Abstract
Cancer research has been righteously and successfully focused on prevention, early detection and identification of specific molecular targets that distinguish the malignant cells from the neighboring benign cells1. However, a major clinical challenge concerns how we can reduce lethal tissue injury caused by intensive chemoradiotherapy during treatment of late-staged metastatic cancers. Here we tested whether induction of adult stem cells repairs chemoradiation-induced tissue injury and prolongs overall survival. We found that intestinal stem cells (ISCs)2 expressed Slit2 and its single-span transmembrane cell-surface receptor Roundabout 1 (Robo1)3,4. Partial genetic deletion of Robo1 decreased intestinal stem cells (ISCs) and caused villus hypotrophy, whereas Slit2 transgene increased ISCs and triggered villus hypertrophy. During lethal dosages of chemoradiation, administering a short pulse of R-spondin 1 (Rspo1; a Wnt agonist)5–14 plus Slit2 reduced ISC loss, mitigated gut impairment and protected animals from death, without concomitantly decreasing tumor sensitivity to chemotherapy. Rspo1 and Slit2 may thus act as therapeutic adjuvants to enhance host tolerance to aggressive chemoradiotherapy for eradicating metastatic cancers.
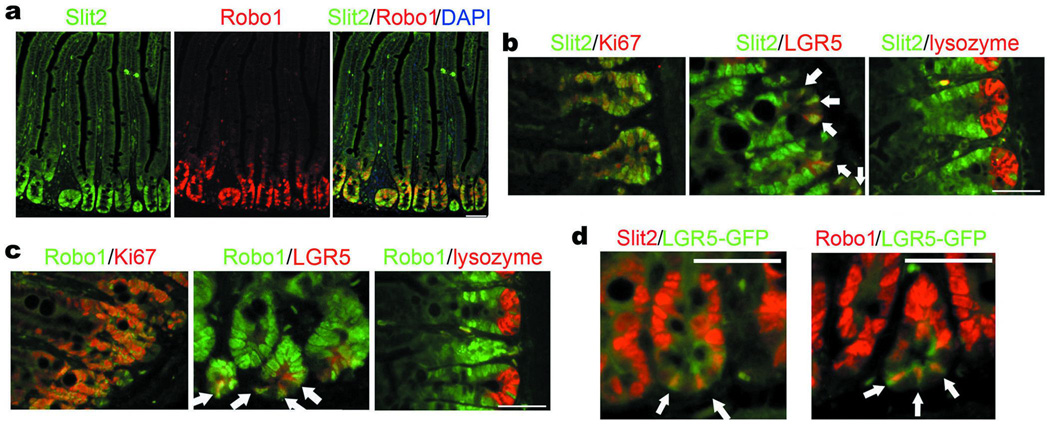
We found, by two-color fluorescent in situ hybridization (FISH), that Slit2 and Robo1 mRNAs not only co-localized, but also were expressed in markedly higher amounts in the crypts when compared to the villi in the small intestines of adult C57BL/6 (Wt) mice (Fig. 1a and Supplementary Fig. 1a). Ki67+-transient amplifying (TA) cells and LGR5+-ISCs expressed Slit2 and Robo1 mRNAs (Fig. 1b, c). In contrast, neither Slit2 nor Robo1 mRNA was visible in lysozyme+-Paneth cells. Using the intestinal specimens harvested from LGR5-EGFP-IRES-creERT2 (LGR5-GFP) mice15, we confirmed that Slit2 and Robo1 mRNAs were expressed by GFPhigh-ISCs at the bottom of the crypt (Fig. 1d). The distribution of Slit2 and Robo1 is apparently higher at the crypts than in the villi of the small intestine, which mirrors the well-characterized gradient of active β-catenin in the crypt-villus axis16.
Figure 1. Expression of Slit2 and Robo1 in mouse small intestine.
a, Expression and co-localization of Slit2 and Robo1 mRNAs in the crypts of small intestine. Slit2 and Robo1 mRNAs in the Wt small intestines were detected by using the DIG- or biotin-conjugated antisense Slit2 and Robo1 mRNA probes. Slides were counterstained with DAPI. Immunofluorescent images were observed under a laser scanning confocal microscope, and the recorded fluorescent images were then merged. b–d, Cellular distribution of mRNAs for Slit2 (b and d) and Robo1 (c and d). Slit2, Robo1, Ki67 (a marker for proliferating TA cells), LGR5 (a marker for ISCs), and lysozyme (a marker for Paneth cells) were found at the crypt of small intestines using FISH (Slit2 and Robo1) and immunofluorescent staining with their respective Abs (Ki67, LGR5 and lysozyme). Alternatively, the intestinal tissues isolated from LGR5-GFP mice were stained by the anti-GFP Ab to detect GFPhigh-cells (d). White arrows indicate LGR5+-cells (a–c) and GFPhigh-cells (d) co-localized with Slit2 or Robo1 mRNA. Results represent at least three separate experiments (five mice/group; 8 weeks old). Bars, 50 µm for a–d.
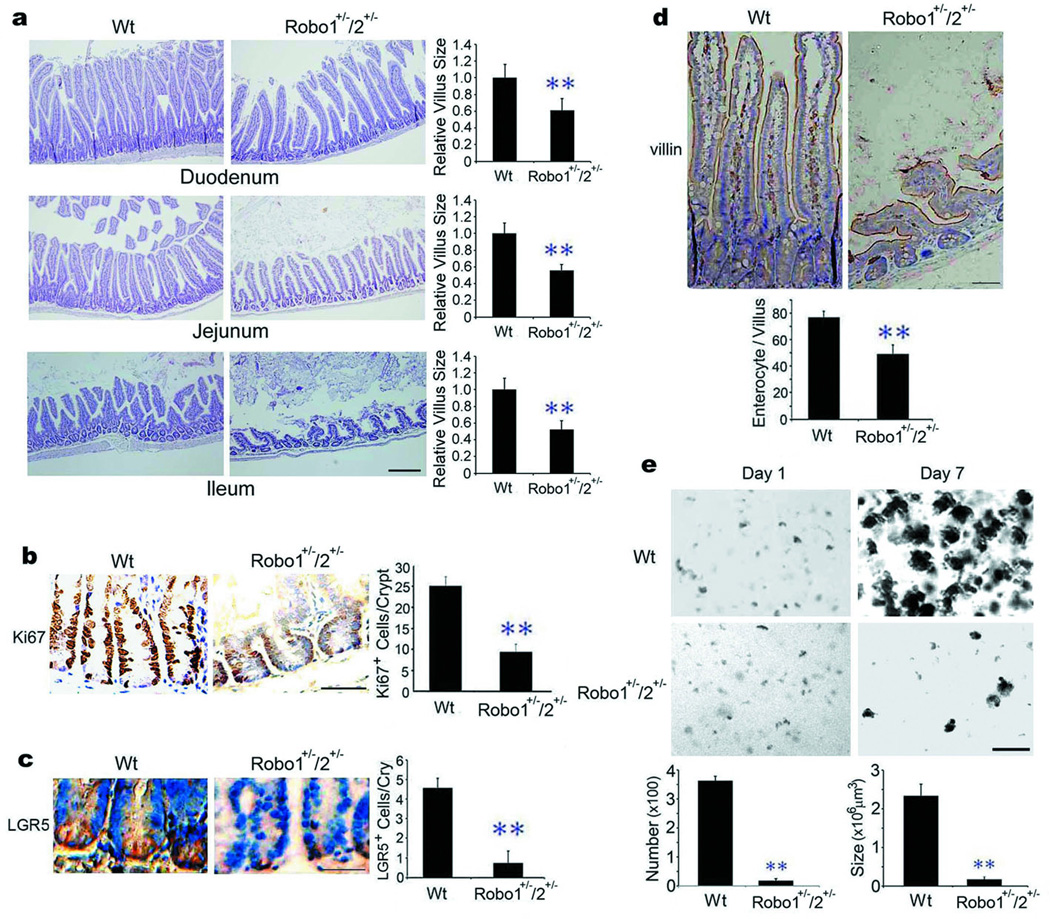
Complete or partial genetic deletion of Robo1 is embryonic lethal while mice with partial genetic deletion of both Robo1 and 2 are viable17,18. Thus, we examined the intestinal morphology in Robo1/2 double heterozygotes (Robo1+/−; Robo2+/− or Robo1/2 mutants). Robo1/2 mutants displayed reduced expression of Robo1 protein, but not Slit2 or α–tubulin (tub; Supplementary Fig. 2a). Notably, Robo2 mRNA was not detectable in the Wt small intestine, even though it clearly was present in the cerebellum (Supplementary Fig. 1b). Compared to Wt littermates, Robo1/2 mutants had noticeably sparser, shorter and floppier villi throughout the entire small intestine (Fig. 2a; Supplementary Fig. 3a), displaying markedly fewer Ki67+-TA cells (Fig. 2b), LGR5+-ISCs (Fig. 2c), villin+-enterocytes (Fig. 2d), and a reduced number of proliferating intestinal cells visualized by bromodeoxyuridine (BrdU) staining (Supplementary Fig. 4a). The mutant animals also had a higher level of endotoxin (Supplementary Fig. 5), a functional indicator of intestinal impairment13. Importantly, Robo1 single heterozygotes (Robo1+/− or Robo1 mutant) phenocopied the Robo1/2 double heterozygotes (Supplementary Fig. 6a–d), whereas no such alterations were detected in the intestines of Robo2 single heterozygotes (Robo2+/− or Robo2 mutant; Supplementary Fig. 7a–d). We also isolated intestinal crypts from Robo1/2 mutants and found that Robo1+/−/2+/− crypts failed to form intestinal organoids, also called mini-guts or enteroids5–8, as compared to their Wt littermates (Fig. 2e and Supplementary Fig. 8a for enlarged images). The expression of LGR5, CD133, Sox9, Bmi1 and mTert mRNAs as ISC markers19 was significantly reduced in the Robo1/2 mutant intestines relative to their Wt counterparts (Supplementary Fig. 9a). As Slit-Robo signaling re-models neovasculature3 and induces epithelial-mesenchymal transition4, we examined and found no aberrant localization and distribution of CD31+-vascular endothelial cells in the vasculatures, including those distributing alongside the TA compartment and the capillary bed in the villi (Supplementary Fig. 10a, d), or of α-smooth muscle actin+-intestinal smooth muscle cells (Supplementary Fig. 11a) in the intestines of Robo1/2 mutants as compared to their Wt littermates. These data collectively indicate that the Slit2-Robo1 interaction induces ISCs during physiologic maintenance of intestinal homeostasis.
Figure 2. Phenotypic aberration in Robo1+/−; Robo2+/− small intestine.
a, Morphology of small intestines. Paraffin-embedded intestinal sections from Wt littermates (Wt) and Robo1/2 mutants were stained with hematoxylin & eosin (H&E). Relative villus sizes were measured and statistically analyzed. b–d, Effects of partial Robo1/2 deficiency on intestinal cells. Intestinal sections from Wt littermates and Robo1/2 mutants were immunohistochemically stained for Ki67+-TA cells (b), LGR5+-ISCs (c) and villin+-enterocytes (d). The numbers of positive cells were counted in each crypt (b–d). e, Formation of intestinal organoids. The intestinal crypts isolated from Wt littermates and Robo1/2 mutants were cultured and the numbers and sizes of intestinal organoids were measured at day 7. Results represent fifty tissue specimens in each group (n=5) and the mean ± S.D. values. Bars, 200 µm for a, e and 50 µm for b–d. **, p<0.01.
To complement our genetic findings, we used R5, a monoclonal antibody (mAb) that binds to Robo1, but not Robo2–4, and neutralizes Slit2 binding to Robo13,4. Wt mice were treated daily for 6 days with an intraperitoneal injection of isotype-matched irrelevant mouse IgG (mIgG) or R5. As expected, R5, but not mIgG, reduced intestinal villus size (Supplementary Fig. 12a) and number (Supplementary Fig. 3b), decreased the numbers of Ki67+-TA cells (Supplementary Fig. 12b), LGR5+-ISCs (Supplementary Fig. 12c), villin+-enterocytes (Supplementary Fig. 12d) and BrdU+-intestinal cells (Supplementary Fig. 4b), without concomitantly affecting the distributions of vascular endothelial cells (Supplementary Fig. 10b, e) and smooth muscle cells (Supplementary Fig. 11b). Compared to mIgG, R5 potently inhibited the in vitro formation of intestinal organoids5–8 isolated from Wt mice (Supplementary Figs. 12e, 8b for enlarged images), the expression of LGR5, CD133, Sox9, Bmi1 and mTert mRNAs19 (Supplementary Fig. 9b).
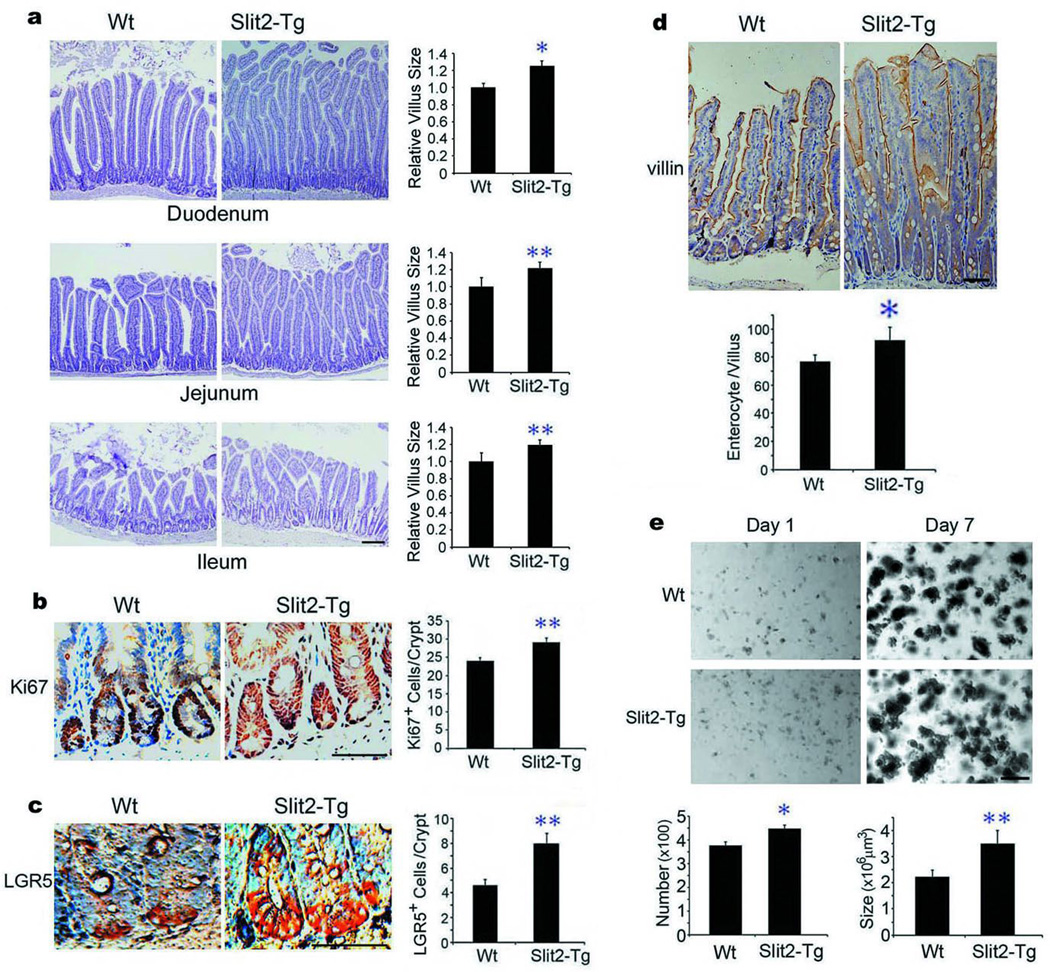
We next asked whether ectopically expressed Slit2 (Robo1 ligand) might augment ISCs and their daughter cells, leading to villus hypertrophy. To this end, we examined the intestinal phenotypic changes in Slit2 transgenic (Slit2-Tg) mice driven by the pCMV promoter for efficient, but non-selective expression of human Slit2 transgene20. As compared to Wt mice, Slit2-Tg mice displayed an increased mosaic expression of Slit2 protein, but not Robo1 orα–tubulin (tub), in the small intestines (Supplementary Fig. 2b). When compared to Wt mice, Slit2-Tg mice had noticeably thicker, longer, enlarged and outnumbered villi (Fig. 3a, Supplementary Fig. 3c), with increased numbers of Ki67+-TA cells (Fig. 3b), LGR5+-ISCs (Fig. 3c), BrdU+-intestinal cells (Supplementary Fig. 4c) and villin+-enterocytes (Fig. 3d), without concomitantly affecting the distribution of CD31+-vascular endothelial cells (Supplementary Fig. 10c, f) or α-smooth muscle actin+-smooth muscle cells (Supplementary Fig. 11c). The compartment of Ki67+-TA cells appeared to be enlarged, which could be caused by accelerated proliferation (Supplementary Fig. 4c) and/or aberrant directional migration of TA cells triggered by Slit-Robo signaling3,4. Notably, the intestinal crypts isolated from Slit2–Tg mice formed more and larger intestinal organoids5–8 relative to their Wt counterparts (Fig. 3e; Supplementary Fig. 8c for enlarged images). Compared to Wt mice, the expression of LGR5, CD133, Sox9, Bmi1 and mTert mRNAs19 (Supplementary Fig. 9c) was significantly augmented in the small intestine of Slit2-Tg mice. Taken together, our results indicate the functional significance of Slit-Robo signaling in intestinal regeneration through modulation of ISCs.
Figure 3. Slit2 transgene enhances small intestinal regeneration.
a, Effects of Slit2 overexpression on intestinal morphology. Small intestines from Wt and Slit2-Tg mice were stained with H&E, and the relative villus sizes were measured and statistically analyzed. b–d, Ectopic Slit2 expression affects the number and distribution of intestinal cells. Small intestines from Wt and Slit2-Tg mice were immunohistochemically stained for Ki67+-TA cells (b), LGR5+-ISCs (c) and villin+-enterocytes (d). The numbers of positive cells were counted in each crypt. e, The intestinal crypts isolated from Wt and Slit2-Tg mice were in vitro cultured and the numbers and sizes of intestinal organoids were measured at day 7. Results represent fifty tissue specimens in each group (five mice/group) and the mean ± S.D. of 10 tissue sections/mouse. Bars, 100 µm for a, e and 50 µm for b–d. *, p<0.05; **, p<0.01.
We further tested whether recombinant Slit2 (rSlit24) could substitute and potentiate recombinant Rspo1 (rRspo1; Supplementary Fig. 13a) for inducing intestinal organoids in vitro. As predicted, rSlit2 acted synergistically with rRspo1 to promote in vitro formation and growth of intestinal organoids in terms of their number and size (Supplementary Figs. 14a, 8d for enlarged images). In the absence of rRspo1 (rRspo1-), rSlit2 alone, at 0.5 or 1 µg/ml, was capable of inducing intestinal organoids. The intestinal crypts isolated from Slit2-Tg mice also formed intestinal organoids without added rRspo1 or rSlit2. Using single cell sorting gated for GFP5, we isolated GFPhigh-ISCs from LGR5-GFP mice15 and found that rSlit2 by itself induced intestinal organoids (Supplementary Figs. 14b, 8e for enlarged images). More importantly, rSlit2 acted cooperatively with rRspo1 in the formation of intestinal organoids in vitro. To further demonstrate whether Slit2 could induce the self-renewal and pluripotential capacities of ISCs, we performed serial passages (up to 4 passages; every two weeks for each passage) of cultured intestinal organoids and found that rSlit2 not only substituted for rRspo1, but also functioned synergistically with rRspo1, using both the isolated Wt intestinal crypts (Supplementary Fig. 15a) and the sorted GFPhigh-ISCs (Supplementary Fig. 15b).
Considering that LGR5 is a specific ISC marker15 and a direct targeting gene of canonical Wnt signaling2,5–8, we treated LGR5-GFP mice with mIgG and R5 and found that compared to untreated (normal) or mIgG-treated LGR5-GFP mice, R5 inhibited the number of GFPhigh-ISCs (~50% inhibition; Supplementary Fig. 16a). Compared to untreated LGR5-GFP mice, treatment with rRspo1 plus rSlit2 increased the number of GFPhigh-ISCs (~30% increase; Supplementary Fig. 16b). Treatment with rRspo1 or rSlit2 alone also slightly augmented the number of GFPhigh-ISCs. Our results thus provide in vivo evidence for the biological importance of our newly discovered Slit2 and Rspo1 cooperation in induction of ISCs and activation of Wnt/β-catenin signaling.
We also tested the functional significance of Robo1 in Rspo1-induced intestinal repair in vivo. Consistent with previous reports9–14, intravenous administration of rRspo1 potently promoted villus growth in the Wt jejunum (Supplementary Fig. 17a). Surprisingly, rRspo1 (0.1 mg/mouse/day for 5 days) failed to accelerate growth of intestinal epithelial cells in Robo1/2 mutants. Compared to their rRspo1-treated Wt counterparts, rRspo1 also failed to significantly augment the numbers of LGR5+-ISCs (Supplementary Fig. 17b) and Ki67+-TA cells (Supplementary Fig. 17c) in the jejunum crypts of Robo1+/−; Robo2+/− mice. A prolonged pulse of rRspo1 (0.1 mg/mouse/day for 10 days) further robustly enhanced crypt size (Supplementary Fig. 18a) and augmented the numbers of Ki-67+-TA cells (Supplementary Fig. 18b), BrdU+-intestinal cells (Supplementary Fig. 18c) and LGR5+-ISCs (Supplementary Fig. 18d) in Wt littermates, but not in Robo1/2 mutants. As predicted, treatment of Wt littermates with rRspo1 markedly induced the cytoplasmic and nuclear translocation of β-catenin (Supplementary Fig. 19a) and elevated the expression of c-Myc (Supplementary Fig. 19b) as compared to Robo1/2 mutants.
Stimulation of Wnt/β-catenin signaling with Rspo1 can ameliorate 5-FU and radiation-induced gut damage, including radiation-induced gastrointestinal syndrome (RIGS)9–13. Relatedly, we found that the therapeutic dosage of 5-fluorouracil (5-FU)9, a well-characterized chemotherapy medicine, drastically shortened the villus length (Supplementary Fig. 20a) and reduced the numbers of LGR5+-ISCs (Supplementary Fig. 20b) and Ki67+-TA cells (Supplementary Fig. 20c) in the Wt jejunum, but not in the Slit2-Tg jejunum. These findings attest to the functional significance of Slit2 for promoting chemotherapy-induced intestinal repair.
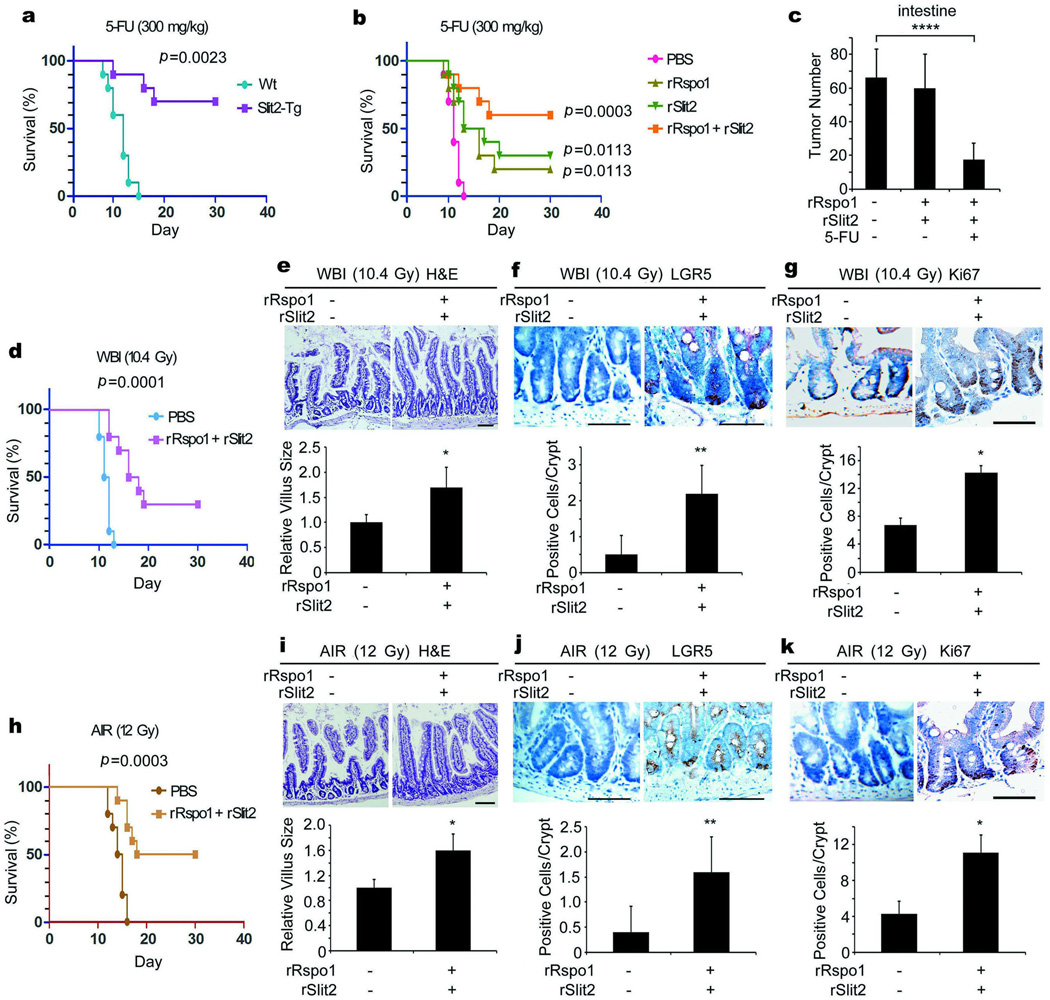
Considering our findings that Slit2 potentiated Rspo1-induced ISCs and synergized with Rspo1 for reducing chemotherapy-induced gut injury, we asked whether Slit2, in combination with Rspo1, could induce ISCs and consequently prolong overall survival in mice receiving lethal challenges of chemoradiation. To our surprise, in mice with the Slit2 transgene, there was a 70% survival rate in mice receiving a lethal dose of 5-FU21, whereas this same dosage caused the death of all Wt mice within two weeks (Fig. 4a). The lethal dose of 5-FU abolished >90% GFPhigh-ISCs (Supplementary Fig. 21a). However, a 3-day treatment of rSlit2 or rRspo1 alone protected ~40% GFPhigh-ISCs. Most exciting was the finding that the same regiment of rRspo1 plus rSlit2 preserved ~80% GFPhigh-ISCs. Our findings thus indicate that Slit2 acts synergistically with Rspo1 for cooperative induction of ISCs, leading to prolongation of overall survival in response to the lethal dose of chemotherapy.
Figure 4. Slit2 and Rspo1 cooperatively induce ISCs, reduce intestinal damage and prolong overall survival.
a, Slit2 transgene increases resistance to 5-FU. A single lethal dose of 5-FU was given to Wt and Slit2-Tg mice and the death rates were recorded. b, c, Slit2 plus Rspo1 prolongs overall survival in tumor-bearing mice. rSlit2 or rRspo1 alone or in combination were given intravenously to DSS-treated ApcMIN/+ mice for 3 days. On day 2, a single lethal dose of 5-FU was given and the death rates were recorded (b). The tumor numbers in the small intestines of surviving mice were also counted at day 30 (c). d–k, Slit2 plus Rspo1 decreases radiation-induced death. Wt mice were treated with rSlit2 plus rRspo1. On day 2, they were irradiated and the death rates were recorded (d, h). The villus sizes (e, i), the numbers of LGR5+-ISCs (f, j), and Ki-67+-TA cells (g, k) in the jejunum were also determined. Results are derived from 10 or 12 mice/group (a–d, h) or the mean ± S.D. of 10 tissue sections/mouse (e–g, i–k). Kaplan-Meier survival curves were constructed and analyzed by a log rank test (a, b, d, h). Bars, 50 µm. *, p<0.05; **, p<0.01; ****, p<0.0001
Because aberrant Wnt signaling causally contribute to colorectal carcinogenesis2, because Slit2 and Robo1 expression inversely correlates with the overall survival rate in colorectal cancer4, and because recurrent gene fusion of Rspo2 and 3 contributes to colorectal cancer22, we explored whether a short 3-day pulse of rSlit2 and/or rRspo1 could accelerate the development of intestinal carcinogenesis and/or decrease its sensitivity to chemoradiotherapy. To test this, ApcMIN/+ mice with spontaneous intestinal adenoma were treated with dextran sulfate sodium (DSS) to induce inflammation related intestinal carcinogenesis, a murine model that is thought to closely mimic multi-factorial human colorectal cancer23. Treatment of DSS-treated ApcMIN/+ mice with rSlit2 or rRspo1 alone led to a 20–30% survival rate upon the lethal dosage of 5-FU (Fig. 4b). Importantly, a combination of rSlit2 and rRspo1 led to a 60% survival rate (p=0.0023 between the rRspo1 plus rSlit2 group compared to either group alone), demonstrating the functional cooperation between Slit2 and Rspo1 leading to increased host tolerance to the lethal dose of chemotherapy in this murine model of carcinogenesis.
Consistent with previous reports using recombinant flagellin in xenografted mouse sarcoma and malignant melanoma24 and adenoviral Rspo1 in xenografted human colorectal carcinoma12, neither acceleration of intestinal cancer development nor desensitization to 5-FU chemotherapy was detected with our rRspo1 and rSlit2 combination treatment (Fig. 4c). In contrast, upon administration of rRspo1 and rSlit2, a single “lethal” dose of 5-FU drastically eliminated the number of intestinal tumors, suggesting that Rspo1 plus Slit2 treatment may act as adjuvants before, during or after intensive chemotherapy for cancer eradication.
Furthermore, the combination of rSlit2 plus rRspo1 led to a 30% survival rate in mice receiving a lethal dose of whole body irradiation (WBI; Fig. 4d) and a 50% survival rate in mice receiving a lethal dose of abdominal irradiation (AIR; Fig. 4h). We also observed concomitant prolongations of the villus length (Fig. 4e, i), augmentations of LGR5+-ISCs (Fig. 4f, j) and Ki67+-TA cells (Fig. 4g, k) in the jejunum, which are in analogous to previous finding that LGR5+-ISCs in the small intestine are resistant to radiation25. Mechanistically, although the lethal dose of WBI abolished >80% GFPhigh-ISCs (Supplementary Fig. 21b), a 3-day treatment of rRspo1 or rSlit2 alone protected ~40% GFPhigh-ISCs while rRspo1 plus rSlit2 preserved >80% GFPhigh-ISCs in LGR5-GFP mice.
In summary, our study indicates that Slit2 and Rspo1 cooperatively induce ISCs for intestinal homeostasis and repair and significantly prolong overall survival following lethal doses of chemoradiotherapy. To the best of our knowledge, the combined Slit2/Rspo1 treatment, used as an adjuvant approach, is the first example to demonstrate the feasibility of inducing endogenous adult tissue-specific stem cells for organ and tissue repair with far-reaching medical impact.
METHODS
Mouse experiments and histology
C57BL6/J (Wt; Stock No. 005304), LGR5-EGFP-IRES-creERT2 (LGR5-GFP; Stock No. 008875)15, and ApcMIN/+ (Stock No. 002020) mice were purchased from Jackson Laboratory. Robo1+/−; Robo2+/− mice17,18 were purchased from MMRRC/University of Missouri and their Wt littermates were used as controls. During the process of expanding our cohort of Robo1+/−; Robo2+/− mice, we unexpectedly obtained several adult single Robo1 (Robo1+/−; less than 3% of total mouse population) or Robo2 (Robo2+/−) heterozygotes that were not embryonically lethal. Notably, we have never obtained any viable Robo1−/− homozygotes after multiple trials. Slit2-Tg mice were generated and characterized as described20. Male and female mice were equally divided without randomization. They were used at 8 weeks old unless specifically indicated. For measurement of cell proliferation, mice were injected with BrdU (1 mg/100 grams of body weight) for 2 h prior to sacrifice. For antibody treatment, Wt mice were injected intraperitoneally with isotype-matched irrelevant mIgG or R5 monoclonal Ab3,4 (1 mg/mouse/day for 6 consecutive days). For induction of inflammation-related intestinal carcinogenesis, ApcMIN/+ mice were treated with 2% dextran sulfate sodium (DSS) in drinking water for one week and were used experimentally two weeks later23. For “chemoradiation” experiments, Wt and DSS-treated ApcMIN/+ mice (10 weeks old) were injected intravenously with Slit2 or Rspo1 alone or in combination (0.1 mg/mouse/day for 3 consecutive days). On day 2, they were given 5-FU intraperitoneally (30 mg/kg/mouse/day for 5 days for the therapeutic dose9 or 300 mg/kg once for the lethal high dose21) or were irradiated (10.4 Gy/mouse once for WBI or 12 Gy/mouse once for AIR12). Alternatively, Slit2-Tg mice20 (10 weeks old) were given the lethal high dose of 5-FU intraperitoneally (300 mg/kg once21). Tissue sections of small intestines were stained with hematoxylin & eosin (H&E) and the relative villus sizes and numbers at the duodenum, jejunum and ileum were double-blindly measured. The length ratios of 1:3:2 of the entire small intestine were defined as the duodenum, jejunum and ileum26, respectively. Serum LPS levels were measured using the limulus amebocyte lysate assay (Lonza). Mouse experiments were approved by the University Committee on Use and Care of Animals (UCUCA) of the University of Michigan.
Fluorescent in situ hybridization
cDNA segments of mouse Slit2 (base pair 3960–4566), mouse Robo1 (base pair 3443–4956) and mouse Robo2 (base pair 2328–4527) were reverse transcribed and labeled with digoxigenin (DIG) or biotin according to the manufacturer’s instructions (Roche). Intestinal tissues were fixed with 4% paraformaldehyde and tissue sections (5-µm thick) were incubated with 1 µg/ml linearized DIG- or biotin-labeled antisense or sense RNA probe27. For immunofluorescent staining, Western Blocking Reagent (11921673001, Roche) was diluted 1 to 10 with 20 mM Tris-HCl, pH 7.4, containing 0.1% Tween-20 (TBST) for blocking non-specific staining and for diluting all antibodies, including 1:1000 dilution of sheep anti-DIG Ab (11333089001, Roche) or mouse anti-biotin mAb (200-002-211, Jackson ImmunoResearch), and 1:200 dilution of HRP-conjugated rabbit anti-sheep Ab (313-035-047, Jackson ImmunoResearch). Tyramide Signal Amplification Kits (Alexa Fluor 488 and 555; Invitrogen) were used according to the manufacturer’s protocol.
Immunofluorescent and immunohistochemical Staining
Intestinal tissues were fixed with 4% paraformaldehyde, sectioned (5-µm thick) and permeabilized with 0.05% Triton X-100 in phosphate buffered saline, pH 7.4 (PBS). Samples were blocked with 1% bovine serum albumin (BSA; Sigma) and incubated with primary Ab at 37°C for 1 h, including Abs against Ki67 (ab15580, abcam), LGR5 (ab75850, abcam), lysozyme (ab36362, abcam), CD31 (ab28364, abcam), α-smooth muscle actin (ab5694, abcam), villin (610358, BD Biosciences), c-Myc (sc-40, Santa Cruz Biotechnology), GFP (NB600-303, Novus Biologicals) and BrdU (B2531, Sigma Aldrich). After washing extensively, samples were incubated with appropriate fluorescent dye- or HRP-conjugated secondary Ab at 37°C for 1 hour. Sections were counterstained with 4',6-diamidino-2-phenylindole (DAPI) or H&E. Slides were then washed and mounted for observation under a scanning confocal microscope (Leica TCS SP2) or a fluorescence stereomicroscope (Leica M205 FA). The immunohistochemical staining data were quantified double blindly using ImageTool Software.
In vitro culture of intestinal crypts
The intestinal crypts of mouse small intestines were isolated28 and cultured in vitro5–8. Specifically, mouse ileums (~6 cm) were dissected out of the animals, flushed with ice-cold sterile PBS, cut open lengthwise and into 1 cm pieces, and transferred into the Petri dish with ice-cold sterile PBS supplemented with 1% Pen/Strep (Invitrogen). This step was repeated twice. They were then transferred to a 50-ml conical tube containing 30 ml of ice-cold sterile shaking buffer (PBS supplemented with 15 mM EDTA and 1% Pen/Strep) and rocked in the cold room for 30 minutes, then vortexed at maximal speed for a total of 2 minutes in 30 second intervals. The shaking buffers containing separated crypts were filtered through a 70-μm filter into a new 50-ml conical tube, counted, transferred to a round-bottom tube (500 crypts/tube), and centrifuged at 200g at 4°C for 10 minutes. After gently removing the supernatant, the crypt pellets were resuspended in 20 µl of the gut media [Advanced DMEM/F12, 1% L-glutamine, 1% Pen/Strep, 10 μM HEPES (all from Invitrogen), 1% N2 supplement (R&D Systems), 2% B27 supplement (Invitrogen), 0.5 µg/ml rRspo1 (Supplementary Fig. 13b), 0.1 µg/ml rNoggin (Supplementary Fig. 14b), 0.1 µg/ml EGF (R&D Systems), 0.1 µg/ml rWnt3a (R&D Systems), or 0.5 or 1 µg/ml rSlit24]. These crypts were mixed with ice-cold 50 µl Matrigel (BD Biosciences) and plated onto one well of the 24-well cell culture plate, using a pre-chilled pipette tip. The plate was placed in the 37°C incubator for 30 minutes and an aliquot of 0.5 ml gut media was overlaid. The media was replaced every 2–4 days.
Isolation and cell sorting of GFP-positive ISCs expressing LGR5-GFP was performed according to the previous publication10. Specifically, the small intestinal crypts of LGR5-GFP mice were isolated and dissociated with TrypLE express (Invitrogen) and 2,000 U/ml DNase (Sigma Aldrich) at 37°C for 30 min. Dissociated cells were passed through a 20-μm cell strainer and washed with sterile PBS. GFPhigh-ISCs were isolated using Moflo cell sorter. An aliquot of 10,000 sorted GFPhigh-ISCs were suspended in 20 µl gut media, mixed well with 50 µl Matrigel, and transferred to one well of 24-well plate. The plate was placed in the 37°C incubator for 30 minutes and an aliquot of 0.5 ml gut media supplemented with Y-27632 (10 μM, to prevent anoikis; Sigma Aldrich) was overlaid. The efficiency of organoids generation from isolated GFPhigh-ISCs was ~3%, which was similar to the previous reports5,29. Indicated growth factors were added every other day and the entire medium was changed every 4 days. For serial passage, the cultured intestinal organoids were removed from the Matrigel culture and mechanically dissociated into the single crypt domains. After mixing with gut media and Matrigel, they were transferred to the new well and an aliquot of 0.5 ml gut media was overlaid. This procedure was repeated during a two week period, without loss of replicating efficiency.
Isolation and quantitation of GFP+-ISCs
The small intestinal crypts of LGR5-GFP mice were isolated and dissociated with TrypLE express (Invitrogen) and 2,000 U/ml DNase (Sigma Aldrich) at 37°C for 30 min. Dissociated cells were passed through a 20-μm cell strainer and washed with sterile PBS. GFPhigh-ISCs were stained with the anti-GFP Ab (NB600-303, Novus Biologicals), followed by an appropriate FITC-conjugated secondary Ab for quatification by flow cytometry.
qRT-PCR
All mouse RT2 qPCR primer pairs were purchased from SA Biosciences. The crypts of mouse small intestine were isolated28 for qRT-PCR as previously described4. Notably, we routinely isolated less amounts of total mRNAs from the atrophied Robo1+/−/2+/− and R5-treated intestines and more amounts of total mRNAs from the hypertrophied Slit2-Tg intestines than the Wt intestines (data not shown). However, the adjusted amounts of total isolated mRNAs were used as the starting materials in the identical quantity for the purpose of comparison.
Immunoblotting
The isolated intestinal crypts were washed with ice-cold phosphate buffered saline, pH 7.4 (PBS) and lysed with ice-cold radioimmunoprecipitation assay lysis buffer [50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM sodium orthovanadate 10 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 2 µg/ml aprotinin, 2 µg/ml leupeptin, 1 µg/ml pepstatin A, 15 µg/ml benzamidine, 0.5% Nonidet P-40, 0.15% bovine serum albumin (BSA) and 10% glycerol] at 4°C for 1 h. Samples were centrifuged at 12,000g for 15 min at 4°C. Samples were subjected to SDS-PAGE, transferred to PVDF membranes (EMD Millipore) and detected with appropriate primary Abs followed by horseradish peroxidase-conjugated goat anti-mouse or rabbit immunoglobulin G (IgG). The blotting signals were detected using SuperSignal West Dura Extended Duration Substrate (Pierce). Quantitative analyses of immunoblotting signals on Fuji Films were obtained by densitometry analysis using LAS4000 Image Software. Notably, we routinely isolated less amounts of total proteins from the atrophied Robo1+/−; Robo2+/− and R5-treated intestines and more amounts of total proteins from the hypertrophied Slit2-Tg intestines than the Wt intestines (data not shown). However, the adjusted amounts of total isolated proteins were used as the starting materials in the identical quantity for the purpose of comparison. The primary Abs against pan-Slit and Robo1 (S1 and R43,4), α-tubulin (T6074, Sigma Aldrich) and β-actin (A1978, BD Biosciences) were used.
Baculovirus expression of Rspo1 and Noggin
The cDNAs of human Rspo1 and Noggin (Open Biosystems) were amplified for construction of 6-His fusion proteins, using the forward primer 5’-TTGCGGCCGCATGCGGCTTGGGCTGTG-3’ and the reverse primer 5’-GGGAATTCGGCAGGCCCTGCAGATGTGAGTGGCC-3’ for Rspo1; and the forward primer 5’-TAGCGGCCGCATGGAGCGCTGCCCC-3’ and the reverse primer 5’-GGGAATTCGCACGAGCACTTGCACTCGGAATGATGG-3’ for Noggin. The inserts of Rspo1 and noggin were digested with NotI/EcoRI. They were ligated into the pVL1392 vector (BD Pharmingen).
Recombinant Rspo1 and Noggin were expressed in Sf9 insect cells using the baculovirus expression system (BaculoGold; BD Pharmingen) and purified to homogeneity from the serum-free supernatant of Sf9 cells infected with their respective viral stocks (MOI ~2 ×108/ml) by Talon metal affinity chromatography (BD Clontech)30. Endotoxin levels of these isolated recombinant proteins were <0.1 unit/mg of proteins measured by limulusamoebocyte lysate (LAL) from Cape Cod.
Statistical analysis
The experimental data were statistically analyzed by Student’s t-test or Mann-Whitney test. Kaplan-Meier survival curves were constructed and analyzed by a log rank test. The p value less than 0.05 or 0.01 was considered statistically significant or very significant, respectively.
Supplementary Material
Acknowledgements
We thank Li Ma for generating recombinant proteins, Michael H. Geng for technical assistances, Xuesong Yang for helping with the in situ hybridization, and Judith Connett for critical reading of the manuscript. This work was supported by grants from the National Institutes of Health (CA126897 and CA181039 to J.- G.G.).
Footnotes
Supplemental Information is available in the online version of the paper.
Author Contributions W.-J.Z. and Z.H.G. carried out all experiments, collected and analyzed data, J.R.S. contributed in vitro intestinal crypt culture, and J.-G.G. proposed hypothesis, designed experiments and wrote the manuscript.
The authors declare competing financial interests.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H, Nusse R. Wnt/β -catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Xiao Y, Ding B, Zhang N, Yuan X, Gui L, Qian K, Duan S, Chen Z, Rao Y, et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4:19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhou WJ, Geng ZH, Chi S, Zhang W, Niu XF, Lan SJ, Ma L, Yang X, Wang LJ, Ding YQ, et al. Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res. 2011;21:609–626. doi: 10.1038/cr.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 6.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung P, Sato T, Merlos-Suárez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, et al. Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 8.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, De Vera J, Narushima S, Beck EX, Palencia S, Shinkawa P, Kim KA, Liu Y, Levy MD, Berg DJ, et al. R-Spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Kim KA, De Vera J, Palencia S, Wagle M, Abo A. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/β-catenin pathway. Proc. Natl. Acad. Sci. USA. 2009;106:2331–2336. doi: 10.1073/pnas.0805159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhanja P, Saha S, Kabarriti R, Liu L, Roy-Chowdhury N, Roy-Chowdhury J, Sellers RS, Alfieri AA, Guha C. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 4:e8014. doi: 10.1371/journal.pone.0008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takashima S, Kadowaki M, Aoyama K, Koyama M, Oshima T, Tomizuka K, Akashi K, Teshima T. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J. Exp. Med. 2011;208:285–294. doi: 10.1084/jem.20101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 15.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 16.Solanas G, Batlle E. Control of cell adhesion and compartmentalization in the intestinal epithelium. Exp. Cell Res. 2011;317:2695–2701. doi: 10.1016/j.yexcr.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Grieshammer U, Le Ma, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev. Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- 18.Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- 19.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye BQ, Geng ZH, Ma L, Geng JG. Slit2 regulates attractive eosinophil and repulsive neutrophil chemotaxis through differential srGAP1 expression during lung inflammation. J. Immunol. 2010;185:6294–6305. doi: 10.4049/jimmunol.1001648. [DOI] [PubMed] [Google Scholar]
- 21.Martine DS, Stolfi RL, Sawyer RC, Sawyer RC, Spiegelman S, Young CW. High-dose 5-fluorouracil with delayed uridine“rescue” in mice. Cancer Res. 1982;42:3964–3970. [PubMed] [Google Scholar]
- 22.Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman V, Jaiswal BS, et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phutthaphadoong S, Yamada Y, Hirata A, Tomita H, Hara A, Limtrakul P, Iwasaki T, Kobayashi H, Mori H. Chemopreventive effect of fermented brown rice and rice bran (FBRA) on the inflammation-related colorectal carcinogenesis in ApcMin/+ mice. Oncol. Rep. 2012;23:53–59. [PubMed] [Google Scholar]
- 24.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua G, Thin TH, Feldman R, Haimovitz-Friedman A, Clevers H, Fuks Z, Kolesnick R. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143:1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan LP, Wang HH, Wang DQ. Cholesterol absorption is mainly regulated by the jejunal and ileal ATP-binding cassette sterol efflux transporters Abcg5 and Abcg8 in mice. J. Lipid Res. 2004;45:1312–1323. doi: 10.1194/jlr.M400030-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Chrisman H, Weijer CJ. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development. 2008;135:3521–3530. doi: 10.1242/dev.023416. [DOI] [PubMed] [Google Scholar]
- 28.Booth C, O’Shea JA, Potten CS. Maintenance of functional stem cells in isolated and cultured adult intestinal epithelium. Exp. Cell Res. 1999;249:359–366. doi: 10.1006/excr.1999.4483. [DOI] [PubMed] [Google Scholar]
- 29.Merlos-Suárez A, Barriga FM, Jung P, Iglesias M, Céspedes MV, Rossell D, Sevillano M, Hernando-Momblona X, da Silva-Diz V, Muñoz P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Wang HB, Wang JT, Zhang L, Geng ZH, Xu WL, Xu T, Huo Y, Zhu X, Plow EF, Chen M, Geng JG. P-selectin primes leukocyte integrin activation during inflammation. Nat. Immunol. 2007;8:882–892. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.