Abstract
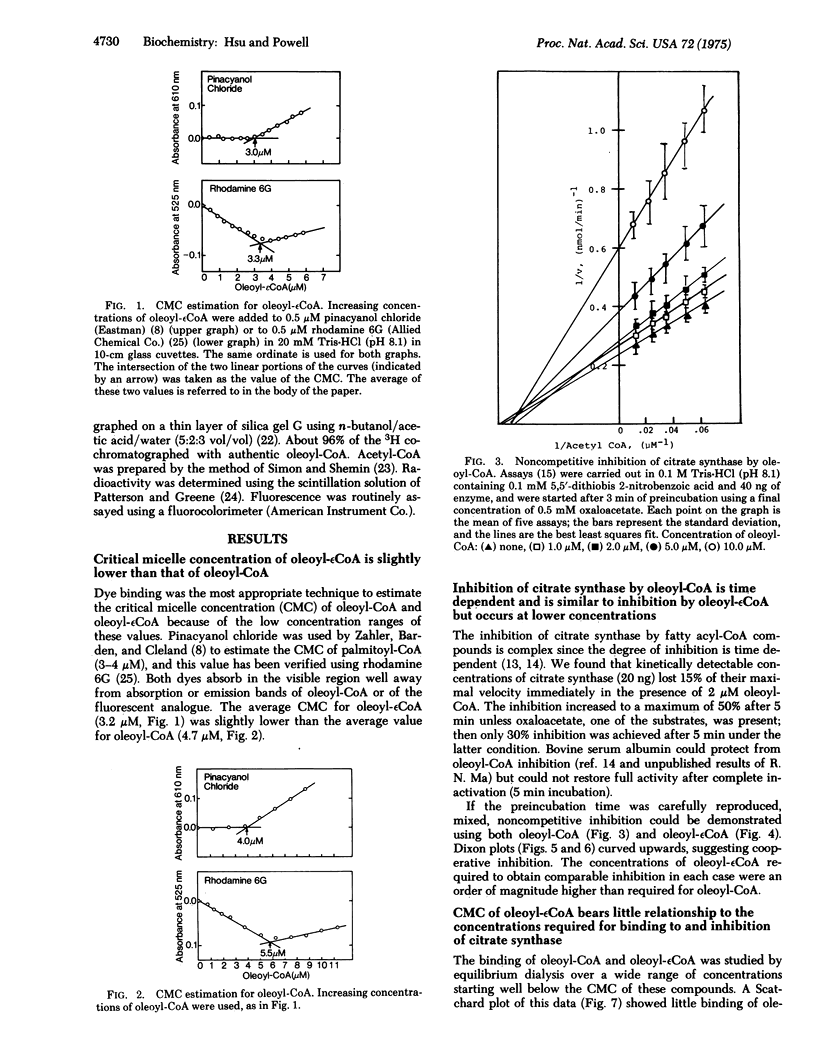
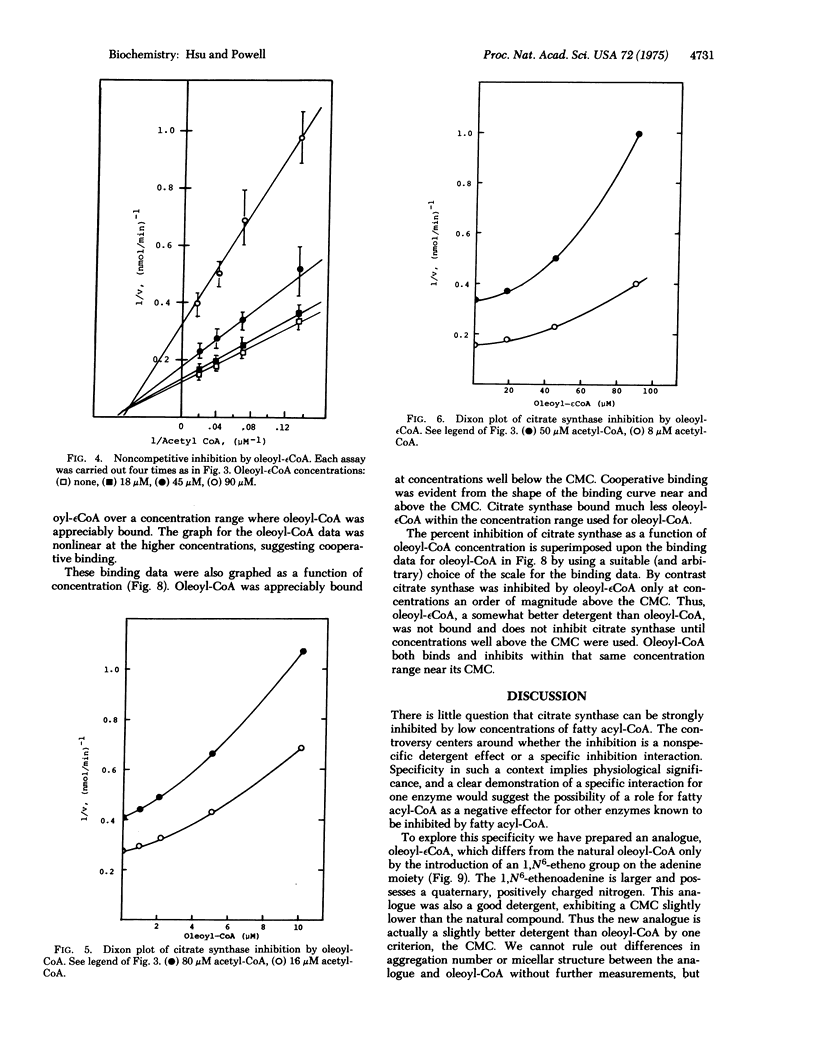
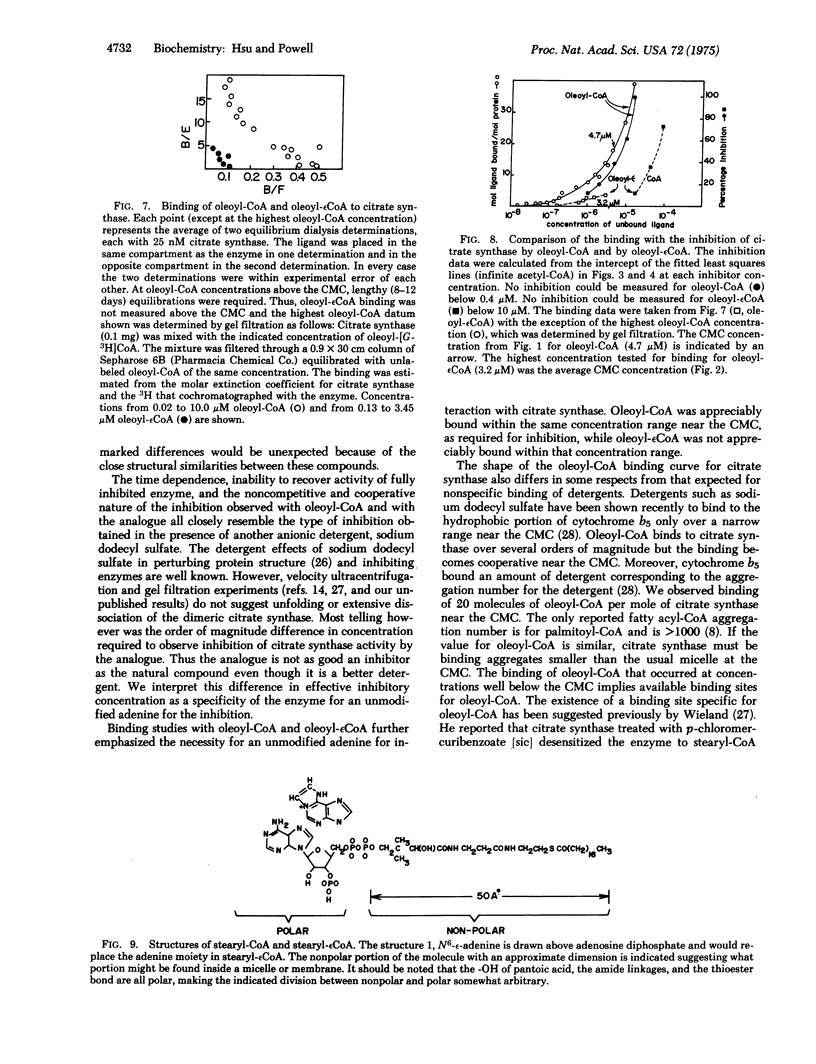
Fatty acyl-CoAs are good detergents (dritical micelle concentrations = 3-4 muM) and can inhibit a number of enzymes, including some involved in fatty acid biosynthesis. The regulatory significance of fatty acyl-CoAs as negative effectors has been questioned largely because of the difficulties in distinguishing possible nonspecific detergent effects from more specific regulatory interactions with these enzymes. A new analogue of oleoyl-CoA, oleoyl-(1, N6-etheno)-CoA, is a better detergent (critical micelle concentration = 3.2 muM) than oleoyl-CoA (critical micelle concentration = 4.7 muM). This new analogue is not as good (by an order of magnitude) an inhibitor of citrate synthase [citrate oxaloacetatelyase (pro-3S-CH2-COO-vectoracetyl-CoA); EC 4.1.3.7] nor is it bound as well oleoyl-CoA. Since the only difference between these two compounds is substitution of 1,N6-ethenoadenine for the adenine of CoA, the difference in inhibition and binding implies a specific interaction between the adenine moiety of oleoyl-CoA and citrate synthase. Moreover, since oleoyl-(1,N6-etheno)CoA is a better detergent than oleoyl-CoA, the detergency of oleoyl-CoA is not the sole cause of the fatty acyl-CoA inhibition of citrate synthase. These results support a physiological role for oleoyl-CoA as a negative effector for citrate synthase. An analogous physiological role for fatty acyl-CoA as negative effectors for other enzymes seems reasonable.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORTZ W. M., LYNEN F. THE INHIBITION OF ACETYL COA CARBOXYLASE BY LONG CHAIN ACYL COA DERIVATIVES. Biochem Z. 1963 Aug 14;337:505–509. [PubMed] [Google Scholar]
- Banis R. J., Tove S. B. Solubilization of a long chain fatty acyl-CoA synthetase from chicken adipose tissue microsomes. Biochim Biophys Acta. 1974 May 29;348(2):210–220. doi: 10.1016/0005-2760(74)90232-x. [DOI] [PubMed] [Google Scholar]
- Barber E. D., Lands W. E. Determination of acyl-CoA concentrations using pancreatic lipase. Biochim Biophys Acta. 1971 Nov 13;250(2):361–366. doi: 10.1016/0005-2744(71)90192-6. [DOI] [PubMed] [Google Scholar]
- Bergeron R., Machida Y., Bloch K. Complex formation between mycobacterial polysaccharides or cyclodextrins and palmitoyl coenzyme A. J Biol Chem. 1975 Feb 25;250(4):1223–1230. [PubMed] [Google Scholar]
- Devaux P. F., Bienvenüe A., Lauquin G., Brisson A. D., Vignais P. M., Vignais P. V. Interaction between spin-labeled acyl-coenzyme A and the mitochondrial adenosine diphosphate carrier. Biochemistry. 1975 Mar 25;14(6):1272–1280. doi: 10.1021/bi00677a028. [DOI] [PubMed] [Google Scholar]
- Goodridge A. G. Regulation of fatty acid synthesis in isolated hepatocytes. Evidence for a physiological role for long chain fatty acyl coenzyme A and citrate. J Biol Chem. 1973 Jun 25;248(12):4318–4326. [PubMed] [Google Scholar]
- Goodridge A. G. Regulation of the activity of acetyl coenzyme A carboxylase by palmitoyl coenzyme A and citrate. J Biol Chem. 1972 Nov 10;247(21):6946–6952. [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi A., Bloch K. Inhibition of glucose 6-phosphate dehydrogenase by palmitoyl coenzyme A. J Biol Chem. 1974 Sep 25;249(18):5793–5800. [PubMed] [Google Scholar]
- Lane M. D., Moss J., Ryder E., Stoll E. The activation of acetyl CoA carboxylase by tricarboxylic acids. Adv Enzyme Regul. 1970;9:237–251. doi: 10.1016/s0065-2571(71)80047-x. [DOI] [PubMed] [Google Scholar]
- Numa S., Ringelmann E., Lynen F. Zur Hemmung der Acetyl-CoA-Carboxylase durch Fettsäure-Coenzym A-Verbindungen. Biochem Z. 1965 Dec 1;343(3):243–257. [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Reitz R. C., Lands W. E., Christie W. W., Holman R. T. Effects of ethylenic bond position upon acyltransferase activity with isomeric cis,cis-octadecadienoyl coenzyme A thiol esters. J Biol Chem. 1968 May 10;243(9):2241–2246. [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Robinson N. C., Tanford C. The binding of deoxycholate, Triton X-100, sodium dodecyl sulfate, and phosphatidylcholine vesicles to cytochrome b5. Biochemistry. 1975 Jan 28;14(2):369–378. doi: 10.1021/bi00673a025. [DOI] [PubMed] [Google Scholar]
- Rous S. The origin of hydrogen in fatty acid synthesis. Adv Lipid Res. 1971;9:73–118. doi: 10.1016/b978-0-12-024909-1.50009-0. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J., Villar-Palasi C., Gilman A. G. Fluorescent modification of adenosine 3',5'-monophosphate: spectroscopic properties and activity in enzyme systems. Science. 1972 Jul 21;177(4045):279–280. doi: 10.1126/science.177.4045.279. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J., Weber G. Fluorescent modification of adenosine-containing coenzymes. Biological activities and spectroscopic properties. Biochemistry. 1972 Sep 12;11(19):3499–3506. doi: 10.1021/bi00769a001. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Palmityl-coenzyme A inhibition of the citrate-condensing enzyme. Biochim Biophys Acta. 1965 Dec 2;106(3):445–455. doi: 10.1016/0005-2760(65)90061-5. [DOI] [PubMed] [Google Scholar]
- Srere P. A. The citrate enzymes: their structures, mechanisms, and biological functions. Curr Top Cell Regul. 1972;5:229–283. doi: 10.1016/b978-0-12-152805-8.50013-7. [DOI] [PubMed] [Google Scholar]
- Sumper M. Control of fatty-acid biosynthesis by long-chain acyl CoAs and by lipid membranes. Eur J Biochem. 1974 Nov 15;49(2):469–475. doi: 10.1111/j.1432-1033.1974.tb03851.x. [DOI] [PubMed] [Google Scholar]
- Sumper M., Träuble H. Membranes as acceptors for palmitoyl CoA in fatty acid biosynthesis. FEBS Lett. 1973 Feb 15;30(1):29–34. doi: 10.1016/0014-5793(73)80612-x. [DOI] [PubMed] [Google Scholar]
- TUBBS P. K. INHIBITION OF CITRATE FORMATION BY LONG-CHAIN ACYL THIOESTERS OF COENZYME A AS A POSSIBLE CONTROL MECHANISM IN FATTY ACID BIOSYNTHESIS. Biochim Biophys Acta. 1963 Oct 22;70:608–609. doi: 10.1016/0006-3002(63)90804-7. [DOI] [PubMed] [Google Scholar]
- Taketa K., Pogell B. M. The effect of palmityl coenzyme A on glucose 6-phosphate dehydrogenase and other enzymes. J Biol Chem. 1966 Feb 10;241(3):720–726. [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Saturated fatty acid biosynthesis and its regulation. Annu Rev Biochem. 1973;42:21–60. doi: 10.1146/annurev.bi.42.070173.000321. [DOI] [PubMed] [Google Scholar]
- WIELAND O., WEISS L. INHIBITION OF CITRATE-SYNTHASE BY PALMITYL-COENZYME A. Biochem Biophys Res Commun. 1963 Sep 10;13:26–31. doi: 10.1016/0006-291x(63)90156-6. [DOI] [PubMed] [Google Scholar]
- Wieland O. In vitro behavior of enzymes. General discussion. Adv Enzyme Regul. 1966;4:281–284. doi: 10.1016/0065-2571(66)90022-7. [DOI] [PubMed] [Google Scholar]
- Wood J. M. Effect of ionic strength on the activity of carnitine palmityltransferase I. Biochemistry. 1973 Dec 18;12(26):5268–5273. doi: 10.1021/bi00750a007. [DOI] [PubMed] [Google Scholar]
- Zahler W. L., Barden R. E., Cleland W. W. Some physical properties of palmityl-coenzyme A micelles. Biochim Biophys Acta. 1968 Sep 2;164(1):1–11. doi: 10.1016/0005-2760(68)90065-9. [DOI] [PubMed] [Google Scholar]
- Ziboh V. A., Dreize M. A., Hsia S. L. Inhibition of lipid synthesis and glucose-6-phosphate dehydrogenase in rat skin by dehydroepiandrosterone. J Lipid Res. 1970 Jul;11(4):346–354. [PubMed] [Google Scholar]



