Abstract
Pilomyxoid astrocytomas is an uncommon aggressive piloid neoplasm, closely related to pilocytic astrocytomas and typically presents in the very young but can occur in older children and rarely in adults. A 12-years-old male presented with focal seizures, headache and vomiting of 10 days duration. Computed tomogram showed a hypo- to hyperdense and peripherally enhancing, solid-cystic lesion in the left temporal lobe. Histopathological examination revealed a characteristic tumor composed of bipolar cells arranged in dyscohesive sheets, angiocentric pattern in a loose myxoid background, with brisk mitotic activity and foci of necrosis. No Rosenthal fibers or eosinophilic granular bodies were seen. The tumor cells showed strong GFAP and scattered p53 positivity, but were negative for EMA. Ki-67 positivity ranged from 30 to 40%, highest reported till date. The patient was treated with radiotherapy and concurrent temozolamide and the tumor recurred after two years.
Keywords: Ki-67, pilomyxoid astrocytoma, recurrence, temporal lobe
Introduction
Pilomyxoid astrocytoma (PMA) is an uncommon progressive piloid neoplasm, closely related to pilocytic astrocytoma (PA).[1] Originally described by Janisch et al.,[2] in 1985 as ‘diencephalic pilocytic astrocytoma with clinical onset in infancy’, the term pilomyxoid astrocytoma was introduced in 1999 by Tihan et al.[3] It has been recognized as a separate entity in WHO classification of Central Nervous System Tumors in 2007 because of its distinct histological features and labeled grade II tumor owing to its aggressiveness.[1] This tumor typically presents in young children (median: 10 months), but can also occur in older children and is rare in adults.[1]
Case Report
A 12-years-old male presented with focal seizures, headache and vomiting of 10 days duration. On clinical examination there were no neurological deficits or papilledema. Computed tomogram (CT) scan of the brain showed a solid-cystic lesion in the left temporal lobe, which was hypo- to hyperdense with peripheral contrast enhancement [Figure 1]. Gross total resection of the tumor was done and sent for histopathological examination. Immediate post-operative period and further recovery was uneventful.
Figure 1.
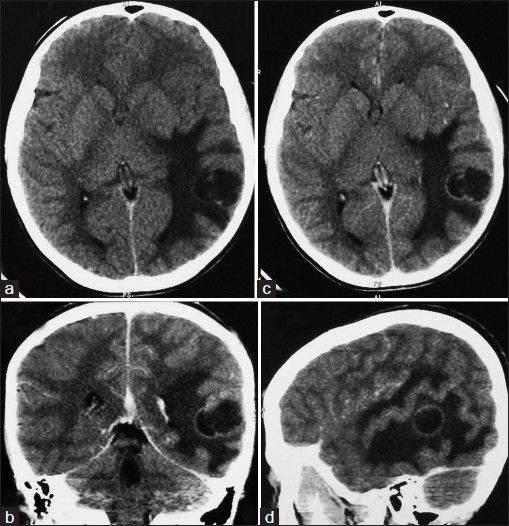
(a-d) Computed tomogram scan of the brain showed a solid-cystic lesion in the left temporal lobe, which was hypo- to hyperdense with peripheral contrast enhancement and perilesional edema.
Microscopic examination showed a cellular tumor, composed of monomorphous, bipolar cells arranged in dyscohesive sheeting pattern [Figure 2a], interspersed with microcytic spaces and abundant myxoid matrix [Figure 2c]. The tumor cells were small to medium-sized, with scant cytoplasm and bipolar, short cytoplasmic processes arranged in an angiocentric fashion, forming pseudorosettes [Figure 2b]. Brisk mitotic activity and foci of necrosis [Figure 2c] was seen. However, no Rosenthal fibers or eosinophilic granular bodies were noted. Immunohistochemical studies showed strong glial fibrillary acid protein (GFAP) positivity [Figure 2a inset], p53 positive in occasional tumor cell nuclei [Figure 2f] and Ki-67 labeling index varied from 30-40%% [Figure 2e] in different areas of the tumor. Tumor cells were immunonegative for epithelial membrane antigen (EMA) [Figure 2d inset].
Figure 2.

(a) Cellular tumor composed of bipolar cells in dyscohesive sheets (H and E, ×40); (b) Prominent angiocentric pattern (H and E, ×40);(c) Foci of necrosis (H and E, ×40); (d) Myxoid matrix (H and E, ×400). (a inset) Tumor cells are positive for GFAP (×40); (d inset) Negative for EMA (×100); (e) Ki-67 labeling index of 30-40% (×100); (f) Scattered tumor cells p53 positive (×100). (g): Radiation-induced geographic necrosis and preserved tumor in repeat excision specimen (H and E, ×40); (h) Ghost outlines of angiocentric tumor pattern (H and E, ×100); (g inset) Preserved angiocentricity and myxoid matrix (H and E, ×100); (i) Ki-67 labeling index of 40-50% (×100).
The patient had received 50Gy radiation with concurrent temozolamide (TMZ) over a period of 5 weeks at 75 mg/m2. The patient presented with recurrence after two years. Histological examination of the repeat gross total excision specimen showed radiation-induced geographic necrosis [Figure 1g and h], ghost outlines of angiocentric tumor pattern [Figure 1h], with preserved tumor islands, retained angiocentricity and myxoid matrix [Figure 1g inset], and Ki-67 labeling index of 40-50% [Figure 1h]. The patient had received 50 Gy radiation with concurrent TMZ over a period of 5 weeks at 75 mg/m2, followed adjuvant TMZ at 150 mg/m2 from day 1 to day 5, cycles repeated every 4 weeks.
Discussion
The review of literature shows less than 100 cases of pilomyxoid astrocytoma being reported till date (PubMed search as on 12th June 2013). The mean age at diagnosis for patients with PMA has been documented to be 18 months.[4] Apart from the commonest site of hypothalamic/chiasmatic region, this entity has also been described in thalamus, cerebellum, brain stem, temporal lobe and spinal cord.[1] Pilomyxoid astrocytoma most commonly presents with symptoms of raised intracranial pressure or parenchymal compression.[4] The presenting symptoms of hypothalamic juvenile PMA are vomiting, feeding difficulties, developmental delay and failure to thrive.[2]
On magnetic resonance imaging, these tumors are well circumscribed without evidence of peritumoral edema or parenchymal infiltration.[5] The majority (84.6%) are solid, with the remainder showing a minimal cystic component. Radiographic evidence of central necrosis is rare and PMA tends to enhance homogeneously on contrast.[4,5] Presentation at younger age, more frequent occurrence in the suprasellar area, mainly solid mass containing non-enhancing portion and more frequent leptomeningeal dissemination, are helpful differential features of PMAs as compared to PAs.[6]
Angiocentric pattern of arrangement of tumor cells and myxoid matrix are characteristic histologic features. Rosenthal fibres or eosinophilic granular bodies, both characteristic of pilocytic astrocytoma are conspicuously absent in PMA. Mitotic figures and glomeruloid vascular tufts can be present and Ki-76 labeling index ranges from 2-20%.[1] The high Ki-76 labeling index of 30-40%, as recorded in this case has hitherto not been reported. The tumor cells and fibrillary background shows strong immunopositivity for GFAP, S-100 and Vimentin.[1] Though the angiocentric/pseudorosette pattern may mimic ependymoma, but absence of perinuclear dot expression of EMA in the present case of PMA differentiated it from ependymoma. Poor prognostic indicators in gliomas, that is necrosis, mitotic figures and vascular proliferation, are not uncommon in PMAs.[7]
The occurrence in the setting of neurofibromatosis type 1 (NF-1) and KIAA1549:BRAF fusions have been reported in PMA.[8,9] Patients with PMA experience shorter progression free survival and higher local recurrence than those with juvenile PA.[4] Intra-cerebral metastasis of an intramedullary PMA,[10] leptomeningeal dissemination[11] and rapid progression to glioblastoma[12] have also been reported. A greater extent of surgical excision is associated with favorable outcome. But gross total excision may not be achievable in hypothalamic PMA and in such cases chemotherapy is instituted.[13] Kim et al., have used cisplatin and vincristine to treat juvenile PMA of the opticohypothalamic region.[14] Terasaki et al., successfully treated leptomeningeal gliomatosis of pilomyxoid astrocytomas with concurrent radiation and TMZ after failed frontline chemotherapy with carboplatin and vincristine.[15] Adjuvant therapy with TMZ was incorporated in the present case, as the tumor's biological behavior was akin to high grade astrocytomas.
It is important to separately recognize PMA from PA due to its propensity to involve younger age group, aggressiveness, higher local recurrence, metastasis, CSF spread and progression. Increased mitotic activity, proliferation index and necrosis or endothelial proliferation necessitates adjuvant therapy. Further follow-up and larger studies are needed to more accurately determine the prognosis of these tumors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Scheithauer BW, Hawkins C, Tihan T, VandenBerg SR, Burger PC. Pilocytic Astrocytoma. In: Louis DN, Ohgaki H, Weistler OD, Cavenee WK, editors. WHO Classification of Tumors of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2007. pp. 243–4. [Google Scholar]
- 2.Janisch W, Schreiber D, Martin H, Gerlach H. Diencephalic pilocytic astrocytoma with clinical onset in infancy. Biological behavior and pathomorphological findings in 11 children. Zentralbl Allg Pathol. 1985;130:31–43. [PubMed] [Google Scholar]
- 3.Tihan T, Fisher PG, Kepner JL, Godfraind C, McComb RD, Goldthwaite PT, et al. Paediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999;58:1061–8. doi: 10.1097/00005072-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Komotar RJ, Mocco J, Carson BS, Sughrue ME, Zacharia BE, Sisti AC, et al. Pilomyxoid astrocytoma: A review. Med Gen Med. 2004;6:42. [PMC free article] [PubMed] [Google Scholar]
- 5.Arslanoglu A, Cirak B, Horska A, Okoh J, Tihan T, Aronson L, et al. MR imaging characteristics of pilomyxoid astrocytomas. AJNR Am J Neuroradiol. 2003;24:1906–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IH, Kim JH, Suh YL, Eo H, Shin HJ, Yoo SY, et al. Imaging characteristics of pilomyxoid astrocytomas in comparison with pilocytic astrocytomas. Eur J Radiol. 2011;79:311–6. doi: 10.1016/j.ejrad.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MW, Eberhart CG, Perry A, Tihan T, Cohen KJ, Rosenblum MK, et al. Spectrum of pilomyxoid astrocytomas: Intermediate pilomyxoid tumors. Am J Surg Pathol. 2010;34:1783–91. doi: 10.1097/PAS.0b013e3181fd66c3. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez FJ, Perry A, Gutmann DH, O’Neill BP, Leonard J, Bryant S, et al. Gliomas in neurofibromatosis type 1: A clinicopathologic study of 100 patients. J Neuropathol Exp Neurol. 2008;67:240–9. doi: 10.1097/NEN.0b013e318165eb75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin A, Rodriguez FJ, Karajannis MA, Williams SC, Legault G, Zagzag D, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eigenbrod S, Thon N, Jansen N, Janssen H, Mielke J, Ruiter M, et al. Intramedullary pilomyxoid astrocytoma with intracerebral metastasis exhibiting oligoden-droglioma-like features. Rare Tumors. 2012;4:e30. doi: 10.4081/rt.2012.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahore A, Kammar A, Dange N, Epari S, Goel A. Diencephalic juvenile pilomyxoid astrocytoma with leptomeningeal dissemination. Turk Neurosurg. 2011;21:222–5. doi: 10.5137/1019-5149.JTN.2638-09.1. [DOI] [PubMed] [Google Scholar]
- 12.Paraskevopoulos D, Patsalas I, Karkavelas G, Foroglou N, Magras I, Selviaridis P. Pilomyxoid astrocytoma of the cervical spinal cord in a child with rapid progression into glioblastoma: Case report and literature review. Childs Nerv Syst. 2011;27:313–21. doi: 10.1007/s00381-010-1171-5. [DOI] [PubMed] [Google Scholar]
- 13.Komotar RJ, Burger PC, Carson BS, Brem H, Olivi A, Goldthwaite PT, et al. Pilocytic and pilomyxoid hypothalamic/chiasmatic astrocytomas. Neurosurgery. 2004;54:72–9. doi: 10.1227/01.neu.0000097266.89676.25. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Kang SS, Jung TY, Jung S. Juvenile pilomyxoid astrocytoma in the opticohypothalamus. J Korean Neurosurg Soc. 2010;48:445–7. doi: 10.3340/jkns.2010.48.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terasaki M, Bouffet E, Maeda M, Sugita Y, Sawamura Y, Morioka M. Successful treatment of leptomeningeal gliomatosis of pilomyxoid astrocytoma after failed frontline chemotherapy. Neurologist. 2012;18:32–5. doi: 10.1097/NRL.0b013e31823d7a92. [DOI] [PubMed] [Google Scholar]


