Abstract
Oncolytic virotherapy is an emerging treatment modality which uses replication competent viruses to destroy cancers. Advances in the past two years include preclinical proof of feasibility for a single-shot virotherapy cure, identification of drugs that accelerate intratumoral virus propagation, new strategies to maximize the immunotherapeutic potential of oncolytic virotherapy, and clinical confirmation of a critical viremic thereshold for vascular delivery and intratumoral virus replication. The primary clinical milestone was completion of accrual in a phase III trial of intratumoral herpes simplex virus therapy using talimogene laherparepvec for metastatic melanoma. Challenges for the field are to select ‘winners’ from a burgeoning number of oncolytic platforms and engineered derivatives, to transiently suppress but then unleash the power of the immune system to maximize both virus spread and anticancer immunity, to develop more meaningful preclinical virotherapy models and to manufacture viruses with orders of magnitude higher yields compared to established vaccine manufacturing processes.
REVIEW ARTICLE
Oncolytic viruses are therapeutically useful anticancer viruses that will selectively infect and damage cancerous tissues without causing harm to normal tissues1. Each virus has a specific cellular tropism that determines which tissues are preferentially infected, and hence, what disease is caused. Rabies virus, for example, damages neurons, hepatitis B virus damages hepatocytes, HIV damages helper T lymphocytes and influenza virus damages airway epithelium. Many, if not most, naturally occurring viruses have a preferential, although nonexclusive, tropism for tumors and tumor cells. This probably has more to do with tumor biology than with virus biology since most tumors have evolved not only to avoid immune detection or destruction, but also to resist apoptosis and translational suppression, which are the key responses used by normal cells to limit a virus infection. Oncolytic viruses can kill infected cancer cells in many different ways, ranging from direct virus-mediated cytotoxicity through a variety of cytotoxic immune effector mechanisms. Conventional concepts of cell death (apoptosis, necrosis or autophagy) are generally inadequate to fully describe the complex cell killing scenarios encountered in virotherapy. This is because the oncolytic virus typically takes over and controls the molecular cell death machinery of the infected cancer cell, allowing death to occur only after available cellular resources have been maximally exploited for the synthesis and assembly of new viruses2. In addition to the killing of infected cells, oncolytic viruses can mediate the killing of uninfected cancer cells by indirect mechanisms such as the destruction of tumor blood vessels, the amplification of specific anticancer immune responses or through the specific activities of transgene-encoded proteins expressed from engineered viruses1.
Specific targeting of cancer cells is obviously the sine qua non for oncolytic virotherapy and can be achieved in several ways. Some viruses such as H1 autonomously replicating parvoviruses, reovirus, Newcastle Disease Virus, Mumps virus, Moloney leukemia virus have a natural preference for cancer cells, whereas such as measles, adenovirus, Vesicular Stomatitis Virus, vaccinia and Herpes Simplex Virus can be adapted or engineered to make them cancer-specific. Surface markers such as EGF receptor, Her2-neu, Folate receptor, Prostate Specific Membrane Antigen and CD20, and nuclear transcription factors PSA, hTERT, COX-2, osteocalcin expressed selectively by tumor cells can be targeted by using them as receptors for virus entry or as essential cofactors for viral gene expression3, 4. Alternatively, oncolytic viruses can be engineered to exploit the defective antiviral defenses of tumor cells as explained below5. Normal cells respond to virus infection by downmodulating their metabolism and/or by undergoing apoptosis, thereby inhibiting virus propagation. Successful viruses use a variety of strategies to combat these innate immune responses, but become non-pathogenic when engineered or evolved to incapacitate their immune combat proteins. Examples include the VSV matrix protein, the NS1 protein of influenza virus, the C and V proteins of paramyxovirus family members, the HSV γ34.5 protein and the proteins encoded in the E1 and E3 regions of the adenovirus genome. Interestingly, as the apoptotic and antimetabolic responses of tumor cells are generally deficient, attenuated viruses with defective immune combat proteins often retain their ability to propagate in tumor cells. An alternative way to ‘target’ viruses to cancer cells is to selectively eliminate their undesirable tropisms by engineering targets for brain, liver or muscle-specific microRNAs into their genomes such that the viral life cycle is selectively blocked in the relevant target tissue6.
Here we provide a critical overview of the current state of the field of oncolytic virotherapy research, emphasizing what we consider to be the most important recent advances and the main challenges going forward. The review is divided into three sections. The first section reviews the clinical oncolytic virotherapy experience to date and illustrates that the approach has genuine promise but that its full potential has yet to be realized. The subsequent sections address the two key stages of a successful oncolytic virotherapy treatment episode, both of which are truly hotbeds of preclinical research innovation: first, delivery of the virus to the tumor; and second, spread of the virus infection through the tumor. Optimizing the efficiency and accuracy of both of these critical processes will doubtless continue to challenge the field for years to come, but there have been many recent developments, several of which are already being translated to determine whether they can improve clinical outcomes.
Because of space constraints, citations have been limited to key manuscripts published since 2007. However, in some cases, we refer to seminal papers published before this time. Where multiple primary manuscripts address the same topic, because of space limitations, we have cited review articles. We apologize to those investigators whose work has not been cited and take full responsibility for these omissions.
Clinical development
The idea of using viruses to treat cancer first began to take hold In the 1950s when tissue culture systems and rodent cancer models were originally developed7. Hundreds of cancer patients were treated with impure oncolytic virus preparations (even infected body fluids) administered by almost every imaginable route8. The viruses were usually arrested by the immune system and did not affect tumor growth, but sometimes the infection took hold and tumors regressed, especially in immunosuppressed patients, although they frequently became sick or died when the infection spread to normal tissues. In one particularly promising study from Osaka University, tumor regressions were reported in 37 of 90 terminal cancer patients treated with a non-attenuated mumps virus9. But this work was not continued beyond the 1970s and the strains of mumps virus used for the work have since been lost (T. Asada, personal communication). The modern era of oncolytic virotherapy, in which virus genomes are engineered to enhance their anti-tumor specificity, can be traced to a 1991 publication in which a thymidine kinase (TK)-negative herpes simplex virus (HSV) with attenuated neurovirulence was shown to be active in a murine glioblastoma model10. Since that first application of virus engineering to an oncolytic HSV, the pace of clinical activities has accelerated considerably, with numerous ongoing or completed trials using oncolytic viruses belonging to at least 10 different virus families (Table 1), and a steady stream of new oncolytic viruses entering the clinical arena11–13.
Table 1.
A listing of current and recently completed oncolytic virotherapy trials. A search was performed on www.clinicaltrials.gov and the clinical trial database of the Journal of Gene Medicine (http://www.wiley.com/legacy/wileychi/genmed/clinical/).
| Virus | Name | Modifications | Phase | Tumor | Route | Combination | Site | Status* (PubMed reference) |
|---|---|---|---|---|---|---|---|---|
| Adenovirus | Oncorine (H101) | E1B-55k-E3− | II | SCCHN | IT | Cisplatin | Multileft | Completed, PMID: 14693057 |
| III | SCCHN | IT | Cisplatin | Multileft | Completed, PMID: 15601557 | |||
| Onyx-015 | E1B-55k-E3B− | I | Lung Mets | IV | - | Mutlileft | Completed, PMID: 11420638 | |
| I | Glioma | Intracavity | - | Mutlileft | Completed, PMID: 15509513 | |||
| I | Ovarian Ca | IP | - | Mutlileft | Completed, PMID: 11896105 | |||
| I | SCCHN | IT | - | Multileft | Completed, PMID: 10741699 | |||
| I | Solid tumors | IV | Enbrel | Mary Crowley | Completed, PMID: 17704755 | |||
| I | Sarcoma | IT | Mitomycin-C Dox, cisplatin | Mayo Clinic | Completed, PMID: 15647767 | |||
| I/II | PanCa | IT | Gemzar | UCLA | Completed, PMID: 12576418 | |||
| II | CRC | IV | - | Mutlileft | Completed, PMID: 12697873 | |||
| II | Hepatobiliary | IT | - | Montefiore | Completed, PMID: 12576437 | |||
| II | CRC, PanCa | IA | - | Mutlileft | Completed, PMID: 12414631 | |||
| II | SCCHN | IT | - | Multileft | Completed, PMID: 11208818 | |||
| II | SCCHN | IT | Cisplatin, 5-FU | Multileft | Completed, PMID: 10932224 | |||
| II | CRC | IV | 5-FU/leucovorin | Stanford | Completed, PMID: 15803147 | |||
| CG7060 | PSA control | I | Prostate Ca | IT | RT | Johns Hopkins | Completed, PMID: 11606381 | |
| CG7870/CV787 | rat probasin-E1A hPSA-E1B E3+ | I/II | Prostate Ca | IV | - | Multileft | Completed, PMID: 16690359 | |
| I/II | Prostate Ca | IV | Docetaxel | Mary Crowley | Terminated, 2005 | |||
| CG0070 | E2F-1, GM-CSF | II/III | Bladder Ca | Intracavity | - | UCSF | Not yet open, PMID: 16397056 | |
| Telomelysin | hTERT | I | solid tumors | IT | - | Mary Crowley | Completed, PMID: 19935775 | |
| Ad5-CD/TKrep | CD/TK | I | Prostate Ca | IT | 5-FC & GCV | Henry Ford, Detroit | Completed, PMID: 12208748 | |
| I | Prostate Ca | IT | 5-FC+GCV+RT | Henry Ford, Detroit | Completed, PMID: 14612551 | |||
| Ad5-D24-RGD | RGD, Delta-24 | I | Ovarian Ca | IP | - | UAB | Completed, PMID: 20978148 | |
| I | Glioma | IT | - | MD Andersen | Recruiting | |||
| I/II | Glioma | IT | - | Erasmus Medical left | Recruiting | |||
| Ad5-SSTR/TK-RGD | SSTR, TK, RGD | I | Ovarian Ca | IP | GCV | UAB | Active, PMID: 16397056 | |
| CGTG-102 | Ad5/3, GM-CSF Delta-24 | I/II | Solid tumors | IT | - | Baylor | Not open, PMID: 20664527 | |
| I | Solid tumors | IT/IV | Metronomic CTX | Docrates Hospital Helsinki | Recruiting | |||
| INGN-007 (VRX-007) | wtE1a, ADP | I | Solid tumors | IT | - | Mary Crowley | Not open, PMID: 19197324 | |
| ColoAd1 | Ad3/11p | I/II | CRC, HCC | - | PsiOxus | Not open, PMID: 18560559 | ||
| Coxsackie Virus (CVA21) | CAVATAK | - | I | Melanoma | IT | - | Viralytics | Completed |
| II | Melanoma | IT | - | Viralytics | Recruiting | |||
| I | SCCHN | IT | Viralytics | Terminated | ||||
| I | Solid tumors | IV | - | Viralytics | Recruiting | |||
| Herpes Simplex Virus | Talimogene Laherparepvec (OncoVEX) | GM-CSF | I | Solid tumors | IT | - | Multileft | Completed, PMID: 17121894 |
| ICP34.5(−) | II | Melanoma | IT | - | Multileft | Completed, PMID: 19915919, 19884534 | ||
| ICP47(−) | III | Melanoma | IT | - | Multileft | Active | ||
| Us11 ↑ | I/II | SCCHN | IT | RT, Cisplatin | Multileft | Completed, PMID: 20670951 | ||
| G207 | ICP34.5(−), ICP6(−) LacZ(+) | I/II | Glioma | IT | - | U of Alabama | Completed, PMID: 18957964, 10845725 | |
| I | Glioma | IT | RT | U of Alabama | Completed | |||
| G47Delta | From G207, ICP47− | I | Glioma | IT | - | Tokyo Hospital | Recruiting, PMID: 11353831 | |
| HSV 1716 (Seprehvir) | ICP34.5(−) | I | Non-CNS solid tumors | IT | - | Cincinnati | Recruiting | |
| I | SCCHN | IT | - | U of Glasgow | Completed, PMID: 18615711 | |||
| I | Glioma | IT | - | U of Glasgow | Completed, PMID: 15334111, 11960316 | |||
| I | Melanoma | IT | - | U of Glasgow | 11229673, 2001 | |||
| I | Mesothelioma | IP | - | UK | not active | |||
| HF10 | HSV-1 HF strain | I | Solid tumors | IT | - | Multileft | Recruiting | |
| I | Pancreatic Ca | IT | - | Nagoya University | Completed, PMID: 21102422 | |||
| I | Breast Ca | IT | - | Nagoya University | Completed, PMID: 16865590 | |||
| I | SCCHN | IT | - | Nagoya University | Completed, PMID: 16923721 | |||
| NV1020 | I | CRC liver mets | IA | - | MSKCC | Completed, PMID: 19018254 | ||
| Measles Virus (Edmonston) | MV-CEA | CEA | I | Ovarian Ca | IP | - | Mayo Clinic | Completed, PMID:20103634 |
| I | Glioma | IT | - | Mayo Clinic | Recruiting | |||
| MV-NIS | NIS | I | Myeloma | IV | CTX | Mayo Clinic | Recruiting | |
| I | Ovarian Ca | IP | - | Mayo Clinic | Recruiting | |||
| I | Mesothelioma | IP | - | U of Minnesota/Mayo Clinic | Recruiting | |||
| I | SCCHN | IT | - | Mayo Clinic | Not open | |||
| Newcastle Disease Virus | NDV-HUJ | - | I/II | Glioma | IV | - | Goldyne Savad Inst | Completed, PMID: 16257582 |
| PV701 | - | I | Solid tumors | IV | - | Ottawa Hospital | Completed, PMID: 16638865 | |
| MTH-68/H | - | II | Solid tumors | Inhalation | - | UCRI | Completed, PMID: 8275514 | |
| NV1020 | I | Solid tummors | IV | - | Multileft | Completed, PMID: 11980996 | ||
| Parvovirus | H-1PV | - | I/II | Glioma | IT/IV | - | University Hospital Heidelberg | Recruiting, PMID: 20299703 |
| Poliovirus (Sabin) | PVS-RIPO | IRES | I | Glioma | IT | - | Duke | Recruiting, PMID: 20299272 |
| Reovirus (Dearing) | Reolysin | - | I/II | Glioma | IT | - | Multileft | Completed, PMID: 18253152 |
| I | Peritoneal Ca | IP | - | Ohio State | Recruiting | |||
| I | Solid tumors | IV | - | Multileft | Completed, PMID: 18981012 | |||
| I | Solid tumors | IV | CTX | Multileft | Recruiting | |||
| I | CRC | IV | FOLFIRI | Multileft | Recruiting | |||
| II | Sarcoma | IV | - | Multileft | Completed | |||
| II | Melanoma | IV | - | Multileft | Suspended | |||
| II | Ovarian, Peritoneal Ca | IV | PTX | Mutlileft | Recruiting | |||
| II | Pancreatic Ca | IV | PTX, CBDCA | Multileft | Recruiting | |||
| II | SCCHN | IV | PTX, CBDCA | Multileft | Not recruiting | |||
| II | Melanoma | IV | PTX, CBDCA | U of Texas | Recruiting | |||
| II | Pancreatic Ca | IV | Gemzar | U of Texas | Recruiting | |||
| II | Lung Ca | IV | PTX, CBDCA | Multileft | Recruiting | |||
| III | SCCHN | IV | PTX, CBDCA | Multileft | Recruiting | |||
| Seneca Valley Virus | NTX-010 | II | Small Cell Lung Ca | IV | - | NCCTG multileft | Recruiting, PMID: 17971529 | |
| Retrovirus | Toca 511 | CD | I/II | Glioma | IT | 5-FC | Multileft | Recruiting, PMID: 16257382 |
| Vaccinia (Wyeth strain) | JX-594 | GM-CSF TK(−) | I | CRC | IV | - | South Korea | Recruiting |
| I | Solid tumors | IV | - | Multileft | Completed | |||
| I | HCC | IT | - | Busan, South Korea | Completed, PMID: 18495536 | |||
| I | Pediatric solid tumors | IT | - | Cincinnnati | Recruiting | |||
| I | Melanoma | IT | - | Busan, South Korea | Completed, PMID: 21772252 | |||
| I/II | Melanoma | IT | - | Multileft | Completed. PMID: 10505851 | |||
| II | HCC | IT | - | Multileft | Not recruiting, data analysis | |||
| IIB | HCC | IV | - | Multileft | Recruiting | |||
| I/II | CRC | IV/IT | Irinotecan | Multileft | Recruiting | |||
| II | CRC | IT | - | Ottawa Hospital | Not yet recruiting | |||
| Vaccinia (Western Reserve) | vvDD-CDSR | TK−, VGF− LacZ, CD Somatostatin R | I | Solid tumors | IT/IV | - | U of Pittsburgh | Recruiting PMID: 15336655 |
| Vaccinia (Lister) | GL-ONC1 (GLV-h68) | Renilla Luciferase GFP, β-gal β-glucoronidase | I | Solid tumors | IV | - | Royal Marsden | Recruiting, PMID: 21779374 |
| I/II | Peritoneal Carcinomatosis | IP | University Hospital Tuebingen | Recruiting | ||||
| I/II | SCCHN | IV | RT,Cisplatin | Moores UCSD Cancer left | Recruiting | |||
| Vesicular Stomatitis Virus (Indiana) | VSV-hIFNβ | IFN beta | I | HCC | IT | - | Mayo Clinic | Recruiting |
The clinical tolerability of oncolytic viruses has overall been excellent, even at today’s highest feasible doses14. Even so, future oncolytic virus trials will likely use even higher doses as manufacturing yields are continually increasing due to a variety of technical advances, such as cell substrate optimization or the use of cell microcarriers and disposable wave bioreactors15–17. Hence, it may be premature to judge whether effective oncolytic virotherapy will be devoid of serious toxicities at clinically effective doses. One unique safety risk is the concern that an oncolytic virus might spread from the treated patient and mutate to regain its pathogenic potential18. However, although virus shedding has sometimes been documented in urine or respiratory secretions, oncolytic virus transmission to contacts and carers has not yet been seen14.
Clinical efficacy
Evidence for efficacy of single-agent oncolytic virotherapy comes from two recent phase 1/2 clinical trials backed up by an number of quite compelling anecdotal reports7, 19–21. In one trial, talimogene laherparepvec, formerly named OncoVEX, which is an oncolytic HSV coding for granulocyte/macrophage colony stimulating factor (GM-CSF) was administered by direct intratumoral injection to patients with metastatic malignant melanoma and led to complete regressions of injected and uninjected lesions in 8 of 50 treated patients19. This study remains the most compelling demonstration that intratumoral administration of an oncolytic virus can powerfully cross prime and amplify anticancer immunity. Perhaps because of its well-known susceptibility to immunotherapy, melanoma appears to be a particularly good target for oncolytic virotherapy, responding well not just to HSV-GMCSF (OncoVEX) but also to vaccinia virus therapy22.
In the second trial, an oncolytic vaccinia virus, JX594, also engineered to express GM-CSF, was administered intratumorally to patients with non-resectable hepatocellular carcinoma, leading to objective responses in 3 of 10 evaluable patients20.
Trials combining oncolytic viruses such as reovirus, vaccinia and HSV with drugs or radiation are giving a high frequency of tumor responses21, 23–25, but it is difficult to know whether the oncolytic viruses are contributing to these responses over and above the active anticancer drugs with which they are being combined. Only through randomized phase 3 trials can this critical question be answered.
So far, clinical trials have failed to provide a clear demonstration that direct viral lysis of infected cells is an important mechanism of tumor destruction14. Thus, the oncolytic paradigm, where a systemically administered virus spreads extensively at sites of tumor growth to cause tumor destruction, remains to be proven. Of major significance in relation to this point, it has recently been determined in a phase I clinical trial that intravenously administered JX594 was recoverable from tumor biopsies only when the viremic threshold dose of 109 infectious units was exceeded26. The critical insight gained from this study is that systemically administered oncolytic viruses can specifically target sites of tumor growth by extravasating from tumor blood vessels and replicating in the tumor. This is a concentration driven process and is therefore detectable only above a threshold virus dose. Direct oncolytic tumor destruction may therefore be tightly linked to the dose of virus administered which for many oncolytic viruses is limited primarily by manufacturing considerations.
Additional insights have been gained from the ongoing and completed clinical trials. First, since the clinical trial results often fall short of hopes and expectations, it is clear that better, more reliably predictive, preclinical models are needed. Specifically there is a need for orthotopic cancer models in immunocompetent animals that are not only susceptible to the oncolytic virus being evaluated, but also mirror the human pathogenesis of the viral infection. Current models are inadequate because they often lack an immune system (cultured cells and human xenograft models), or are not susceptible to the virus in question, although exceptions do exist (e.g. vaccinia).
A second additional insight is that iterative phase I clinical trials may become standard practice in the oncolytic virotherapy field. Conventional drugs are typically perfected before they enter clinical testing but oncolytic viruses are more akin to motor cars with multiple component parts, all of which are constantly subject to improvement, refinement and perfection through engineering efforts. Iterative phase I trials provide the only mechanism whereby the steady stream of new engineering modifications that only slightly change the product specification can be accommodated into the development pipeline.
Another important recent insight from the clinic is that it is clinically feasible to monitor virus spread by reporter transgene expression monitoring, a useful source of pharmacokinetic data which can be especially helpful during early stage clinical development. The progress of an oncolytic virus infection can be monitored in rodents by post-mortem analysis of the changing biodistribution of virus-infected cells at multiple timepoints, but this is impractical in oncolytic virus–treated human subjects. Hence, little has been learned of the reasons for inferior outcomes of oncolytic virotherapy in humans versus rodents. Reporter genes have therefore been engineered into oncolytic virus genomes to facilitate repetitive, noninvasive determination of the number and location of virus-infected cells in the body 27, 28.
When we administered an oncolytic measles virus encoding the soluble extracellular domain of carcinoembryonic antigen (MV-CEA) intraperitoneally to patients with refractory ovarian cancer, serum CEA monitoring studies suggested that the virus infected only a small number of tumor cells, and was not undergoing significant amplification in vivo29. Reporter genes compatible with radioactive tracers have also been tested in humans. Oncolytic HSVs are amenable to PET imaging via the HSV TK, which phosphorylates specific positron-emitting substrates, trapping them inside the cell, as demonstrated in a clinical trial of HSV TK gene therapy for glioblastoma30, although clinical validation for tracking the spread of a replication competent oncolytic virus is still awaited. The gene encoding the thyroidal sodium iodide symporter (NIS), which concentrates radioactive iodide, has been inserted into the genomes of several oncolytic viruses such as adenovirus, measles, VSV, HSV, and vaccinia have been used preclinically in conjunction with various radioisotopes (125I, 123I, 124I and 99mTcO4) to monitor in vivo spread31. This versatile NIS approach was recently validated in a clinical study in which 99mTcO4-based SPECT/computed tomography (CT) imaging was used to monitor the intratumoral spread of an oncolytic adenovirus coding for NIS32. In an approach known as radiovirotherapy, we demonstrated that it will also be feasible in the future to increase the potency of a NIS-expressing virus by administering 131I, which delivers high-energy beta particles, into the infected tumor33.
Delivering oncolytic viruses to the tumor
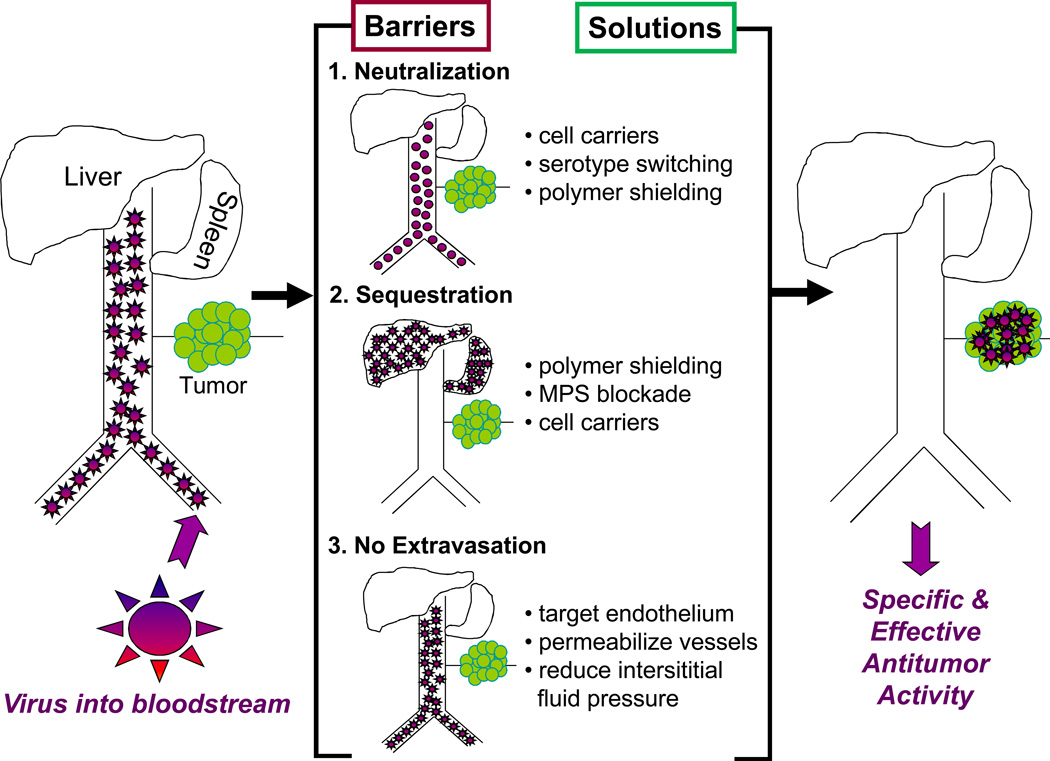
Although several ongoing trials are emphasizing intratumoral delivery, systemic delivery will be absolutely required for treatment of metastatic cancer. The goal of systemic therapy is to exceed the ‘viremic threshold’ above which the virus nucleates a critical number of intratumoral infectious centers whose expansion and coalescence leads to tumor destruction. Current research is therefore focused on minimizing oncolytic virus sequestration in liver and spleen, evading neutralization by serum factors, targeting the viruses to the vascular endothelial cells lining tumor blood vessels and selectively enhancing their permeability.
Minimizing sequestration in the liver and spleen
Intravenously administered viruses are rapidly cleared from the circulation as a result of sequestration by the mononuclear phagocytic system (MPS) in the liver and spleen. Before clearance, they are typically coated (opsonized) with antibodies, complement, coagulation factors and/or other serum proteins that facilitate their recognition by splenic macrophages and hepatic Kupffer cells. These ‘decorated’ particles bind to receptors (e.g., Fcγ receptors, complement receptor 1 (CR1), CR3 or scavenger receptors) on macrophages/endothelial cells, resulting in receptor-mediated phagocytosis and accelerated clearance from the circulation34. Some viruses, for example adenoviruses, can bind directly to scavenger receptors on Kupffer cells inducing proinflammatory cytokines that can result in serious dose-limiting toxicities35, 36.
Strategies to minimize sequestration include chemical modification of the coat proteins of the viruses by conjugation of biocompatible polymers, such as polyethylene glycol (PEG) and N-[2-hydroxypropyl]methaacrylamide (HPMA)37, 38. Both PEG and HPMA are already used clinically to prolong the circulation times of proteins and liposomes and to reduce off target toxicities39. Polymer coating can destroy virus infectivity, which can be restored by re-engineering receptor binding ligands onto the surface of the shielded particles40. For example, PEGylation of VSV glycoprotein pseudotyped lentiviral vectors increased vector circulation half-life fivefold and dramatically inhibited complement inactivation41. PEGylated adenovirus 5 (Ad5) is cleared fourfold slower than unmodified Ad542. The length of the PEG influences outcome; 20 kDa PEG but not 5kDa PEG can detarget oncolytic Ad5 from Kupffer cells and hepatocytes without inducing liver enzymes43. HPMA-cloaked adenovirus vectors are also protected from neutralizing antibodies and have a prolonged circulatory half-life44. An alternative approach to minimize sequestration of viruses (e.g. HSV) that are readily bound by IgM and complement proteins is to deplete these serum factors by pretreating with cobra venom factor or cyclophosphamide45–47.
Virus sequestration by the MPS is saturable44. Sequestration by the liver and spleen can therefore be inhibited either by pre-conditioning to saturate MPS scavenger receptors or by poisoning the macrophages/endothelial cells. Predosing of mice with polyinosinic acid, which binds to scavenger receptors on endothelial cells or macrophages in the liver and spleen, can reduce MPS sequestration of adenoviruses48. Clodronate-loaded liposomes can also deplete liver Kupffer cells and splenic macrophages of mice. Oncolytic adenoviral therapy has been combined with clodronate liposomes for depletion of Kupffer cells to enhance therapeutic outcome49, 50 Other MPS blocking strategies include preadministration of gadolinium chloride (GdCl3) or gamma globulins42, 51. In one study, GdCl3 prolonged the circulatory half-life of an Ad5 vector, with a 100-fold difference in blood levels at 60 minutes51. Predosing with high doses of intravenous adenoviral particles is toxic to Kupffer cells, which decline substantially in numbers by 4 hours, greatly reducing MPS clearance of a second dose52, 53.
Evading neutralization by serum factors
Many of the barriers viruses encounter following intravenous administration (e.g. neutralizing antibodies, inactivation by complement or scavenging by Kupffer cells) can be overcome by hiding oncolytic viruses inside carrier cells. Two approaches have shown promise in pre-clinical models: infusing ex vivo infected tumor cell lines54 or using normal primary cells that can home to tumor beds55. Permissive tumor cells have the advantage of being easy to propagate and genetically modified, are productive virus factories in vivo and could in theory be used as an ‘off the shelf’ product. In one study, we demonstrated that lethally irradiated myeloma cells infected with an oncolytic measles virus were therapeutically potent when administered intravenously to myeloma-bearing mice with protective titers of antimeasles antibodies56.
Mesenchymal stem cells57 (MSCs) are another cell type that have been used both preclinically and in small clinical trials58 to deliver oncolytic viruses to tumor beds. MSCs have been shown to preferentially engraft into solid tumors59 and we recently have shown they could efficiently deliver oncolytic measles viruses to intraperitoneal ovarian cancer deposits in the presence of neutralizing antiviral antibodies60.
Cellular carriers should ideally be combined with oncolytic viruses that will not kill the carrier before it has infiltrated into the tumor. Some viruses can piggyback on cells found normally in the circulation. Dendritic cells and T cells when admixed with reovirus, carried and delivered their oncolytic cargo even in the face of neutralizing antibodies55, 61. VSV and measles virus can be delivered to tumor beds by loading onto T cells and when bound to these cells, VSV particles are protected from neutralizing antibodies62, 63. Technology for routine isolation of assorted white cells from blood products is widely available clinically and thus may make the implantation of carrier cell approaches in the clinic more practical going forward.
Selectively increasing the permeability of tumor blood vessels
The EPR effect was first described in 1986 when it was shown that the leaky vasculature of tumors could be exploited to allow the passage of macromolecules from the lumenal side of the blood vessels into tumor tissues64, 65. Leakiness is due to the presence of fenestrae (50–80 nm) and intercellular gaps between tumor endothelial cells (200–900 nm compared with 2–6 nm in normal blood vessels), facilitating extravasation of macromolecules, viruses and nanoparticles66–69. However, poor lymphatic drainage and dense stromal tissue increase the interstitial fluid pressure in tumors impeding virus extravasation and diffusion.
Vascular permeability can be increased by preadministration of interleukin 2 (IL-2), tumor necrosis factor alpha (TNF-α), histamine or a bradykinin analog65, 68, 70. Giving chemotherapy can reduce the intratumoral interstitial fluid pressure by killing tumor cells, thereby enhancing extravasation without directly impacting vessel permeability68. In one recent study, a combination of vascular endothelial growth factor (VEGF) and metronomic doses of paclitaxel or cisplatin increased the vascular permeability of the tumor endothelium and improved the delivery of Sindbis vector to tumors71. Multiple injections of VEGF165 resulted in superior reovirus infection of proliferating tumor endothelium, thereby increasing therapeutic activity of in a syngeneic B16 melanoma model72. Systemic IL-2 accompanied by depletion of T regulatory cells (Tregs) also enhanced the extravasation of oncolytic viruses in B16 metastases in the lungs of mice73.
Targeting the viruses to tumor vessel endothelium
In addition to being structurally different from normal vessels68, the tumor vasculature is antigenically distinct74–76. Targets visible from the lumenal side include antigens overexpressed on tumor endothelial cells (e.g., αvβ3 integrins, VEGF receptor 2, prostate-specific membrane antigen (PSMA), urokinase plasminogen activator receptor, phosphatidylserine, E-selectin, vascular cell adhesion molecule (VCAM), tissue factor, endosialin and endoglin (CD105)75, 76. High-affinity protein or peptide ligands are available for targeting most of these endothelial markers. Other targets include structural elements that are exposed during vessel formation and remodeling, for example, laminin (targeted by L36 single chain Fv (scFv)77) and fibronectin (targeted by the E19 scFv)78. Importantly, most of the above mentioned vascular targets are not expressed exclusively on tumor blood vessel endothelium.
Chemical or genetic modifications to oncolytic viruses have been used to selectively target the tumor cell surface, de-target sensitive tissues or create dual target viruses to enhance both vascular targeting and tumor infection (transductional targeting). For instance, we previously genetically modified measles virus79 to display a variety of polypeptide ligands on its surface facilitating infection of tumor cells overexpressing the targeted receptor3. Polymer coating has been used to mask natural attachment proteins, re-directing virus infection by chemically coupling therapeutic antibodies (e.g., cetuximab) that bind tumor cells80.
A scFv against E selectin was conjugated onto polymer-coated adenoviral particles to enhance their binding to activated endothelial cells in inflamed areas or in tumors81. Oncolytic measles viruses expressing vascular targeting peptides, the amino terminal fragment of urokinase plasminogen activator, or cyclic RGD and echistatin, which bind to αvβ3 integrin receptor, were shown in our laboratory to infect tumor vessel endothelial cells in vivo82, 83. Echistatin binds the αvβ3 receptor with 1000-fold higher affinity than cyclic RGD84 and this is associated with enhanced ability of the virus to infect tumor vasculature. Recently, we also reported that VSV can naturally interact with tumor blood vessel endothelium in CT26 colorectal tumors in Balb/c mice85. By 24 hours post intravenous infusion of VSV, vascular perfusion had shut down at the core of the tumors and VSV antigen was detected in the blood vessels. To improve transfer of oncolytic viruses from blood vessel lumen to tumor parenchyma, the concept of heterocellular fusion between endothelial cells and underlying tumor cells has also been explored86. Adenoviral vectors encoding a fusogenic membrane glycoprotein driven by the human endothelial receptor tyrosine kinase promoter have been shown in vivo to trigger fusion between endothelial cells and epithelial cells, facilitating transendothelial virus penetration86.
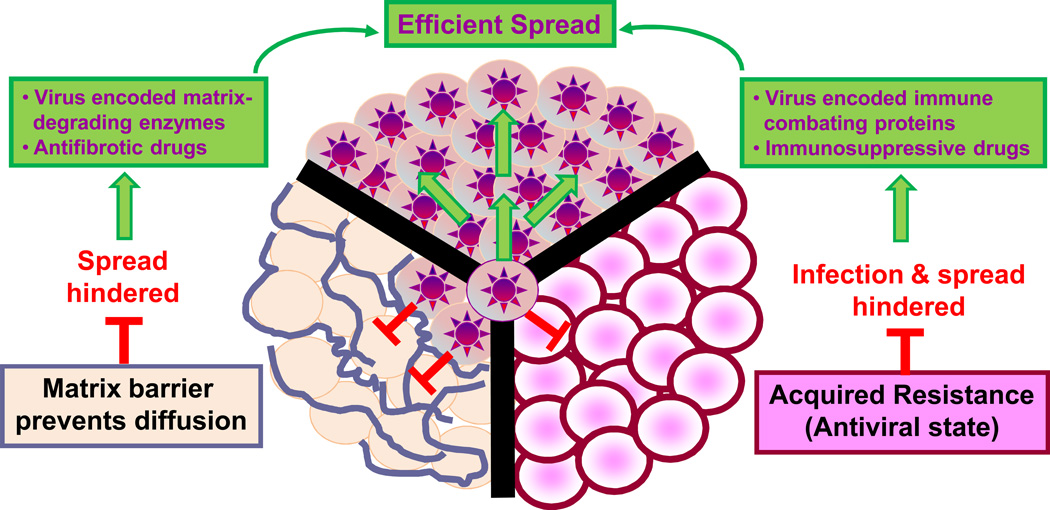
Intratumoral oncolytic virus spread
Mammalian cells have evolved to resist virus infections. A typical infection involves attacks on cellular defenses by viral gene products (virulence proteins), defensive parries by the host cell through the elaboration of anti-viral proteins and further counter attacks by the virus. Viral virulence genes encode proteins that suppress host defense systems, facilitate virus spread between cells and usurp cell metabolic processes. Oncolytic viruses are selected or engineered to be attenuated in normal tissues often by mutation or deletion of virus virulence genes75, 87. Thus, an oncolytic virus entering a normal cell triggers the cellular anti-viral response but is unable to counterattack so the infection is quickly eliminated. The antiviral response involves production of proteins that counteract the virus by acting directly against the virus88, 89, communicating with adjacent cells90 or jump-starting apoptotic programs91. Interferons and their receptors are key players in this anti-viral response, re-programming the physiological properties of infected and surrounding cells, inducing cell cycle arrest, providing anti-angiogenic signals, promoting apoptosis, inhibiting protein synthesis and activating the immune system.
In contrast to normal cells, the successful tumor cell has often eliminated/inactivated key gene products that have the dual role of controlling critical cell growth/death programs and aiding in resisting virus infections92. Because of these tumor-specific mutations, oncolytic viruses—despite their defective virulence genes—can initiate productive infections in cancer cells. Occasionally, cancer cells are completely devoid of antiviral activity87 but partial inactivation is more common resulting in only limited sensitivity to oncolytic virus therapy93.
Enhancing oncolytic virus growth in tumors through genetic arming
Incorporation of virulence genes from other virus strains or repairing previously attenuated/deleted virulence genes can overcome residual antiviral responses found in some tumors93, 94. Although this could compromise excellent safety profile of oncolytic viruses, it is expected that the approach can be finetuned to enhance clinical oncolytic virus therapy. A related approach to improve oncolytic virus potency is to combine viruses with complementing virulence proteins95. An interferon-sensitive oncolytic vesicular stomatitis virus (VSV) replicated efficiently in refractory tumor cells when they were co-infected with an oncolytic poxvirus encoding a secreted interferon antagonist. We achieved a similar ’ping pong’ synergy effect using an engineered fusogenic VSV to accelerate the intratumoral spread (through cell fusion) of an oncolytic poxvirus95. Because VSV has an RNA genome and vaccinia has a DNA genomes, the exchange of virulence genes leading to a pathogenic ‘super virus’ seems very unlikely. Clinical development of poxviruses encoding an interferon antagonist (B18R protein) is currently underway96 and two interferon-responsive oncolytic viruses (Oncovex and Reolysin) are in advanced stage trials. If approved as single agents, these agents could be combined to create more effective oncolytic virus therapy regimens.
Chemical sensitizers to enhance oncolytic virus growth in tumor cells
Another tactic to neutralize residual antiviral activities in oncolytic virus resistant cancers is through the use of small molecules. Several groups (including our own) have shown that histone deacetylase inhibitors (HDAC inhibitors) can suppress the residual IFN responsiveness of tumor cells, thereby increasing oncolytic virus potency without compromising specificity97–100. We have identified additional compounds enhancing oncolytic virus growth in refractory tumor cells using high-throughput screening. The previously uncharacterized compound, 3,4-dichloro-5-phenyl-2,5-dihydrofuran-2-on, whose mechanism of action remains the subject of intense ongoing investigations, enhanced the growth of various oncolytic viruses on a spectrum of tumor cells by blunting their interferon101. Several chemically unrelated compounds were isolated in this screen but their cellular targets have not yet been defined. A similar screen with an attenuated herpes simplex virus (HSV) lacking ribonucleotide reductase identified two molecules (dipyridamole and dilazep) that inhibit the cellular equilibrative nucleoside transporter-1 (ENT1), thereby inducing cellular ribonucleotide reductase102.
Rapamycin potentiates the growth of several oncolytic viruses in rodent tumor models, mainly by disrupting the TORC1 [TOR (target of rapamycin) complex 1]-dependent production of interferon and/or disrupting the phosphotidylinositol 3-kinase AKT pathway103–105. Cyclophosphamide has also been shown to improve oncolytic virus efficacy through several mechanisms. It dampens the innate antiviral response, slows the generation of anti–oncolytic virus neutralizing antibodies, may target T-regs and may affect tumor vasculature enhancing oncolytic virus extravasation106–108. Even so, combining drugs with viruses is not without risk and could promote off-target infections, compromising safety107.
Improving virus spread in tumors
Some oncolytic viruses are particularly well equipped to spread within and between tumors. For instance, vaccinia virus generates multiple virus ’subspecies’ adapted in different ways for efficient spread. The extracellular enveloped or ’cloaked’ form (EEV) facilitates widespread dissemination and to some extent avoidance of neutralizing antibodies109 whereas the cell associated (CEV) form has an actin tail that propels the virus into adjacent tumor cells110. Other viruses, such as oncolytic measles, spread by fusing infected with uninfected cells111, 112. Engineering this capacity (cell fusion) into other virus platforms can improve therapy113 but can also lead to increased unwanted pathology114.
Movement of viruses through tumors can be impeded by dense intratumoral connective tissue115, 116. Recently, it has been shown that losartan, an US Food and Drug Administration (FDA; Silver Spring, MD)-approved angiotensin II receptor antagonist/antihypertensive agent can enhance the intratumoral spread of an oncolytic HSV by disrupting transforming growth factor beta 1 (TGF-β1) signaling, which decreases collagen production, although several weeks of antifibrotic activity would be needed to impact clinical oncolytic virus outcomes117. Hyaluronan is a sulfated glycosaminoglycan and key component of the tumor extracellular matrix. Injecting hyaluronidase into tumors enhances the spread and efficacy of oncolytic adenoviruses118. A hyaluronidase-expressing oncolytic adenovirus demonstrated improved spread and activity in a human melanoma xenograft model119. Lastly, damage caused to tumors by cytotoxic agents, radiation or apoptosis inducers can lead to creation of voids and channels that facilitate virus spread116.
Engineering tumor selectivity into oncolytic virus backbones
Many of the earliest engineered oncolytic viruses were based upon the adenovirus backbone and were designed to take advantage of ‘tumor-specific’ promoter elements120. For example, the telomerase reverse transcriptase promoter is inactive in essentially all adult somatic tissues but is robustly expressed in cancer cells102. An alternative strategy is to use a promoter element that targets both the cancer and an expendable adult tissue (e.g., prostate121). This approach was extended to HSV122 and more recently to replication-competent retroviral oncolytic vectors13. Expression profiling is providing new leads for promoters that could be used for oncolytic virus regulation123. Poxviruses are not amenable to transcriptional targeting because they replicate entirely in the cell cytoplasm and regulate their transcription independently of the host cell transcriptional machinery.
Transductional targeting (discussed previously) can also be used to eliminate toxicities, particularly when the oncolytic virus binds an ubiqutious receptor. VSV was pseudotyped with the surface glycoprotein from a non-neurotropic lymphocytic choriomeningitis virus (LCMV) or retargeted measles virus, thereby eliminating its neurotoxicity without compromising its ability to infect and kill cancer cells124, 125. Modification of the hypervariable loop of the adenovirus hexon protein ablates the ability of that virus to infect normal hepatocytes but not tumor cells126.
Given the potential off-target effects of transcriptional and transductional targeting, other tropism modifying strategies are of interest. One exciting new strategy is the application of microRNA targeting to oncolytic viruses127, 128 which takes advantage of differential expression of certain microRNA species in tumor and normal tissues. Insertion of liver-specific microRNA binding sites in the 3´ untranslated region (UTR) of the E1A gene of an oncolytic adenovirus eliminated its hepatotoxicity without destroying tumor cell killing activity128, 129.
MicroRNA regulation of oncolytic virus tropism was first described in RNA viruses that cannot be controlled through transcriptional targeting130–132. Particularly dramatic are the results with coxsackievirus A21 (CVA21), a very potent oncolytic virus in mice that also causes fatal myositis due to off-target infection of normal muscle. We demonstrated that inclusion of muscle-specific microRNA targets into the 3´ UTR of CVA21 eliminated muscle toxicity but did not compromise anticancer activity131. One potential issue with this approach is that microRNA targets can mutate during oncolysis so it may be prudent to use a second selectivity strategy to minimize the chances of toxic escape variants arising during therapy. An oncolytic adenovirus was regulated by both transcriptional targeting (telomerase promoter) and microRNA targeting of the E1 gene133. Dual targeting approaches may facilitate the generation of potent but highly specific oncolytic strains encoding wild-type virulence proteins. The positioning of microRNA targets is another critical determinant for their effectiveness in attenuating virus replication. Inclusion of microRNA targets in VSV can eliminate unwanted neurotoxicity in mice; however, it is successful only when positioned at the extreme end of the viral genome controlling the expression of the L or polymerase gene of the virus130.
Oncolytic virus replication can also be targeted by regulation of viral protein translation. The potently oncolytic chimeric poliovirus, PVS-RIPO (live-attenuated poliovirus type 1 (Sabin) vaccine containing an internal ribosome entry site (IRES) element from human rhinovirus type 2), lacked neurotoxicity because translation from the inserted rhinovirus IRES is selectively blocked in neurons134. Translational control through the IRES element of another picornavirus, encephalomyocarditis virus, appears to play a role in its oncolytic specificity135. The level and activation state of eukaryotic initiation factor 4E (eIF4E) contribute to the initiation and progression of a variety of cancers and some viruses actively promote eIF4E activation136. Incorporation of complex 5´ UTRs responsive to the levels of cellular eIF4E has therefore been used to target HSV122 and adenovirus137.
Conditional manipulation of protein stability is also being used to regulate oncolytic virus expression. Fusion of a ‘destabilizing domain’ was used to create chimeric proteins that are inherently unstable149,138. A cell-permeable synthetic small molecule ligand called Shield-1 ((S)-(R)-3-(3,4-dimethoxyphenyl)-1-(3-(2-morpholinoethoxy)phenyl)propyl 1-((S)-2-(3,4,5-trimethoxyphenyl)butanoyl)piperidine-2-carboxylate), can bind the destabilizing domain and reverse this instability allowing regulated production of imaging proteins (e.g., luciferase) or TNF-α in animal tumor models138–140. This provides an attractive system for fine-tuned expression of therapeutic transgenes to control the spread of an oncolytic virus.
Controlling adaptive immunity and clearance of oncolytic viruses
When reviewing the history of the oncolytic virus field, it is notable that immunosuppressed patients have generally responded better to oncolytic virus therapy than those with an intact immune system, but this increased oncolytic activity was often associated with unacceptable toxicity7. Impairment of the adaptive antiviral immune response is therefore a double-edged sword but can be used to advantage, provided the virus is so specific for the tumor that it cannot damage normal tissues. Virus-targeting technologies have now advanced to the point where combining virotherapy with immunosuppressive drugs has become an appealing approach by which to enhance their antitumor activity. There are many immunosuppressive drugs to choose from in this regard, but cyclophosphamide is currently the most favored because it is potently toxic to both T and B lymphocytes, has direct antitumor activity, has been very widely used since 1949 for both cancer therapy and for immunosuppression, and is very reasonably priced141. Several preclinical studies have shown that cyclophosphamide can retard immune clearance of oncolytic viruses, enhance persistence of virus infection and prolong therapeutic efficacy142; the approach is now being evaluated in clinical trials.
Enhancing antitumor immunity
Immune evasion by tumors, recognized as one of the ‘Hallmarks of Cancer’”, represents an important target for new cancer therapeutics143. Tumors produce immunosuppressive cytokines (e.g., TGFβ) and recruit immune inhibitory cells (e.g., T-regs), thereby paralyzing the antitumor immune response144, 145. Oncolytic viruses may uniquely combine tumor debulking activity (via direct tumor lysis and/or vascular attack) with potent activation of adaptive and innate immune responses. Targeted infection of the tumor leads to a localized inflammatory response triggering an immune storm directly within the malignancy, facilitating immune recognition of cancer specific neo-antigens145. As discussed in the earlier section on clinical trial results, work with the oncolytic vaccinia virus JX-594 and more recently with Amgen’s oncolytic herpes virus, talimogene laherparepvec, both armed with GM-CSF, suggests clinical benefit can be attained when localized oncolytic activity is coupled with immune cell recruitment19, 145, 146.
The idea of an oncolytic vaccine combining virus-mediated tumor destruction with immune recognition of tumor antigens is attractive but requires careful orchestration as the activated immune system may prematurely suppress therapeutic virus replication147. Recent work suggests that even limited infection of distant lymph node metastases may lead to enhanced therapeutic benefit. Bridle and colleagues159 have shown that an oncolytic rhabdovirus expressing a tumor antigen can robustly boost a primed anti-tumor immune response, but only if given systemically when the virus can access both the tumor and distant lymph tissues145, 148. Recentlxzy, rather striking results have been observed in tumor-bearing animals ‘vaccinated’ with an oncolytic rhabdovirus expressing a complex library of cDNAs encoding normal cellular antigens149. Although it is unlikely that a library of viruses expressing thousands of unique sequences could become a therapeutic product, this work does suggest that future oncolytics that express a small number of carefully selected tumor antigens should be tested in the clinical setting. Towards that goal, three VSVs encoding melanoma specific antigens that induce IL-17 recall responses were selected from a library of VSV-cDNA, that when used in combination but not alone were as efficacious as the parental complete VSV-cDNA library150. Several groups are now combining adoptive cell therapy with oncolytic viruses reasoning that virus-mediated tumor cell destruction should enhance the activity of the transferred cells145, 151.
Which virus for which indication?
Given that naturally occurring viruses have such widely differing structures, lifecycles and tropisms, resulting in a diversity of highly distinctive clinical manifestations, it would seem logical that each oncolytic virus would be ideally suited to a specific malignancy. It is therefore somewhat surprising that there are to date very few examples of this specific matching of a given oncolytic virus with a specific class of malignancy, and most of the oncolytic viruses currently in development show a relatively broad spectrum of antitumor activity, typically against both epithelial and hematological malignancies. Certain oncolytic viruses were initially developed with the expectation that they would be better suited to a given broad class of malignancies, but this has subsequently proven not to be the case. Thus, oncolytic adenoviruses were considered better suited for therapy of epithelial malignancies but are now showing activity against hematologic cancers152, 153; herpes simplex viruses were developed originally for brain cancer therapy but are now showing promise in a variety of non-central nervous system tumors, including sarcomas and epithelial malignancies153, 154; and measles viruses were originally considered an ideal candidate for hematologic malignancies but have also proven to have broad spectrum activity against epithelial malignancies and sarcomas155.
Obviously, where viruses are engineered to target specific cell surface receptors or nuclear transcription factors, their utility is thereafter limited to tumors that express the relevant target, but to date there has been a definite preference for clinical translation of oncolytic viruses with a broader spectrum antitumor activity. This may be a consequence of the safety concerns being more difficult to address for fully retargeted viruses that stringently target a single type of tumor. Although it may seem counterintuitive that a virus engineered to restrict its host range might have greater pathogenic potential than the parent virus, several examples indicate the association of loss of pathogenic potential (attenuation) with the broadening of virus host range156, 157. Thus, assumptions about safety and host range cannot be trusted and have to be tested experimentally in appropriate animal species to directly address this question.
It is apparent from the above discussion that safety considerations are ever-present in preclinical oncolytic virus studies and may also be drivers of the choice of virus for a given indication. Different viruses have differing toxicities, and genetically manipulating these viruses may result in unexpected toxicities, such as an instance in which insertion of the IL-4 gene into a murine poxvirus resulted in 100% lethality in pre-vaccinated animals that were previously completely immune to the wild-type virus158. Natural and engineered virus tropisms, virus mutability and capacity for evolution, immunomodulatory/antiapoptotic and cytotoxic gene products, virus transmissibility, prevalence of antiviral immunity in the population and availability of drugs or antisera to eliminate unwanted or persistent infections are all important factors to be considered in the safety analysis of oncolytic viruses that are candidates for clinical translation.
CONCLUSIONS
Oncolytic viruses are structurally and biologically diverse, spreading through tumors and killing tumor cells by multiple mechanisms and with different kinetics. Because of their large size and immunogenicity they are constrained by physical barriers and by host immunity, but they can also cross-prime and amplify antitumor immunity, serving as a cancer immunotherapy. Overall, the field has been slow to develop but recent clinical trial data has been promising and a first-in-class USA approval is expected soon for a recombinant herpes simplex virus being tested in a randomised phase III clinical trial that recently completed accrual. This virus (Talimogene Laherparepvec, previously OncoVEX) is administered by intralesional injection to patients with metastatic malignant melanoma and spreads locally, cross-priming the antimelanoma immune response, but does not spread systemically to distant sites of tumor growth. Thus the talimogene study is primarily exploiting the oncolytic virus as tumor-debulking immunotherapy and does not clinically validate the ‘oncolytic paradigm’ where systemic and intratumoral spread of the infection lead to tumor debulking as a prelude to immune-mediated eradication of minimal residual disease. However, a more direct validation of the oncolytic paradigm may soon come from ongoing clinical trials testing intravascular OV delivery in immunotherapy-resistant tumors using, for example, reovirus, vaccinia, and measles viruses. And for the future, there is a long and growing list of new or improved versions of oncolytic viruses that have been ingeniously selected, engineered and honed for systemic therapy, several of which will doubtless, in the fullness of time, join the growing arsenal of clinically approved anticancer drugs.
Looking beyond the expected clinical approval of oncolytic viruses as single agents, there is enormous scope for the development of more complex protocols to achieve superior treatment outcomes. Preclinical studies provide a very strong basis for this assertion, demonstrating numerous synergistic interactions that can overcome the various barriers constraining oncolytic viruses, such as the use of cell carriers to optimize virus delivery60 or of immunosuppressive drugs159 to enhance their intratumoral spread. One particularly interesting prospect is that new drugs will be developed capable of potently suppressing the innate immune responses of virus infected cells. Elucidation of the intracellular signaling pathways of innate immunity has been progressing very rapidly in recent years, so the stage is now set for this important area of drug discovery.
But as the field comes closer to its lofty goal of a single shot virotherapy cure for cancer160, it is very likely that we will encounter significant treatment-related toxicities. The minimal toxicity in clinical trials to date is often cited as a strength of the oncolytic virotherapy approach, but in the absence of rapid destructive intratumoral virus spread, which is the ultimate goal, it is hardly surprising the treatment has seemed innocuous. With increased potency and more reliable efficacy, toxicity will surely follow, and hence the need for ever more stringent virus targeting technology to ensure that the destructive power of these exciting new drugs is focused exclusively on the tumor.
The most important technical challenges that continue to attract the attention of the oncolytic research community are the optimization/enhancement of systemic virus delivery, intratumoral virus spread and cross-priming of anticancer immunity. However, the harmonization of solutions to these problems is perhaps a greater challenge still, although definitely achievable160. Suppressing immunity may increase intratumoral spread, but diminishes cross-priming of the anticancer immune response. Conversely, enhancing immunity may improve cross-priming but the price paid is to limit intratumoral virus spread, the basis of oncolytic tumor debulking. Many of the ‘solutions’ that have been developed to date have been analyzed in artificial model systems that are not powered to reveal the positive and negative consequences of a given modification to all aspects of the overall treatment paradigm. This points to another major challenge for the field which is to develop better model systems that really do reliably mirror the human oncolytic virotherapy scenario. Mouse xenograft models lack a functional immune system, and immunocompetent mouse tumor models are frequently misleading because the viruses being tested behave differently in mice and humans. Thus, many oncolytic viruses cannot infect mouse cells so they lack activity in syngeneic mouse models, while others preferentially infect mouse vs. human cells so their anticancer activity (and toxicity) is not transferrable to human trials. The use of oncolytic agents such as vaccinia virus which are capable of infecting mouse and human cells with equal efficiency is a potential solution to this problem and may prove to be an important factor for the acceleration of their clinical development. In addition the development of transgenic mouse models susceptible to “human specific” viruses remains an important goal.
The biggest overall challenges facing the field at the current time have less to do with the development of new technology solutions for virus delivery and spread than with how to get them clinically tested. There are so many elegant solutions available for hypothetical problems that it can be demoralizing for scientists to see their engineering efforts lost in the morass. Clinical testing of each new virus modification is simply not realistic because of the enormous amount of work and expense required – manufacture, pharmacology/toxicology testing, protocol development and regulatory approval - to move each new product into phase I trials. Take for example oncolytic adenoviruses where a PubMed search shows 100 publications in the past 10 years on almost as many unique adenovirus configurations representing multiple serotypes (for antibody evasion) with or without engineered fiber modifications (for transductional targeting), hexon modifications (to eliminate hepatic sequestration), polymeric coats (for shielding), gene deletions (for physiological targeting), transgene insertions (to combat innate immunity, enhance adaptive immunity, promote spread, increase cytotoxicity or facilitate noninvasive monitoring). Every one of these modifications can be classified as a new product and the modifications are coming so fast that an oncolytic virus that was state of the art a few years ago may today be considered archaic even before it has completed phase II clinical testing.
Figure 1.
Barriers to efficient oncolytic virus delivery via the bloodstream (virus neutralization by serum factors, sequestration by the mononuclear phagocytic system or lack of extravasation) and solutions to circumvent them.
Figure 2.
Factors constraining intratumoral virus spread (host innate or acquired immunity and extracellular matrix) and solutions to circumvent them.
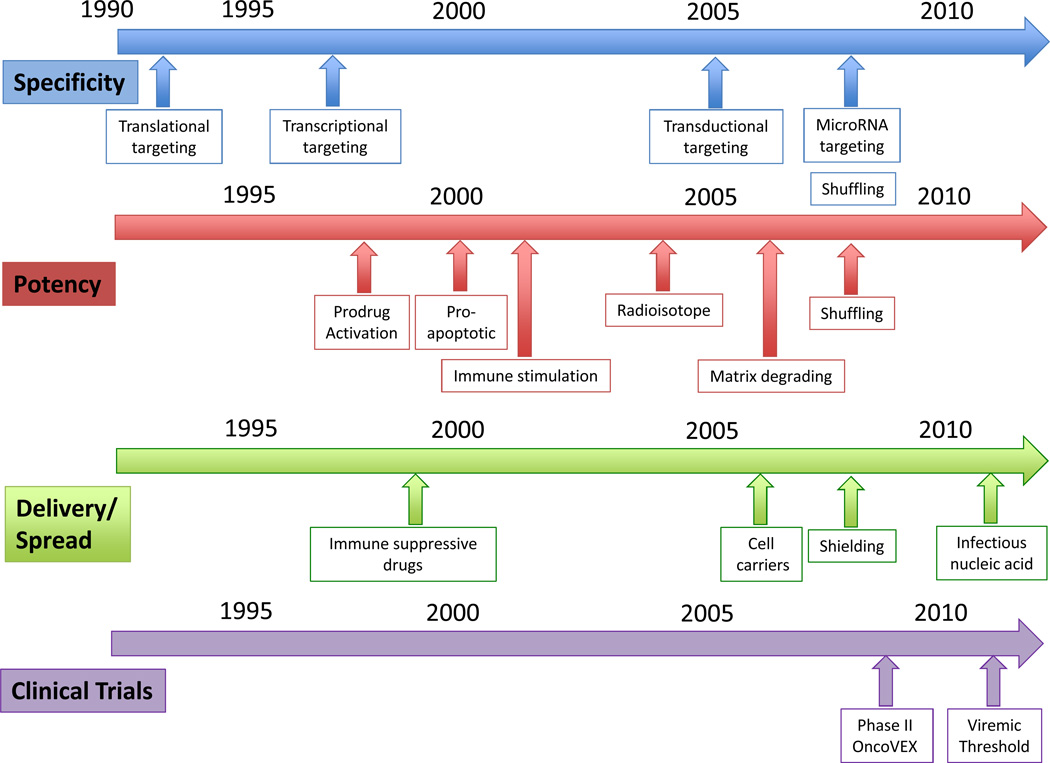
Figure 3. A timeline of milestones in the development of oncolytic virotherapy to improve virus specificity, potency, delivery and spread.
SPECIFICITY: (1) Translational targeting: 1991, Engineering of a replication-competent HSV attenuated for neurovirulence for glioma treatment10. (2) Transcriptional targeting: 1997, targeting of HSV using albumin promoter/enhancer for hepatoma cells161 and Ad using Prostate specific antigen (PSA) promoter for prostate cancer cells162, (3) Transductional targeting: 2005, targeting entry and cytopathic effects of oncolytic measles virus by display of single-chain antibody on the virus attachment protein, (4) MicroRNA targeting: 2008, to control unwanted toxicity of picornarvirus131 and vesicular stomatitis virus132 while retaining antitumor activity, (5) DNA shuffling, 2008, Mixing a pool adenoviral serotypes and passaging the pools under conditions that invite recombination between serotypes to generate tumor selective virus163.
POTENCY: (1) Prodrug activation: 1998, an oncolytic adenovirus expressing cytosine deaminase and HSV-Tk designed to work in combination with 5-FC and Ganciclovir, (2) Proapoptotic genes: 2000, introduction of the adenovirus death protein (ADP) into an oncolytic adenovirus to enhance its cytotoxicity164, (3) Immune stimulation: 2001, oncolytic HSV encoding IL-12 and GM-CSF to recruit T lymphocyte-mediated antitumor immune response165, (4) Radioisotope: 2004, an oncolytic measles virus encoding the human sodium iodide symporter (NIS) which concentrates beta-emitting (radiovirotherapy) and gamma-emitting isotopes (imaging)33, (5) 2006, Matrix degrading proteins: adenovirus encoding relaxin protein to enhance virus intratumoral spread166, (6) Shuffling: 2008, Mixing a pool adenoviral serotypes and passaging the pools under conditions that invite recombination between serotypes to generate more potent adenovirus ColoAd1 163.
DELIVERY & SPREAD: (1) Immune suppressive drugs: 1999, Addition of cyclophosphamide to combat and innate and adaptive antiviral immunity to enhance intratumoral spread of HSV, (2) Cell carriers: 2006, use of cytokine induced killer cells to deliver oncolytic vaccinia virus to tumor, resulting in synergistic antitumor activity167, (3) Shielding: 2008, Polymer coating and retargeting of oncolytic adenovirus for ovarian cancer to enhance viral pharmacokinetics40, (4) Infectious Nucleic Acid: 2011, delivery of oncolytic picornarvirus using infectious nucleic acid (RNA) to successfully achieve sustained viremia and tumor regression.
CLINICAL TRIALS: (1) Activity: 2009, Phase II trial with intralesional injection of oncolytic HSV, OncoVEX (talimogene laherparepvec ), in melanoma patients. 26% complete response (8 out of 50), with durability in both injected and uninjected lesions including visceral sites19. Undergoing Phase III evaluation. (2) Viremic Threshold: 2011, Intravenous delivery of JX-594, oncolytic vaccinia virus, in patients with metastatic tumor, demonstrating the need for a viremic threshold to be reached for efficient virus delivery to tumors26.
ACKNOWLEDGMENTS
SJR and KWP gratefully acknowledges funding support from the Mayo Foundation, Mayo Clinic Comprehensive Cancer Center (CA15083), NIH/NCI (CA100634, CA129966, CA118488, CA129193, CA136547, CA136393), Richard M. Schulze Family Foundation, Al and Mary Agnes McQuinn, Minnesota Partnership for Biotechnology and JCB is supported by the Ontario Institute for Cancer Research, the Terry Fox Foundation and the Ottawa Regional Cancer Foundation.
Footnotes
Conflict of Interest Statement:
Dr. Bell is the Chief Scientific Officer of Jennerex Biotherapeutics.
REFERENCES
- 1.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DM K, CE S, P P. In: Fields Virology. Edn. Fourth Edition. DM K, et al., editors. Vol. I. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 3.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev. 2009;61:554–571. doi: 10.1016/j.addr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther. 2009;9:1163–1176. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- 6.Kelly EJ, Russell SJ. MicroRNAs and the regulation of vector tropism. Mol Ther. 2009;17:409–416. doi: 10.1038/mt.2008.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 8.Southam CM. Present status of oncolytic virus studies. Trans N Y Acad Sci. 1960;22:657–673. doi: 10.1111/j.2164-0947.1960.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 9.Asada T. Treatment of human cancer with mumps virus. Cancer. 1974;34:1907–1928. doi: 10.1002/1097-0142(197412)34:6<1907::aid-cncr2820340609>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 11.Au GG, Lindberg AM, Barry RD, Shafren DR. Oncolysis of vascular malignant human melanoma tumors by Coxsackievirus A21. Int J Oncol. 2005;26:1471–1476. doi: 10.3892/ijo.26.6.1471. [DOI] [PubMed] [Google Scholar]
- 12.Rudin CM, et al. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin Cancer Res. 2011;17:888–895. doi: 10.1158/1078-0432.CCR-10-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai CK, Kasahara N. Replication-competent retrovirus vectors for cancer gene therapy. Front Biosci. 2008;13:3083–3095. doi: 10.2741/2910. [DOI] [PubMed] [Google Scholar]
- 14.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 15.Schoofs G, et al. A high-yielding serum-free, suspension cell culture process to manufacture recombinant adenoviral vectors for gene therapy. Cytotechnology. 1998;28:81–89. doi: 10.1023/A:1008021428969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knop DR, Harrell H. Bioreactor production of recombinant herpes simplex virus vectors. Biotechnol Prog. 2007;23:715–721. doi: 10.1021/bp060373p. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JA, Brown EL, Duncan PA. Approaches to the release of a master cell bank of PER.C6 cells; a novel cell substrate for the manufacture of human vaccines. Dev Biol (Basel) 2006;123:165–176. discussion 183–197. [PubMed] [Google Scholar]
- 18.Russell SJ. Replicating vectors for cancer therapy: a question of strategy. Semin Cancer Biol. 1994;5:437–443. [PubMed] [Google Scholar]
- 19.Senzer NN, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 20.Park BH, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 21.Eager RM, Nemunaitis J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011;18:305–317. doi: 10.1038/cgt.2011.7. [DOI] [PubMed] [Google Scholar]
- 22.Mastrangelo MJ, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 23.Harrington KJ, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- 24.Harrington KJ, Vile RG, Melcher A, Chester J, Pandha HS. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–98. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heo J, et al. Sequential Therapy With JX-594, A Targeted Oncolytic Poxvirus, Followed by Sorafenib in Hepatocellular Carcinoma: Preclinical and Clinical Demonstration of Combination Efficacy. Mol Ther. 2011 doi: 10.1038/mt.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breitbach CJ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011 doi: 10.1038/nature10358. In press. [DOI] [PubMed] [Google Scholar]
- 27.Serganova I, Ponomarev V, Blasberg R. Human reporter genes: potential use in clinical studies. Nucl Med Biol. 2007;34:791–807. doi: 10.1016/j.nucmedbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 29.Galanis E, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs A, et al. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358:727–729. doi: 10.1016/s0140-6736(01)05904-9. [DOI] [PubMed] [Google Scholar]
- 31.Dingli D, Russell SJ, Morris JC., 3rd In vivo imaging and tumor therapy with the sodium iodide symporter. J Cell Biochem. 2003;90:1079–1086. doi: 10.1002/jcb.10714. [DOI] [PubMed] [Google Scholar]
- 32.Barton KN, et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther. 2008;16:1761–1769. doi: 10.1038/mt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dingli D, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 34.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 35.Haisma HJ, et al. Scavenger receptor A: a new route for adenovirus 5. Mol Pharm. 2009;6:366–374. doi: 10.1021/mp8000974. [DOI] [PubMed] [Google Scholar]
- 36.Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;1(11 Suppl):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 37.Fisher KD, Seymour LW. HPMA copolymers for masking and retargeting of therapeutic viruses. Adv Drug Deliv Rev. 2010;62:240–245. doi: 10.1016/j.addr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Eto Y, Yoshioka Y, Mukai Y, Okada N, Nakagawa S. Development of PEGylated adenovirus vector with targeting ligand. Int J Pharm. 2008;354:3–8. doi: 10.1016/j.ijpharm.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 40.Morrison J, et al. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol Ther. 2008;16:244–251. doi: 10.1038/sj.mt.6300363. [DOI] [PubMed] [Google Scholar]
- 41.Croyle MA, et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol. 2004;78:912–921. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alemany R, Suzuki K, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 43.Doronin K, Shashkova EV, May SM, Hofherr SE, Barry MA. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Human gene therapy. 2009;20:975–988. doi: 10.1089/hum.2009.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green NK, et al. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256–1263. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda K, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nature medicine. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda K, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakimoto H, et al. The complement response against an oncolytic virus is species-specific in its activation pathways. Mol Ther. 2002;5:275–282. doi: 10.1006/mthe.2002.0547. [DOI] [PubMed] [Google Scholar]
- 48.Haisma HJ, Bellu AR. Pharmacological interventions for improving adenovirus usage in gene therapy. Mol Pharm. 2011;8:50–55. doi: 10.1021/mp100310h. [DOI] [PubMed] [Google Scholar]
- 49.Shashkova EV, Doronin K, Senac JS, Barry MA. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer research. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- 50.Koski A, et al. Systemic adenoviral gene delivery to orthotopic murine breast tumors with ablation of coagulation factors, thrombocytes and Kupffer cells. J Gene Med. 2009;11:966–977. doi: 10.1002/jgm.1373. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler RJ, et al. Correction of the nonlinear dose response improves the viability of adenoviral vectors for gene therapy of Fabry disease. Human gene therapy. 2002;13:935–945. doi: 10.1089/10430340252939041. [DOI] [PubMed] [Google Scholar]
- 52.Manickan E, et al. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Tao N, et al. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 54.Power AT, Bell JC. Taming the Trojan horse: optimizing dynamic carrier cell/oncolytic virus systems for cancer biotherapy. Gene Ther. 2008;15:772–779. doi: 10.1038/gt.2008.40. [DOI] [PubMed] [Google Scholar]
- 55.Ilett EJ, et al. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16:689–699. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu C, Russell SJ, Peng KW. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol Ther. 2010;18:1155–1164. doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dwyer RM, Khan S, Barry FP, O’Brien T, Kerin MJ. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res Ther. 2010;1:25. doi: 10.1186/scrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Castro J, et al. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther. 2010;17:476–483. doi: 10.1038/cgt.2010.4. [DOI] [PubMed] [Google Scholar]
- 59.Ling X, et al. Mesenchymal Stem Cells Overexpressing IFN-beta Inhibit Breast Cancer Growth and Metastases through Stat3 Signaling in a Syngeneic Tumor Model. Cancer Microenviron. 2010;3:83–95. doi: 10.1007/s12307-010-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mader EK, et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ilett EJ, et al. Internalization of Oncolytic Reovirus by Human Dendritic Cell Carriers Protects the Virus from Neutralization. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiao J, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 64.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer research. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 65.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Hobbs SK, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hashizume H, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fox ME, Szoka FC, Frechet JM. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc Chem Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barnett FH, et al. Selective delivery of herpes virus vectors to experimental brain tumors using RMP-7. Cancer gene therapy. 1999;6:14–20. doi: 10.1038/sj.cgt.7700003. [DOI] [PubMed] [Google Scholar]
- 71.Tseng JC, Granot T, DiGiacomo V, Levin B, Meruelo D. Enhanced specific delivery and targeting of oncolytic Sindbis viral vectors by modulating vascular leakiness in tumor. Cancer gene therapy. 2010;17:244–255. doi: 10.1038/cgt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kottke T, et al. Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice. J Clin Invest. 2010;120:1551–1560. doi: 10.1172/JCI41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kottke T, et al. Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors using systemically delivered oncolytic virus. Mol Ther. 2008;16:1217–1226. doi: 10.1038/mt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Driessen WH, Ozawa MG, Arap W, Pasqualini R. Ligand-directed cancer gene therapy to angiogenic vasculature. Adv Genet. 2009;67:103–121. doi: 10.1016/S0065-2660(09)67004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thorne SH, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5:436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 77.Sanz L, et al. Single-chain antibody-based gene therapy: inhibition of tumor growth by in situ production of phage-derived human antibody fragments blocking functionally active sites of cell-associated matrices. Gene Ther. 2002;9:1049–1053. doi: 10.1038/sj.gt.3301725. [DOI] [PubMed] [Google Scholar]
- 78.Palumbo A, et al. A chemically modified antibody mediates complete eradication of tumours by selective disruption of tumour blood vessels. Br J Cancer. 2011;104:1106–1115. doi: 10.1038/bjc.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakamura T, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 80.Morrison J, et al. Cetuximab retargeting of adenovirus via the epidermal growth factor receptor for treatment of intraperitoneal ovarian cancer. Human gene therapy. 2009;20:239–251. doi: 10.1089/hum.2008.167. [DOI] [PubMed] [Google Scholar]
- 81.Bachtarzi H, et al. Targeting adenovirus gene delivery to activated tumour-associated vasculature via endothelial selectins. J Control Release. 2011;150:196–203. doi: 10.1016/j.jconrel.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ong HT, et al. Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol Ther. 2009;17:1012–1021. doi: 10.1038/mt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jing Y, et al. Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor. Cancer Res. 2009;69:1459–1468. doi: 10.1158/0008-5472.CAN-08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumar CC, et al. Biochemical characterization of the binding of echistatin to integrin alphavbeta3 receptor. J Pharmacol Exp Ther. 1997;283:843–853. [PubMed] [Google Scholar]
- 85.Breitbach CJ, et al. Targeting Tumor Vasculature With an Oncolytic Virus. Mol Ther. 2011 doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen HH, et al. Active adenoviral vascular penetration by targeted formation of heterocellular endothelial-epithelial syncytia. Mol Ther. 2011;19:67–75. doi: 10.1038/mt.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stojdl DF, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 88.Schoggins JW, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011 doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haller O, Kochs G, Weber F. Interferon, Mx, and viral countermeasures. Cytokine Growth Factor Rev. 2007;18:425–433. doi: 10.1016/j.cytogfr.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vandevenne P, Sadzot-Delvaux C, Piette J. Innate immune response and viral interference strategies developed by human herpesviruses. Biochem Pharmacol. 2010;80:1955–1972. doi: 10.1016/j.bcp.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Lu MY, Liao F. Interferon-stimulated gene ISG12b2 is localized to the inner mitochondrial membrane and mediates virus-induced cell death. Cell Death Differ. 2010 doi: 10.1038/cdd.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stojdl DF, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 93.Haralambieva I, et al. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther. 2007;15:588–597. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Altomonte J, et al. Exponential enhancement of oncolytic vesicular stomatitis virus potency by vector-mediated suppression of inflammatory responses in vivo. Mol Ther. 2008;16:146–153. doi: 10.1038/sj.mt.6300343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le Boeuf F, et al. Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther. 2010;18:888–895. doi: 10.1038/mt.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 97.Chang HM, et al. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci U S A. 2004;101:9578–9583. doi: 10.1073/pnas.0400567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen TL, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci U S A. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Otsuki A, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16:1546–1555. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 100.Mactavish H, et al. Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. PLoS One. 2010;5:e14462. doi: 10.1371/journal.pone.0014462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diallo JS, et al. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol Ther. 2010;18:1123–1129. doi: 10.1038/mt.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Passer BJ, et al. Identification of the ENT1 Antagonists Dipyridamole and Dilazep as Amplifiers of Oncolytic Herpes Simplex Virus-1 Replication. Cancer Research. 2010;70:3890–3895. doi: 10.1158/0008-5472.CAN-10-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alain T, et al. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci U S A. 2010;107:1576–1581. doi: 10.1073/pnas.0912344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lun X, et al. Myxoma virus virotherapy for glioma in immunocompetent animal models: optimizing administration routes and synergy with rapamycin. Cancer Res. 2010;70:598–608. doi: 10.1158/0008-5472.CAN-09-1510. [DOI] [PubMed] [Google Scholar]
- 105.Lun XQ, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67:8818–8827. doi: 10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qiao J, et al. Cyclophosphamide Facilitates Antitumor Efficacy against Subcutaneous Tumors following Intravenous Delivery of Reovirus. Clinical Cancer Research. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kottke T, et al. Improved Systemic Delivery of Oncolytic Reovirus to Established Tumors Using Preconditioning with Cyclophosphamide-Mediated Treg Modulation and Interleukin-2. Clinical Cancer Research. 2009;15:561–569. doi: 10.1158/1078-0432.CCR-08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurozumi K, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99:1768–1781. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 109.Kirn DH, Wang Y, Liang W, Contag CH, Thorne SH. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 2008;68:2071–2075. doi: 10.1158/0008-5472.CAN-07-6515. [DOI] [PubMed] [Google Scholar]
- 110.Reeves PM, et al. Variola and monkeypox viruses utilize conserved mechanisms of virion motility and release that depend on abl and SRC family tyrosine kinases. J Virol. 2011;85:21–31. doi: 10.1128/JVI.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoffmann D, Wildner O. Enhanced killing of pancreatic cancer cells by expression of fusogenic membrane glycoproteins in combination with chemotherapy. Mol Cancer Ther. 2006;5:2013–2022. doi: 10.1158/1535-7163.MCT-06-0128. [DOI] [PubMed] [Google Scholar]
- 112.Patel B, et al. Differential Cytopathology and Kinetics of Measles Oncolysis in Two Primary B-cell Malignancies Provides Mechanistic Insights. Mol Ther. 2011 doi: 10.1038/mt.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Israyelyan A, et al. Herpes simplex virus type-1(HSV-1) oncolytic and highly fusogenic mutants carrying the NV1020 genomic deletion effectively inhibit primary and metastatic tumors in mice. Virol J. 2008;5:68. doi: 10.1186/1743-422X-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown CW, et al. The p14 FAST protein of reptilian reovirus increases vesicular stomatitis virus neuropathogenesis. J Virol. 2009;83:552–561. doi: 10.1128/JVI.01921-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sauthoff H, et al. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systemically at late time points. Human gene therapy. 2003;14:425–433. doi: 10.1089/104303403321467199. [DOI] [PubMed] [Google Scholar]
- 116.Yun CO. Overcoming the extracellular matrix barrier to improve intratumoral spread and therapeutic potential of oncolytic virotherapy. Curr Opin Mol Ther. 2008;10:356–361. [PubMed] [Google Scholar]
- 117.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ganesh S, Gonzalez-Edick M, Gibbons D, Van Roey M, Jooss K. Intratumoral Coadministration of Hyaluronidase Enzyme and Oncolytic Adenoviruses Enhances Virus Potency in Metastatic Tumor Models. Clinical Cancer Research. 2008;14:3933–3941. doi: 10.1158/1078-0432.CCR-07-4732. [DOI] [PubMed] [Google Scholar]
- 119.Guedan S, et al. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol Ther. 2010;18:1275–1283. doi: 10.1038/mt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toth K, Dhar D, Wold WS. Oncolytic (replication-competent) adenoviruses as anticancer agents. Expert Opin Biol Ther. 2010;10:353–368. doi: 10.1517/14712590903559822. [DOI] [PubMed] [Google Scholar]
- 121.Muthana M, et al. Use of macrophages to target therapeutic adenovirus to human prostate tumors. Cancer Res. 2011;71:1805–1815. doi: 10.1158/0008-5472.CAN-10-2349. [DOI] [PubMed] [Google Scholar]
- 122.Lee CYF, et al. Transcriptional and Translational Dual-regulated Oncolytic Herpes Simplex Virus Type 1 for Targeting Prostate Tumors. Molecular Therapy. 2010;18:929–935. doi: 10.1038/mt.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Foka P, et al. Novel tumour-specific promoters for transcriptional targeting of hepatocellular carcinoma by herpes simplex virus vectors. J Gene Med. 2010;12:956–967. doi: 10.1002/jgm.1519. [DOI] [PubMed] [Google Scholar]
- 124.Muik A, et al. Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J Virol. 2011 doi: 10.1128/JVI.02511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ayala Breton C, Barber GN, Russell S, Peng KW. Retargeting Vesicular Stomatitis Virus Using Measles Virus Envelope Glycoproteins. Human gene therapy. 2011 doi: 10.1089/hum.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shashkova EV, May SM, Doronin K, Barry MA. Expanded anticancer therapeutic window of hexon-modified oncolytic adenovirus. Mol Ther. 2009;17:2121–2130. doi: 10.1038/mt.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leber MF, et al. MicroRNA-sensitive Oncolytic Measles Viruses for Cancer-specific Vector Tropism. Mol Ther. 2011 doi: 10.1038/mt.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cawood R, Wong SL, Di Y, Baban DF, Seymour LW. MicroRNA controlled adenovirus mediates anti-cancer efficacy without affecting endogenous microRNA activity. PLoS One. 2011;6:e16152. doi: 10.1371/journal.pone.0016152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cawood R, et al. Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog. 2009;5:e1000440. doi: 10.1371/journal.ppat.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kelly EJ, Nace R, Barber GN, Russell SJ. Attenuation of Vesicular Stomatitis Virus Encephalitis through MicroRNA Targeting. J Virol. 2009;84:1550–1562. doi: 10.1128/JVI.01788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kelly EJ, Hadac EM, Greiner S, Russell SJ. Engineering microRNA responsiveness to decrease virus pathogenicity. Nat Med. 2008;14:1278–1283. doi: 10.1038/nm.1776. [DOI] [PubMed] [Google Scholar]
- 132.Edge RE, et al. A let-7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther. 2008;16:1437–1443. doi: 10.1038/mt.2008.130. [DOI] [PubMed] [Google Scholar]
- 133.Sugio K, et al. Enhanced safety profiles of the telomerase-specific replication-competent adenovirus by incorporation of normal cell-specific microRNA-targeted sequences. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2008. [DOI] [PubMed] [Google Scholar]
- 134.Yang X, et al. Evaluation of IRES-mediated, cell-type-specific cytotoxicity of poliovirus using a colorimetric cell proliferation assay. J Virol Methods. 2009;155:44–54. doi: 10.1016/j.jviromet.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roos FC, et al. Oncolytic targeting of renal cell carcinoma via encephalomyocarditis virus. EMBO Mol Med. 2010;2:275–288. doi: 10.1002/emmm.201000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oliere S, et al. Vesicular stomatitis virus oncolysis of T lymphocytes requires cell cycle entry and translation initiation. J Virol. 2008;82:5735–5749. doi: 10.1128/JVI.02601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stoff-Khalili MA, et al. Cancer-specific targeting of a conditionally replicative adenovirus using mRNA translational control. Breast Cancer Res Treat. 2008;108:43–55. doi: 10.1007/s10549-007-9587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Banaszynski LA, Sellmyer MA, Contag CH, Wandless TJ, Thorne SH. Chemical control of protein stability and function in living mice. Nat Med. 2008;14:1123–1127. doi: 10.1038/nm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Glass M, Busche A, Wagner K, Messerle M, Borst EM. Conditional and reversible disruption of essential herpesvirus proteins. Nat Methods. 2009;6:577–579. doi: 10.1038/nmeth.1346. [DOI] [PubMed] [Google Scholar]
- 140.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stepkowski SM. Molecular targets for existing and novel immunosuppressive drugs. Expert Rev Mol Med. 2000;2:1–23. doi: 10.1017/S1462399400001769. [DOI] [PubMed] [Google Scholar]
- 142.Chiocca EA. The host response to cancer virotherapy. Current opinion in molecular therapeutics. 2008;10:38–45. [PubMed] [Google Scholar]
- 143.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 144.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Melcher A, Parato K, Rooney CM, Bell JC. Thunder and Lightning: Immunotherapy and Oncolytic Viruses Collide. Mol Ther. 2011 doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mastrangelo MJ, Maguire HC, Lattime EC. Intralesional vaccinia/GM-CSF recombinant virus in the treatment of metastatic melanoma. Adv Exp Med Biol. 2000;465:391–400. doi: 10.1007/0-306-46817-4_34. [DOI] [PubMed] [Google Scholar]
- 147.Diaz RM, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 148.Bridle BW, Hanson S, Lichty BD. Combining oncolytic virotherapy and tumour vaccination. Cytokine Growth Factor Rev. 2010;21:143–148. doi: 10.1016/j.cytogfr.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 149.Kottke T, et al. Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. Nature medicine. 2011;17:854–859. doi: 10.1038/nm.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pulido J, et al. Using virally expressed melanoma cDNA libraries to identify tumor-associated antigens that cure melanoma. Nature biotechnology. 2012;30:337–343. doi: 10.1038/nbt.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kottke T, et al. Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer. Mol Ther. 2008;16:1910–1918. doi: 10.1038/mt.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Senac JS, et al. Infection and killing of multiple myeloma by adenoviruses. Human gene therapy. 2010;21:179–190. doi: 10.1089/hum.2009.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X, Zhao L, Hang Z, Guo H, Zhang M. Evaluation of HSV-1 and adenovirus vector-mediated infection, replication and cytotoxicity in lymphoma cell lines. Oncol Rep. 2011;26:637–644. doi: 10.3892/or.2011.1306. [DOI] [PubMed] [Google Scholar]
- 154.Kanai R, Wakimoto H, Cheema T, Rabkin SD. Oncolytic herpes simplex virus vectors and chemotherapy: are combinatorial strategies more effective for cancer? Future Oncol. 2010;6:619–634. doi: 10.2217/fon.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–241. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Byrnes AP, Griffin DE. Large-plaque mutants of Sindbis virus show reduced binding to heparan sulfate, heightened viremia, and slower clearance from the circulation. J Virol. 2000;74:644–651. doi: 10.1128/jvi.74.2.644-651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee P, Knight R, Smit JM, Wilschut J, Griffin DE. A single mutation in the E2 glycoprotein important for neurovirulence influences binding of sindbis virus to neuroblastoma cells. J Virol. 2002;76:6302–6310. doi: 10.1128/JVI.76.12.6302-6310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen N, et al. Poxvirus interleukin-4 expression overcomes inherent resistance and vaccine-induced immunity: pathogenesis, prophylaxis, and antiviral therapy. Virology. 2011;409:328–337. doi: 10.1016/j.virol.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Peng KW, et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2012 doi: 10.1038/gt.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Naik S, et al. Curative one-shot systemic virotherapy in murine myeloma. Leukemia. 2012 doi: 10.1038/leu.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Miyatake S, Iyer A, Martuza RL, Rabkin SD. Transcriptional targeting of herpes simplex virus for cell-specific replication. J Virol. 1997;71:5124–5132. doi: 10.1128/jvi.71.7.5124-5132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rodriguez R, et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Research. 1997;57:2559–2563. [PubMed] [Google Scholar]
- 163.Kuhn I, et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS One. 2008;3:e2409. doi: 10.1371/journal.pone.0002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Doronin K, et al. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol. 2000;74:6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wong RJ, et al. Cytokine gene transfer enhances herpes oncolytic therapy in murine squamous cell carcinoma. Human gene therapy. 2001;12:253–265. doi: 10.1089/10430340150218396. [DOI] [PubMed] [Google Scholar]
- 166.Kim JH, et al. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. Journal of the National Cancer Institute. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 167.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]