suspended in 5% FBS DMEM and were seeded in 96-well plates. After pre-incubation of 24 h, the mixed cell culture was treated with 5-FC (0.1, 0.3, 0.5, 1, and 5 mmol/L) for 4 days. After treatment with 5-FC, MTT assay was performed to measure cell viability. (C) To confirm the difference of therapeutic effects resulting from the ratio of cancer cells to hNSCs, HeLa, HB1. F3, HB1.F3.CD, and HB1. F3.CD.IFN-β cells were seeded in different ratios (cancer cells : stem cells = 3:1, 1:1, 1:3) in 96-well plates. After pre-incubation of 24 h, cells were treated with 5-FC at final concentration of 1 mmol/ml for 4 days. The results were presented as means ± SD. The One-way ANOVA was performed and P < 0.05 was considered statistically significant. *, P < 0.05 vs. 5-FC or HB1.F3; #, P < 0.05 vs. HB1.F3.CD.
Finally, to study whether the ratios of the stem cells to HeLa cells affect the intensity of anti-cancer effect on HeLa cervical cancer cells, HeLa cells and stem cells were co-cultured at several ratios (the ratios of HeLa cells to stem cells; 3:1, 1:1, 1:3, and stem cell alone) and treated with 5-FC to measure the cell viability of mixed culture as shown in Fig. 1C. When the ratio of cancer cells to stem cells was 3:1, the cell viability of co-culture system was not changed by the type of stem cells. However, when relatively more stem cells were co-cultured with cancer cells, the intensity of anticancer effects was appeared to be increased. In particular, this tendency was more obvious when using HB1.F3.CD.IFN-β cells than HB1.F3.CD cells (Fig. 1C).
In vitro migration assay
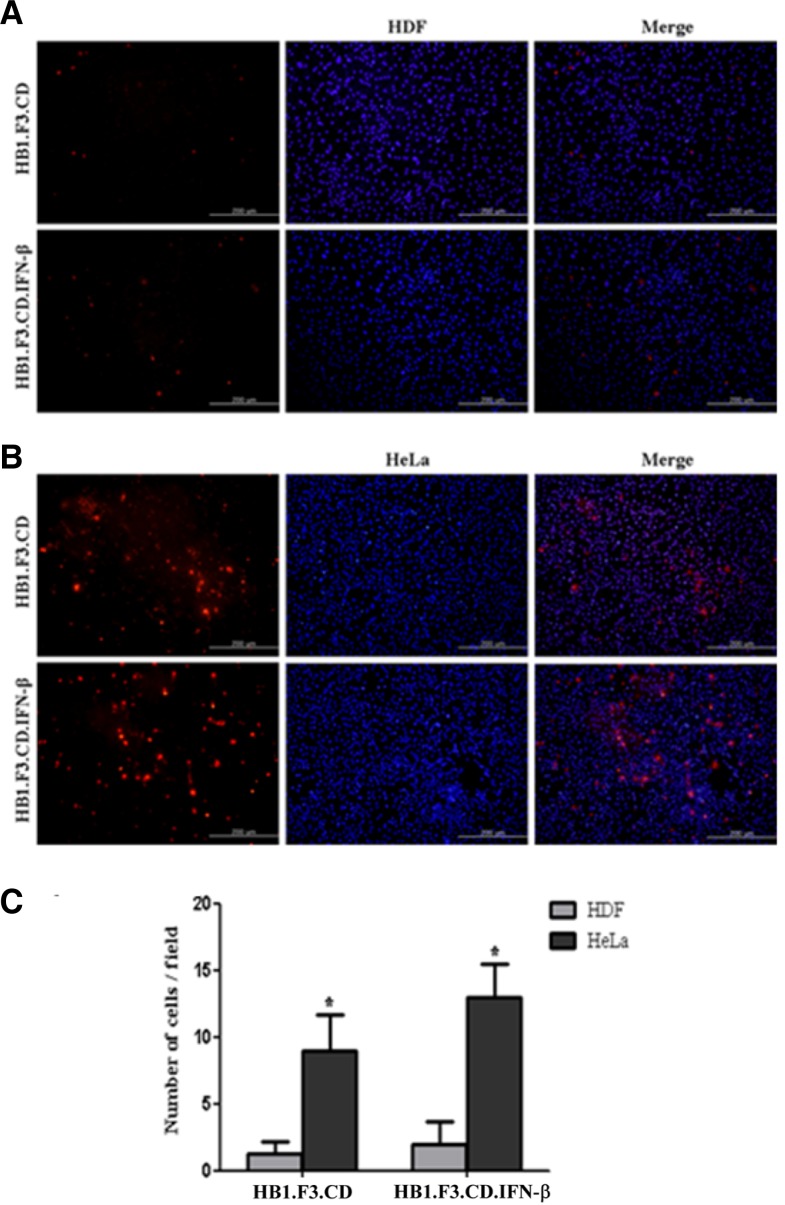
The migratory capability of GESTECs toward HeLa cells in comparison to HDF was investigated by a modified transwell migration assay (Figs. 2A and 2B). HDF is a kind of fibroblasts, which are the most common cells of connective tissue in animals. Therefore, these fibroblasts were adopted as normal cells for a negative control. Using fluorescence microscopy, the number of migrated GESTECs (HB1.F3.CD and HB1.F3.CD. IFN-β cells) was examined. GESTECs showed to significantly migrate to ward HeLa cells instead of moving to HDF as a control as demonstrated in Figs. 2A and 2B. This migratory effect of the stem cells to HeLa cells was calculated compared to HDF, suggesting that some factors which HeLa cells secrete may attract GESTECs toward HeLa cells (Fig. 2C).
Fig. 2.
Tumor tropism of HB1.F3.CD and HB1.F3. CD.IFN-β cells toward HeLa cells. Transwell migration assay was performed so as to assess the capability of migration to cervical cancer cells. HeLa cells or human dermal fibroblasts (HDF) were seeded in 24-well plates and cultured with DMEM containing 10% FBS at 37°C for 6 h. Then cells were incubated with new DMEM with 1% FBS. On the following day, HB1.F3.CD and HB1.F3.CD. IFN-β cells (1 × 105 cells/well) labeled with 2 mM of CMDiI were plated in the upper chambers of the trans-well and cultured at 37°C for 16 h. The next day, HeLa cells and HDF were stained by addition of 200 ng/ml DAPI. Each well was washed with PBS and non-migrated hNSCs on the upper face of the transwell were removed with cotton swabs. Cells stained with CM-DiI and DAPI were examined by fluorescence microscopy. Magnification, × 200. (A) HDF cells were cultured in lower chambers. (B) HeLa cells were cultured in lower chambers instead of HDF cells. (C) The number of migrated cells was counted and the results were presented as means ± SD. The t-test was performed and P < 0.05 was considered statistically significant. *, P < 0.05 vs. HeLa.
Confirmation of chemoattractant ligands and receptors
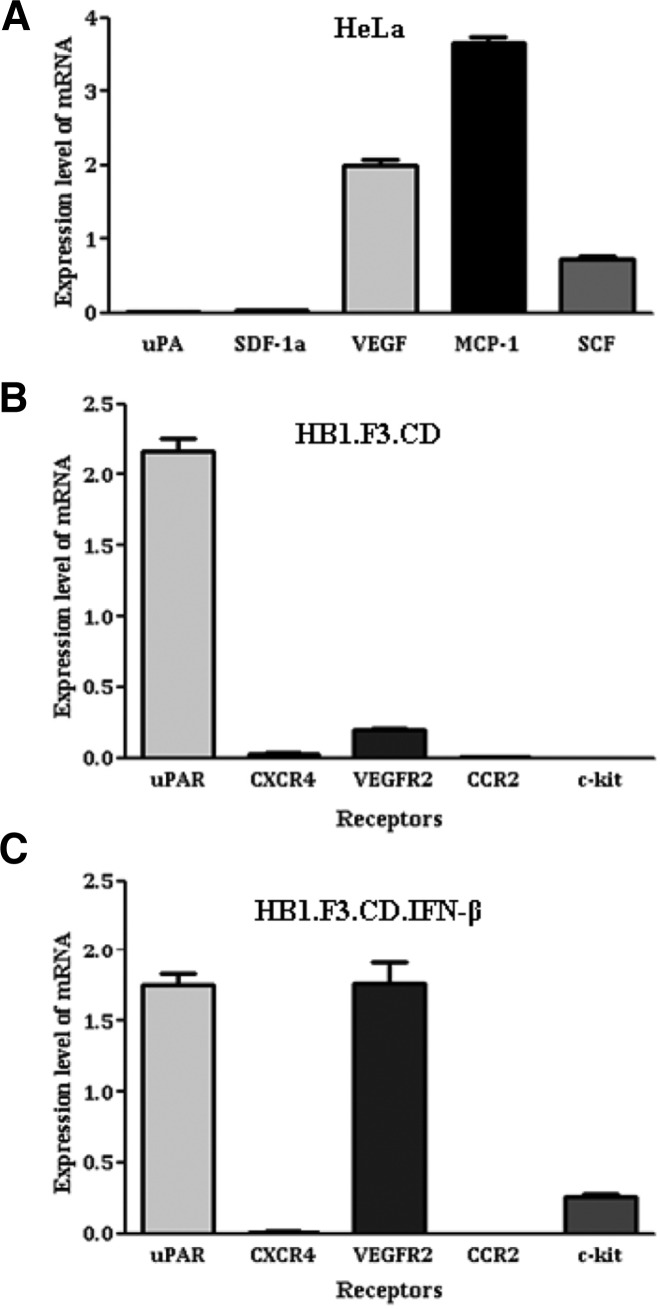
To examine what chemoattractant factors are related with the migration of GESTECs toward HeLa cells and how much amount of them is expressed, we performed real-time PCR for several ligands and their receptors. The relative expression levels of several ligands and their receptors were showed in Figs. 3A–3C. HeLa cells expressed VEGF, MCP-1 and SCF as chemoattractant ligands (Fig. 3A). Among several receptors, HB1.F3.CD cells expressed uPAR and VEGFR2, and HB1. F3.CD.IFN-β cells expressed uPAR, VEGFR2, and c-kit as demonstrated in Figs. 3B and 3C. Although HeLa cells expressed a large amount of MCP-1, MCP-1 did not affect the migratory characteristics because both GESTECs did not express CCR2, a receptor of MCP-1. Although HeLa cells produced SCF to a certain extent, c-kit (a receptor of SCF) was not expressed by HB1.F3.CD cells. However it was produced in a small amount by HB1.F3.CD.IFN-β cells (Fig. 3C). VEGF was considerably expressed by HeLa cells and VEGFR2, its related receptor, was also expressed by both HB1.F3.CD and HB1. F3.CD.IFN-β cells as shown in Figs. 3A–3C. In particular, HB1. F3.CD.IFN-β cells expressed a significant amount of VEGFR2 (Fig. 3C). Taken together, these results suggest that the pair of VEGF and VEGFR2 is considered to be a crucial factor for a selective migration between HeLa cells and GESTECs.
Fig. 3.
Expression of chemoattractant ligands by HeLa cells and their receptors by hNSCs. The ligands and their receptors are following: stem cell factor (SCF)/c-Kit, stromal cell-derived factor 1 (SDF-1)/CXC chemokine receptor 4 (CXCR4), vascular endothelial growth factor (VEGF)/VEGF receptor 2 (VEGFR2), uro-plasminogen activator (uPA)/uPA receptor (uPAR) and monocyte chemotactic protein-1 (MCP-1)/CC receptor 2 (CCR2). The presence of these chemoattractants ligands and related receptors were detected by real time-PCR. Total RNA was extracted using TriZol from cultured HeLa cells and stem cells. cDNA was synthesized by reverse transcription reaction using random primers from 1 μg of total RNA by M-MLV RT. Real-time PCR was performed in 20 μl reaction mixture containing primer, Rox as a reference dye, cDNA, and 2× SYBR green premix. The real-time PCR condition is 95°C for 5 min 1 cycle, 40 cycles in 95°C for 15 s, 58°C for 20 s, 72°C for 15 s. (A) Ligands expressed in HeLa cells. (B) Receptors expressed in HB1.F3.CD cells. (C) Receptors expressed in HB1.F3.CD.IFN-β cells.
Inhibition of migration of GESTECs toward HeLa cells
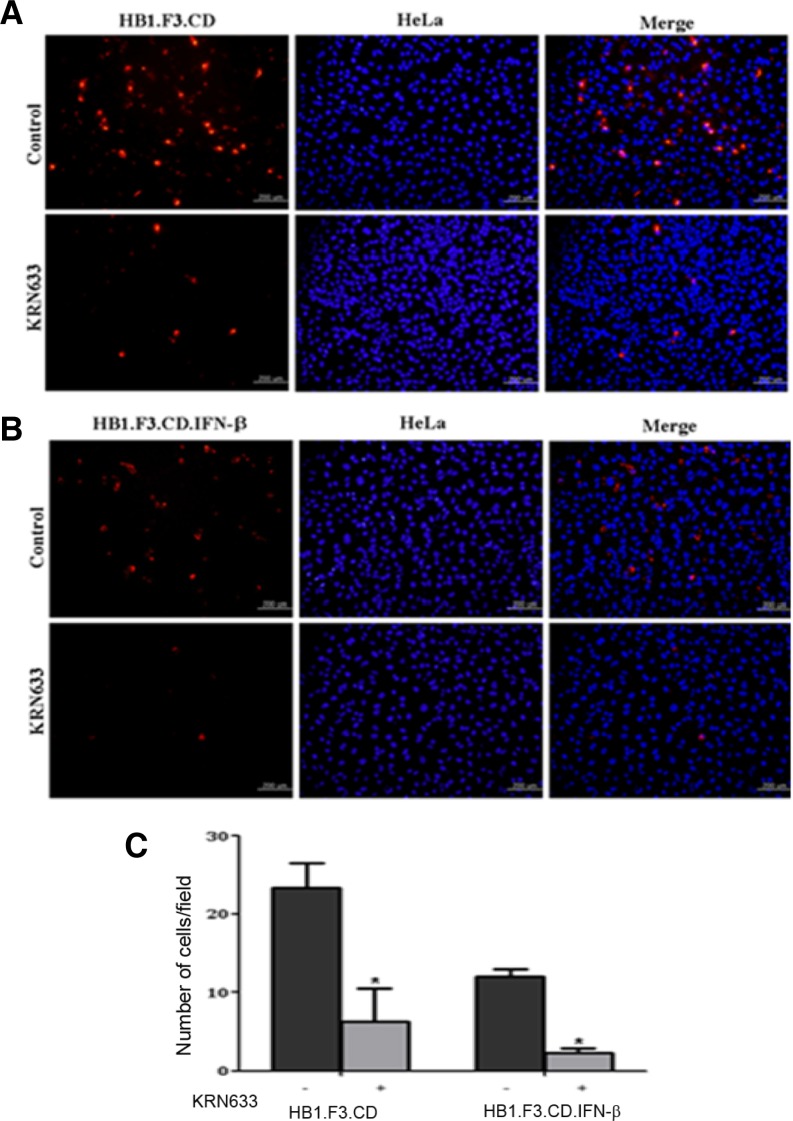
To verify whether VEGF plays a crucial role in the migration of GESTECs toward HeLa cells, KRN633, a selective inhibitor of VEGFR2, was treated to GESTECs. After treatment, these stem cells were analyzed by the modified migration assay. The migration capacity of KRN633-treated GESTECs was compared with that of non-treated GESTECs. When these stem cells were treated with KRN633, the number of migrated HB1.F3.CD and HB1.F3.CD.IFN-β cells toward HeLa cells was much fewer than that of non-treated GESTECs, which was declined to 48.6% and 63.2%, respectively (Figs. 4A and 4B). This migratory capacity of the stem cells to HeLa cells was calculated in the presence or absence of KRN633 (Fig. 4C), suggesting that VEGFR2 expressed by the GESTECs play a key role in the stem cell migration toward HeLa cervical cancer cells by working as a receptor of VEGF expressed by HeLa cells.
Fig. 4.
Inhibited migration of hNSCs toward HeLa cells with the treatment of KRN633, a VEGFR2 inhibitor. The cultured hNSCs were treated with KRN633 for 6 h. After that, the transwell migration assay was performed as described above. The number of migrated cells was counted and the results were presented as means ± SD. Magnification, × 200. *P < 0.05 vs. KRN633 non-treated GESTECs. (A) Migrated HB1.F3.CD cells. (B) Migrated HB1.F3.CD.IFN-β cells. (C) The number of migrated stem cells.
DISCUSSION
In the previous studies, the engineered stem cells expressing suicide genes have been proved to have a potential in gene therapy for treating several kinds of cancers by functioning as cellular vehicles for an effective delivery of therapeutic agents in vivo and in vitro (Yi et al., 2011a; 2011b; 2011c; 2012b).
In the present study, we investigated the effect of CD or CD/IFN-β expressed by stem cells on HeLa cells and a selective migratory ability of therapeutic stem cells to the cancer cells. First, we studied the anti-cancer effects of CD gene and IFN-β transduced into hNSCs in the presence of 5-FC. In the cell viability assay, 5-FC had no cytotoxic effect on HeLa cervical cancer cells as expected because it is a non-toxic prodrug. But, when HeLa cells were treated with 5-FC and the GESTECs expressing CD gene, the cell viability was significantly reduced. This result suggests that 5-FU was effectively converted from 5-FC by CD gene transduced into the stem cells and caused its potent anti-cancer effect on HeLa cells, and therefore, this system can be used for gene therapy on cancer. Particularly, when IFN-β and 5-FC simultaneously acted on cervical cancer, they created synergistic anti-cancer effects, which were considered to be related with the interaction of 5-FU and IFN-β. In the previous study, it was determined that 5-FU increased the susceptibility of tumor cells to IFN-β by enhancing IFN pathway (Chawla-Sarkar et al., 2003). In detail, when tumor cells were treated with 5-FU and type1 IFN, 5-FU up-regulated the expression of IFN type I receptor and interferon stimulated genes (ISGs), resulting in a marked apoptosis of cancer cells (Chawla-Sarkar et al., 2003; Eguchi et al., 2000; Kondo et al., 2005; Lee et al., 2011; Matsumura et al., 2005).
Second, we also investigated the ability of migration of stem cells toward cancer cells. In in vitro migration assays, we observed that stem cells which were loaded in the upper chamber of a transwell migrated to lower chamber containing HeLa cervical cancer cells. In the previous studies, the parental stem cells, HB1.F3, was also proved to have the migrating capability towards various types of cancer, indicating that this cell line can have an advantage as a delivery vehicle for anti-tumor treatment by possessing a tendency to effectively migrate to cancer cells (Aboody et al., 2000; Kang et al., 2012b; Kim et al., 2012b; Lee et al., 2011). From the results of migration assay, it was assumed that chemoattractant factors which cervical cancer cells secrete might make HB1.F3.CD and HB1.F3.CD.IFN-β cells migrate toward cancer cells, resulting in the delivery of therapeutic genes to the right tumor site. In other studies, several factors such as SCF, VEGF and uPA were previously known to play a chemoattractive role in the migration of stem cells to cancer cells (Gutova et al., 2008; Zengel et al., 2010; Zhao et al., 2008). In the current study, chemoattractant ligands such as MCP-1, SCF, and VEGF were confirmed to be expressed in HeLa cells. Meanwhile, HB1.F3.CD cells were determined to produce VEGFR2, a receptor of VEGF, and HB1.F3.CD.IFN-β cells were shown to express c-kit, a receptor of SCF, as well as VEGFR2. Although both HB1.F3.CD and HB1.F3.CD.IFN-β cells significantly expressed uPAR, a receptor of uPA, HeLa cells were determined to not nearly express uPA, its relevant ligand. Therefore, among several chemoattractant ligands and receptors, the pair of VEGF and VEGFR2 may be closely related in tumor tropism of GESTECs that selectively deliver the suicide enzyme and anti-cancer cytokine genes to HeLa cervical cancer cells. VEGF is a signal protein stimulating vasculogenesis and angiogenesis and also known to play several roles such as cell proliferation and migration (Barleon et al., 1996; Parenti et al., 1996).
Finally, to verify the role of VEGF in cell migration, we inhibited the function of VEGFR2 produced by stem cells with KRN633, a selective inhibitor of VEGFR2, and confirmed that the number of migrated stem cells was decreased to 48.6% in case of HB1.F3.CD cells and 63.2% in case of HB1.F3.CD. IFN-β cells, respectively, compared to the case of non-treated GESTECs. Therefore, this result indicated that VEGF and VEGFR2 play a key role in the migration of stem cells expressing CD and/or IFN-β toward HeLa cervical cancer cells as shown in other studies (Ferrara, 2004; Tammela et al., 2005; Zeng et al., 2001).
In conclusion, both GESTECs, HB1.F3.CD and HB1.F3.CD. IFN-β cells, produced a strong anti-cancer effect on HeLa cervical cancer cells. HB1.F3.CD.IFN-β cells expressing both CD and IFN-β have more effective therapeutic effects than HB1.F3.CD cells expressing CD only. In other words, when 5-FU converted from 5-FC by CD gene and IFN-β simultaneously affect HeLa cervical cancer cells, they have a synergetic anti-cancer activity. In addition, the GESTECs may selectively migrate toward the cervical cancer cells through the interaction between VEGF and VEGFR2 expressed by HeLa cells and these stem cells, respectively. Therefore, it is possible to consider that therapeutic stem cells expressing CD and/or IFN-β with an application of a non-toxic prodrug, 5-FC, may have a therapeutic potential by effectively reducing HeLa cervical cancer cells via their selective tumor tropism mainly induced by VEGF action.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ009599), Rural Development Administration, Republic of Korea.
REFERENCES
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboody KS, Bush RA, Garcia E, Metz MZ, Najbauer J, Justus KA, Phelps DA, Remack JS, Yoon KJ, Gillespie S, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS One. 2006;1:e23. doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- Eguchi H, Nagano H, Yamamoto H, Miyamoto A, Kondo M, Dono K, Nakamori S, Umeshita K, Sakon M, Monden M. Augmentation of antitumor activity of 5-fluorouracil by interferon alpha is associated with up-regulation of p27Kip1 in human hepatocellular carcinoma cells. Clin Cancer Res. 2000;6:2881–2890. [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Francis SA, Katz ML. The HPV Vaccine: a comparison of focus groups conducted in South Africa and Ohio Appalachia. Matern Child Health J. 2013;17:1222–1229. doi: 10.1007/s10995-012-1116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutova M, Najbauer J, Frank RT, Kendall SE, Gevorgyan A, Metz MZ, Guevorkian M, Edmiston M, Zhao D, Glackin CA, et al. Urokinase plasminogen activator and urokinase plasminogen activator receptor mediate human stem cell tropism to malignant solid tumors. Stem Cells. 2008;26:1406–1413. doi: 10.1634/stemcells.2008-0141. [DOI] [PubMed] [Google Scholar]
- Jung S, Yi L, Kim J, Jeong D, Oh T, Kim CH, Kim CJ, Shin J, An S, Lee MS. The role of vimentin as a methylation biomarker for early diagnosis of cervical cancer. Mol Cells. 2011;31:405–411. doi: 10.1007/s10059-011-0229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Xu CJ, Wu CQ, Liu XS, Zhong CP, Zhang XH, Qiao SY, Gu JR. A novel strategy to compensate the disadvantages of live vaccine using suicide-gene system and provide better antitumor immunity. Vaccine. 2006;24:2141–2150. doi: 10.1016/j.vaccine.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Kang NH, Hwang KA, Kim SU, Kim YB, Hyun SH, Jeung EB, Choi KC. Potential antitumor therapeutic strategies of human amniotic membrane and amniotic fluid-derived stem cells. Cancer Gene Ther. 2012a;19:517–522. doi: 10.1038/cgt.2012.30. [DOI] [PubMed] [Google Scholar]
- Kang NH, Hwang KA, Yi BR, Lee HJ, Jeung EB, Kim SU, Choi KC. Human amniotic fluid-derived stem cells expressing cytosine deaminase and thymidine kinase inhibits the growth of breast cancer cells in cellular and xenograft mouse models. Cancer Gene Ther. 2012b;19:412–419. doi: 10.1038/cgt.2012.15. [DOI] [PubMed] [Google Scholar]
- Kang NH, Yi BR, Lim SY, Hwang KA, Baek YS, Kang KS, Choi KC. Human amniotic membrane-derived epithelial stem cells display anticancer activity in BALB/c female nude mice bearing disseminated breast cancer xenografts. Int J Oncol. 2012c;40:2022–2028. doi: 10.3892/ijo.2012.1372. [DOI] [PubMed] [Google Scholar]
- Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, Cho BK, Kim M, Menon LG, Black PM, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- Kim SU, Jeung EB, Kim YB, Cho MH, Choi KC. Potential tumor-tropic effect of genetically engineered stem cells expressing suicide enzymes to selectively target invasive cancer in animal models. Anticancer Res. 2011;31:1249–1258. [PubMed] [Google Scholar]
- Kim JH, Kim JY, Kim SU, Cho KG. Therapeutic effect of genetically modified human neural stem cells encoding cytosine deaminase on experimental glioma. Biochem Biophys Res Commun. 2012a;417:534–540. doi: 10.1016/j.bbrc.2011.11.155. [DOI] [PubMed] [Google Scholar]
- Kim KY, Yi BR, Lee HR, Kang NH, Jeung EB, Kim SU, Choi KC. Stem cells with fused gene expression of cytosine deaminase and interferon-beta migrate to human gastric cancer cells and result in synergistic growth inhibition for potential therapeutic use. Int J Oncol. 2012b;40:1097–1104. doi: 10.3892/ijo.2011.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Nagano H, Wada H, Damdinsuren B, Yamamoto H, Hiraoka N, Eguchi H, Miyamoto A, Yamamoto T, Ota H, et al. Combination of IFN-alpha and 5-fluorouracil induces apoptosis through IFN-alpha/beta receptor in human hepatocellular carcinoma cells. Clin Cancer Res. 2005;11:1277–1286. [PubMed] [Google Scholar]
- Lace MJ, Anson JR, Klingelhutz AJ, Harada H, Taniguchi T, Bossler AD, Haugen TH, Turek LP. Interferon-beta treatment increases human papillomavirus early gene transcription and viral plasmid genome replication by activating interferon regulatory factor (IRF)-1. Carcinogenesis. 2009;30:1336–1344. doi: 10.1093/carcin/bgp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Kim Y, Jo MY, Kim HS, Jin Y, Kim SU, Jin J, Joo KM, Nam DH. Combined treatment of tumor-tropic human neural stem cells containing the CD suicide gene effectively targets brain tumors provoking a mild immune response. Oncol Rep. 2011;25:63–68. [PubMed] [Google Scholar]
- Matsumura Y, Yashiro M, Ohira M, Tabuchi H, Hirakawa K. 5-Fluorouracil up-regulates interferon pathway gene expression in esophageal cancer cells. Anticancer Res. 2005;25:3271–3278. [PubMed] [Google Scholar]
- Nakamura K, Yamamoto A, Kamishohara M, Takahashi K, Taguchi E, Miura T, Kubo K, Shibuya M, Isoe T. KRN633: A selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase that suppresses tumor angiogenesis and growth. Mol Cancer Ther. 2004;3:1639–1649. [PubMed] [Google Scholar]
- Niess H, Bao Q, Conrad C, Zischek C, Notohamiprodjo M, Schwab F, Schwarz B, Huss R, Jauch KW, Nelson PJ, et al. Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma. Ann Surg. 2011;254:767–774. doi: 10.1097/SLA.0b013e3182368c4f. discussion 774–765. [DOI] [PubMed] [Google Scholar]
- Parenti A, Donnini S, Morbidelli L, Granger HJ, Ziche M. The effect of linomide on the migration and the proliferation of capillary endothelial cells elicited by vascular endothelial growth factor. Br J Pharmacol. 1996;119:619–621. doi: 10.1111/j.1476-5381.1996.tb15718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Yi BR, Kang NH, Hwang KA, Kim SU, Jeung EB, Choi KC. Antitumor therapeutic effects of cytosine deaminase and interferon-beta against endometrial cancer cells using genetically engineered stem cells in vitro. Anticancer Res. 2011a;31:2853–2861. [PubMed] [Google Scholar]
- Yi BR, Kim SU, Kim YB, Lee HJ, Cho MH, Choi KC. Antitumor effects of genetically engineered stem cells expressing yeast cytosine deaminase in lung cancer brain metastases via their tumor-tropic properties. Oncol Rep. 2011b;27:1823–1828. doi: 10.3892/or.2012.1721. [DOI] [PubMed] [Google Scholar]
- Yi BR, O SN, Kang NH, Hwang KA, Kim SU, Jeung EB, Kim YB, Heo GJ, Choi KC. Genetically engineered stem cells expressing cytosine deaminase and interferon-beta migrate to human lung cancer cells and have potentially therapeutic anti-tumor effects. Int J Oncol. 2011c;39:833–839. doi: 10.3892/ijo.2011.1126. [DOI] [PubMed] [Google Scholar]
- Yi BR, Choi KJ, Kim SU, Choi KC. Therapeutic potential of stem cells expressing suicide genes that selectively target human breast cancer cells: evidence that they exert tumoricidal effects via tumor tropism (review) Int J Oncol. 2012a;41:798–804. doi: 10.3892/ijo.2012.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi BR, Hwang KA, Kang NH, Kim SU, Jeung EB, Kim HC, Choi KC. Synergistic effects of genetically engineered stem cells expressing cytosine deaminase and interferon-beta via their tumor tropism to selectively target human hepatocarcinoma cells. Cancer Gene Ther. 2012b;19:644–651. doi: 10.1038/cgt.2012.45. [DOI] [PubMed] [Google Scholar]
- Yi BR, Park MA, Lee HR, Kang NH, Choi KJ, Kim SU, Choi KC. Suppression of the growth of human colorectal cancer cells by therapeutic stem cells expressing cytosine deaminase and interferon-beta via their tumor-tropic effect in cellular and xenograft mouse models. Mol Oncol. 2013;7:543–554. doi: 10.1016/j.molonc.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Sanyal S, Mukhopadhyay D. Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J Biol Chem. 2001;276:32714–32719. doi: 10.1074/jbc.M103130200. [DOI] [PubMed] [Google Scholar]
- Zengel P, Ramp D, Mack B, Zahler S, Berghaus A, Muehlenweg B, Gires O, Schmitz S. Multimodal therapy for synergic inhibition of tumour cell invasion and tumour-induced angiogenesis. BMC Cancer. 2010;10:92. doi: 10.1186/1471-2407-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, Kim SU, Aboody KS. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res. 2008;6:1819–1829. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]