Abstract
Retinoic acids (13-cis and 13-trans) are known teratogens, and their precursor is retinol, a form of vitamin A. In 1995, Rothman et al demonstrated an association between excessive vitamin A, >10,000 IU/day, during the first trimester of pregnancy and teratogenic effects, particularly in the central nervous system. However, vitamin A deficiency has long been known to be deleterious to the mother and fetus. Therefore, there may be a narrow therapeutic ratio for vitamin A during pregnancy that has not previously been fully appreciated. Neurodevelopmental disorders may not be apparent by macroscopic brain examination or imaging, and proving the existence of a behavioral teratogen is not straightforward. However, an excess of retinoic acid and some neurodevelopmental disorders are both associated with abnormalities in cerebellar morphology. Physical and chemical evidence strongly supports the notion that beta carotene crosses the placenta and is metabolized to retinol. Only very limited amounts of beta carotene are stored in fetal fat cells as evidenced by the fact that maternal fat is yellow from beta carotene, whereas non-brown neonatal fat is white. Furthermore, newborns of carotenemic mothers do not share the yellow complexion of their mothers. The excess 13-trans retinoic acid derived from metabolized beta carotene in the fetus increases the concentration of the more teratogenic 13-cis retinoic acid since the isomerization equilibrium is shifted to the left. Therefore, this paper proposes that consideration be given to monitoring all potential sources of fetal 13-cis and 13-trans retinoic acid, including nutritional supplements, dietary retinol, and beta carotene, particularly in the first trimester of pregnancy.
Keywords: maternal nutrition, beta carotene, retinol, retinoic acid, neurodevelopmental disorders, vitamin A
Introduction
The purpose of this paper is to raise awareness among parents, educators, and medical practitioners that maternal consumption of precursors of retinoic acid may be associated with a subset of children who suffer from neurodevelopmental disorders. This notion is based on the known teratogenic effects of retinoic acids on the central nervous system,1–3 the calculated dose of retinol and beta carotene ingested by the mother during early pregnancy, and strong evidence that the fetus metabolizes beta carotene to retinoids and has limited ability to store beta carotene in fat cells.4–7
In 1995, Rothman et al demonstrated that high maternal dietary intake of vitamin A in the form of retinol, ie, >10,000 IU/day, particularly in the first trimester, may be associated with cranial-neural-crest birth defects.8 Using a smooth regression curve, this study of 22,748 pregnant women and their offspring identified 1 infant in 57 with malformations that could be attributed to the intake of supplements of preformed vitamin A at a dose >10,000 IU/day. The frequency of defects was concentrated among babies born to women who had consumed high levels of vitamin A before the seventh week of gestation. Methodologies used in this study have been criticized, including aggregating heart defects as neural crest defects, estimating missing information on retinol supplementation and demonstrating the vitamin A threshold of >10,000 IU/day.9 Subsequent studies have disputed the 10,000 IU/day threshold based on non-human primate studies and small clinical trials in humans.10
Evidence to support the notion that a toxin is responsible for some neurodevelopmental disorders
A toxin that may cause neurodevelopmental disorders must be able to cross not only the placenta, but also the blood brain barrier (BBB). Because the brain is the most selective and protected organ in the human body, agents that can cross the BBB must meet specific criteria. Lipinski has proposed such criteria, which include a molecular weight < 400 Daltons, 3 hydrogen donors, 7 hydrogen acceptors, and Kow < 5.11 Retinoic acids, which are derivatives of retinol, meet Lipinski’s criteria and are known to cause central nervous system defects in the fetus.
Since retinoic acids are protein bound and not easily metabolized and excreted in humans, the fetus is subjected to constant exposure to the toxic effects of these agents. Furthermore, as a child develops, neural mitosis slows considerably, and the concentration of toxins cannot be diluted by increasing cytoplasm through cell division, thus prolonging the exposure to a toxin.
Finally, neonatal levels of 13-cis retinoic acid are proportionately higher than all-trans retinoic acid levels when compared to maternal levels, suggesting that, in newborns, the equilibrium of cis and trans isomers is shifted toward the more teratogenic 13-cis isomer.12 This is an important finding and points to a deficit in studies that do not demonstrate an association between excess vitamin A and birth defects, as most do not measure levels of retinoic acid isomers in the fetus or newborn.
Evidence that links retinoic acid to cerebellar lesions and neurodevelopmental disorders
The cerebellum was once thought to be a region of the brain involved only with sensory motor function, coordination, and balance, but it is now known to contribute to executive functions, language, working memory, and attention probably via the extensive fronto-corticocerebellar circuits.13 Patients who suffer from cerebellar dysgenesis demonstrate language and communication difficulties.14 Retinoic acid regulates cell differentiation within the central nervous system, and excessive levels of retinoic acid are well known to create abnormalities in the hindbrain of the developing primate embryo, including the cerebellum.15 Imaging studies in patients with neurodevelopmental disorders are not conclusive for a definitive lesion; however, in a number of studies, the cerebellum is abnormal.16 Therefore, an excess of retinoic acid could produce abnormalities in the fetal cerebellum, similar to those found in primates that could be expressed as symptoms of a neurodevelopmental disorder.
The Fate of Beta Carotene in the Fetus
At this time, the storage, metabolism, and excretion of beta carotene in the fetus is not completely understood, but very strong evidence suggests that the fetus metabolizes beta carotene and has limited ability to store beta carotene. A number of studies have shown that, in cord blood (umbilical vein and artery), levels of beta carotene are ≤10% of maternal levels.2,17–20 In the past, this low level of beta carotene in cord blood has been attributed to decreased permeability of beta carotene across the placenta. However, a more likely interpretation is that the fetus rapidly converts beta carotene to retinol, and the excess retinol is stored in liver stellate cells as retinol ester.21,22 Dimenstein et al postulated that the large difference in maternal and fetal beta carotene levels may reflect enhanced metabolism by the fetus, but the clinical significance of retinoic acid levels was not fully appreciated.5,6 Beta carotene has a molecular weight of 537 Daltons, no hydrogen bond donors or acceptors, neutral charge, and XLog P3 of 13.5, all of which favor crossing the placenta into the fetal circulation.23,24 Even considering the growth of the fetus and decreased unidirectional permeability of beta carotene from mother to fetus, it is highly unlikely that the maternal/fetal gradient of beta carotene of >10:1 could be maintained during an average gestation period if the fetus stored significant amounts of beta carotene. In passive placenta transfer, if stores accumulate without metabolism in the fetus, concentrations on either side of the placenta tend to equalize.25 Over time, even droplets of water filling a pail containing a sponge will eventually saturate the sponge and overflow. Those familiar with the uptake and distribution of inhalation anesthetics will appreciate the analogy to this physiology.26
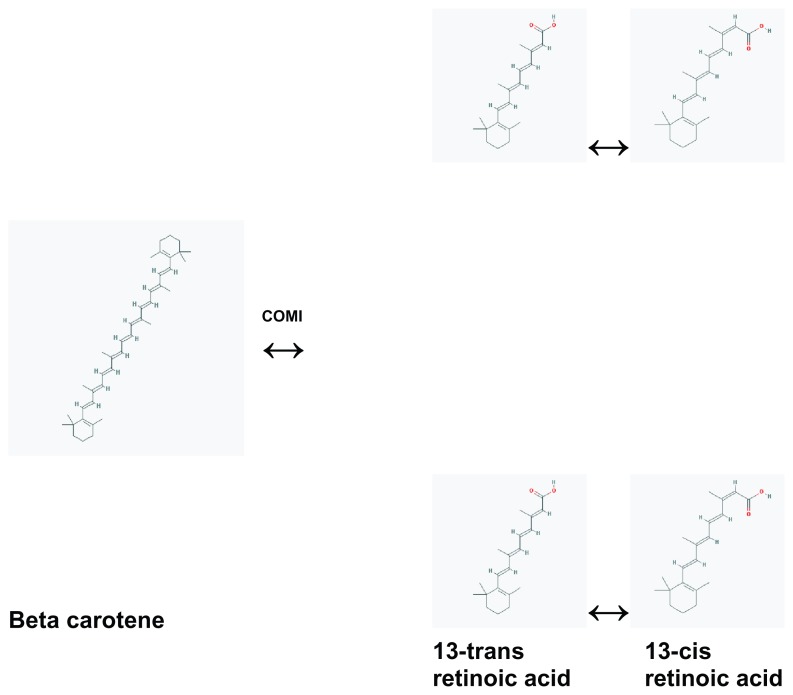
Beta carotene undergoes symmetrical and asymmetrical cleavage. Symmetrical cleavage by beta-carotene-15,15′-oxygenase (COMI) produces two molecules of retinol, which can be subsequently oxidized to retinoic acid.4 The 13-cis and 13-trans isomers of retinoic acid exist in equilibrium (Fig. 1). In addition, studying fetal mice with knock out lesions for COMI and retinol binding protein, Kim et al “demonstrate unequivocally that beta carotene can serve as an alternative vitamin A source for the in situ synthesis of retinoids in developing tissues by the action of CMOI.”4
Figure 1.
Proposed metabolic pathway of beta carotene in the human fetus.
The equation for isomerization with 13-trans retinoic acid as the energy-favored conformation is:
Clearly, an increase in the concentration of 13-trans retinoic acid will shift the equilibrium to the left, increasing production of teratogenic 13-cis retinoic acid. All things considered, the chemistry and physiology strongly indicate that the extremely large gradient between maternal and fetal beta carotene levels is caused by fetal metabolism and limited storage of beta carotene especially when one compares the gradients found in other molecules that cross the placenta.20,27 (Table 1).
Table 1.
Comparison of mean maternal and cord plasma levels of selected molecules that cross the placenta in μmol/L ± SD.20,27
| Maternal | Cord | |
|---|---|---|
| Beta carotene1 | 1.15 ± 0.89 | 0.044 ± 0.04 |
| Retinol1 | 1.72 ± 0.49 | 0.51 ± 0.27 |
| Alpha tocopherol2 | 20.65 ± 4 | 7.21 ± 1.9 |
| Cholesterol2 | 4.83 ± 1 | 2.17 ± 0.6 |
Notes:
Samples obtained within 48 hours after birth;
maternal samples between 10–20 weeks gestation.
Supporting the notion that there is limited storing of beta carotene in neonates, non-brown neonatal fat is white, whereas, in adults and children, it is orange-yellow due to the beta carotene stored in the fat cells.28 In addition, the skin tone of neonates born to carotenemic mothers is not yellow; and, at the time of this writing, a Pub Med search of the terms “carotenemia newborn” retrieved no citations, suggesting that the condition has never been reported.7 Thus, if the liver and binding proteins become saturated with retinol ester and retinol and fetal adipocytes do not store sufficient beta carotene, the fetus is exposed to additional teratogenic retinoic acid.29 In summary strong evidence exits that the human fetus expresses COMI which cleaves beta carotene into retinoids and that fetal beta carotene storage is limited. These deductions support the proposition that an increase in fetal beta carotene exposure could increase teratogenic fetal retinoic acid burden.
A proposition that under conditions of lower oxygen tension the isomerization reaction of beta carotene may favor 13-cis retinoic acid
This author speculates that levels of teratogenic 13-cis retinoic acid increase when the cleavage and isomerization reactions occur under conditions of lower oxygen tension, as found in utero and in smokers. Indeed, excessive ingestion of beta carotene in smokers many of who have lower partial pressure of oxygen in the blood than non smokers is associated with the development of lung cancer; and, in the laboratory, beta carotene cleavage products in liver cells subjected to oxidative stress in the form of hypoxia/reoxygenation produce abnormal hepatocyte metaphases.30–32 Of course, not all carcinogens are teratogens, but some are. The proof of this concept could be established in the laboratory.
Brief Survey of Potential Maternal Sources of Vitamin A
Explanation of RDA for vitamin A in foods
The recommended daily allowance (RDA) for vitamin A in foods is defined as the amount needed to provide 97% or more of all groups with a sufficient quantity of vitamin A to stay healthy.33 Since male adults have the highest requirement for vitamin A, which is 3,000 IU, the RDA is calculated to this group (Table 2).
Table 2.
RDA for Vitamin A.
| Male | Female | |
|---|---|---|
| Infants | ||
| 0.6 months | 400 | |
| 7–12 months | 500 | |
| 1–3 years | 1,000 | |
| Adults | 3,000 | 2,300 |
Note: 1 IU = 0.3 μg retinol. Institute of Medicine.
Prenatal vitamins as a source of retinol
Due to concern about birth defects caused by folic acid deficiency, multivitamins are now prescribed during the prenatal period.34,35 The type of prenatal vitamins recommended varies, and some obstetricians recommend a general multivitamin. Some contain no vitamin A, while others contain as much as 5,000 IU of retinol or 4,000 IU of beta carotene, a precursor of vitamin A (Table 3).
Table 3.
Vitamin A content of multivitamins.
| Vitamin A (IU) | Retinol | Beta carotene | |
|---|---|---|---|
| Walgreens gold seal multiple vitamins | 5000 | + | − |
| Centrum materna | 1000 | + | − |
| 2500 | − | + | |
| Nature made | 4000 | − | + |
| One-a-day women’s prenatal | 2000 | + | − |
| 2000 | − | + |
Fortified foods
Many cereals, dairy products, and soups are fortified with vitamin A. Examples are listed in Table 4.
Table 4.
Foods fortified with vitamin A.
| % RDA | Vitamin A (IU) | Retinol | Beta carotene | |
|---|---|---|---|---|
| Campbell’s soup beef (8 oz) | 35 | 1050 | ? | ? |
| Slim Fast chocolate (11 oz) | 35 | 1050 | + | − |
| Quaker oats instant Oatmeal (28 gm) | 25 | 750 | + | − |
| Vitamin A fortified milk (8 oz) | 10 | 300 | + | − |
| Smart balance light (14 gm) | 10 | 300 | 85% | 15% |
| Fleishmann’s original margarine (14 gm) | 10 | 300 | 70% | 30% |
| Land-O-Lakes butter (14 gm) | 8 | 240 | + | − |
Non-fortified foods
Liver is the food source with the highest quantity of vitamin A by weight. The amount of vitamin A in the liver varies between species, but it is not a common food in a regular diet and is, therefore, unlikely alone to be associated with teratogenic retinoic acid levels. On the other hand, the amount of vitamin A in free-range beef is 7 times greater than in grain-fed animals.36 Thus, if free-range beef is eaten on a regular basis in combination with other foods, vitamins, and beta carotene, significant increases in retinoic acid could result. Raw foods that are high in beta carotene include carrots, sweet potatoes, peppers, and leafy vegetables. Processed foods with concentrated forms of beta carotene include vegetable juices. Examples of non-fortified foods that are high in vitamin A or beta carotene are listed in Table 5.37
Table 5.
Foods with high vitamin A content.
| % DV1 | Vitamin A (IU) | Retinol | Beta carotene | |
|---|---|---|---|---|
| Turkey liver (100 gm) | 1507 | 75,333 | + | − |
| Paprika (7 gm) | 74 | 3,691 | − | + |
| Sweet potato (medium) | 438 | 21,908 | − | + |
| Carrot (medium) | 204 | 10,191 | − | + |
| Spinach (100 gm) | 188 | 9,385 | − | + |
| Lettuce (100 gm) | 150 | 7,492 | − | + |
| Cantaloupe (100 gm) | 68 | 3,382 | − | + |
| Carrot juice (8 oz) | 1000 | 49,988 | − | + |
| V8 vegetable juice (8 oz) | 40 | 1,996 | − | + |
Note:
Based on a daily value of 5,000 IU of Vitamin A. USDA National Nutrient Database.
Calculation of vitamin A and beta carotene intake during pregnancy
The absorption, metabolism, storage, and excretion of beta carotene and retinol in the mother are not completely understood; however, most investigators agree that consumption of these forms of vitamin A is related to maternal serum levels.38–40 In one study, maternal diet influenced cord levels of beta carotene, but not vitamin A.41 Absorption of beta carotene from raw carrots and a low fat diet is very low compared to carrot juice.42 According to current literature, 6 μg of ingested beta carotene convert to 1 retinol equivalent, or 1 μg retinol, but ratios have ranged from 3.3:1 to 26:1.43,44
Based on the discussion above, it may be apparent that maternal ingestion of non-beta carotene sources of vitamin A in foods + fortified foods + nutritional supplements would not likely elevate retinoic acid levels in the fetus to teratogenic levels. Obviously, excesses, such as a daily diet of liver or a non-compliant excess of multivitamins, could tip the balance.
For years, ingestion of what some may consider excesses of beta carotene has been deemed safe.45,46 Carotenemia has rarely been associated with ill health.47–49 In fact, juicing vegetables has become popular among health food advocates. However, if the fetus cannot store beta carotene and converts most of it to retinoic acids, levels could become teratogenic, especially when added to the retinoic acids derived from other sources.
Potential flaws in the concepts presented in this paper
A basic proposition of this paper is that the fetus metabolizes beta carotene and its ability to store beta carotene is limited resulting in potential excesses of teratogenic retinoic acids. It is possible that specialized gating mechanisms for beta carotene may exist; and in cases of low levels of maternal retinol complexes, the gate opens to allow sufficient beta carotene to cross the placenta so that the fetus can convert it to retinol. Finally, if the concepts proposed in this paper are true, they may be relevant to an extremely small subset of children with neurodevelopmental disorders and may require a very large population to show an effect.
Conclusion and Implications for Care
High levels of fetal retinoic acid, a metabolite of vitamin A, are known to be teratogenic and may be associated with developmental defects of the central nervous system, particularly involving the cerebellum, resulting in neurodevelopmental disorders. Previous concepts that support the indiscriminate intake of foods during pregnancy that contain high levels of beta carotene should be reexamined, because strong evidence suggests that the fetus has limited capacity to store beta carotene in fat and likely metabolizes it to retinoic acids. Since cis and trans retinoic acid are in equilibrium, an increase in the concentration of 13-trans retinoic acid is associated with an increase in the concentration of the more teratogenic 13-cis isomer. Also, it is proposed that at low oxygen tensions, as found in the fetus and in smokers, the enzymatic cleavage of beta carotene may produce excess 13-cis retinoic acid. It is very difficult to prove that a single behavioral teratogen exists. Clearly, genetic and environmental factors confound investigations. This author believes that awareness and simple monitoring of all potential sources of retinoic acid during the first trimester of pregnancy, including nutritional supplements, dietary vitamin A from animal sources, and beta carotene, may decrease the incidence of neurodevelopmental disorders.
Acknowledgements
The author would like to thank Kathy Gage of Duke University for editorial assistance in the preparation of this manuscript.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Snodgrass SR. Vitamin neurotoxicity. Mol Neurobiol. 1992 Spring;6(1):41–73. doi: 10.1007/BF02935566. [DOI] [PubMed] [Google Scholar]
- 2.Sapin V, Alexandre MC, Chaib S, et al. Effect of vitamin A status at the end of term pregnancy on the saturation of retinol binding protein with retinol. Am J Clin Nutr. 2000 Feb;71(2):537–43. doi: 10.1093/ajcn/71.2.537. [DOI] [PubMed] [Google Scholar]
- 3.Luo T, Wagner E, Crandall JE, Drager UC. A retinoic-acid critical period in the early postnatal mouse brain. Biol Psychiatry. 2004 Dec 15;56(12):971–80. doi: 10.1016/j.biopsych.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Kim YK, Wassef L, Chung S, et al. Beta-Carotene and its cleavage enzyme beta-carotene-15,15′-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J. 2011 May;25(5):1641–52. doi: 10.1096/fj.10-175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimenstein R, Trugo NM, Donangelo CM, Trugo LC, Anastacio AS. Effect of subadequate maternal vitamin-A status on placental transfer of retinol and beta-carotene to the human fetus. Biol Neonate. 1996;69(4):230–4. doi: 10.1159/000244315. [DOI] [PubMed] [Google Scholar]
- 6.Spiegler E, Kim YK, Wassef L, Shete V, Quadro L.Maternal-fetal transfer and metabolism of vitamin A and its precursor beta-carotene in the developing tissues Biochim Biophys Acta May 19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polifka JE, Dolan CR, Donlan MA, Friedman JM. Clinical teratology counseling and consultation report: high dose beta-carotene use during early pregnancy. Teratology. 1996 Aug;54(2):103–7. doi: 10.1002/(SICI)1096-9926(199606)54:2<103::AID-TERA6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. N Engl J Med. 1955 Nov 23;333( 21):1369–73. doi: 10.1056/NEJM199511233332101. [DOI] [PubMed] [Google Scholar]
- 9.Werler MM, Lammer EJ, Mitchell AA. Teratogenicity of high vitamin A intake. N Engl J Med. 1996 May 2;334(18):1195–6. doi: 10.1056/NEJM199605023341813. author reply 1197. [DOI] [PubMed] [Google Scholar]
- 10.Miller RK, Hendrickx AG, Mills JL, Hummler H, Wiegand UW. Periconceptional vitamin A use: how much is teratogenic? Reprod Toxicol. 1998 Jan-Feb;12(1):75–88. doi: 10.1016/s0890-6238(97)00102-0. [DOI] [PubMed] [Google Scholar]
- 11.Lipinski CA. Drew University Medicinal Chemistry Special Topics Course. 1999. [Google Scholar]
- 12.Berggren Soderlund M, Fex GA, Nilsson-Ehle P. Concentrations of retinoids in early pregnancy and in newborns and their mothers. Am J Clin Nutr. 2005 Mar;81(3):633–6. doi: 10.1093/ajcn/81.3.633. [DOI] [PubMed] [Google Scholar]
- 13.Hodge SM, Makris N, Kennedy DN, et al. Cerebellum, language, and cognition in autism and specific language impairment. J Autism Dev Disord. 2010 Mar;40(3):300–16. doi: 10.1007/s10803-009-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavano A, Fabbro F, Borgatti R. Language and social communication in children with cerebellar dysgenesis. Folia Phoniatr Logop. 2007;59(4):201–9. doi: 10.1159/000102932. [DOI] [PubMed] [Google Scholar]
- 15.Makori N, Peterson PE, Hendrickx AG. 13-cis-retinoic acid causes patterning defects in the early embryonic rostral hindbrain and abnormal development of the cerebellum in the macaque. Teratology. 2001 Feb;63(2):65–76. doi: 10.1002/1096-9926(200102)63:2<65::AID-TERA1011>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry. 2008 Jun;23(4):289–99. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 17.de Barros Silva SS, Rondo PH, Erzinger GS. Beta-carotene concentrations in maternal and cord blood of smokers and non-smokers. Early Hum Dev. 2005 Apr;81(4):313–7. doi: 10.1016/j.earlhumdev.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Yeum KJ, Ferland G, Patry J, Russell RM. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J Am Coll Nutr. 1998 Oct;17(5):442–7. doi: 10.1080/07315724.1998.10718791. [DOI] [PubMed] [Google Scholar]
- 19.Baker H, Thind IS, Frank O, DeAngelis B, Caterini H, Louria DB. Vitamin levels in low-birth-weight newborn infants and their mothers. Am J Obstet Gynecol. 1977 Nov 1;129(5):521–4. doi: 10.1016/0002-9378(77)90090-4. [DOI] [PubMed] [Google Scholar]
- 20.Schulz C, Engel U, Kreienberg R, Biesalski HK. Vitamin A and beta-carotene supply of women with gemini or short birth intervals: a pilot study. Eur J Nutr. 2007 Feb;46(1):12–20. doi: 10.1007/s00394-006-0624-9. [DOI] [PubMed] [Google Scholar]
- 21.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008 Jan;88(1):125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003 Apr;28(2):105–12. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- 23.Audus KL. Controlling drug delivery across the placenta. Eur J Pharm Sci. 1999 Jul;8(3):161–5. doi: 10.1016/s0928-0987(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 24.The PubChem Project. [Accessed September 15, 2011]. pubchem.ncbi.nlm.nih.gov/
- 25.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43(8):487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 26.Lowe HJaE, Edward A. The Quantitative Practice Of Anesthesia: Use Of Closed Circuit. Baltimore: Williams & Wilkins; 1981. [Google Scholar]
- 27.Kiely M, Cogan PF, Kearney PJ, Morrissey PA. Concentrations of tocopherols and carotenoids in maternal and cord blood plasma. Eur J Clin Nutr. 1999 Sep;53(9):711–5. doi: 10.1038/sj.ejcn.1600838. [DOI] [PubMed] [Google Scholar]
- 28.Observation Pca.
- 29.Ganguly C, Mukherjee KL. Relationship between maternal serum vitamin A and vitamin A status of the corresponding fetuses. J Trop Pediatr. 1988 Dec;34(6):313–5. doi: 10.1093/tropej/34.6.313. [DOI] [PubMed] [Google Scholar]
- 30.Alija AJ, Bresgen N, Sommerburg O, Langhans CD, Siems W, Eckl PM. Beta-carotene breakdown products enhance genotoxic effects of oxidative stress in primary rat hepatocytes. Carcinogenesis. 2006 Jun;27(6):1128–33. doi: 10.1093/carcin/bgi342. [DOI] [PubMed] [Google Scholar]
- 31.Alija AJ, Bresgen N, Bojaxhi E, Vogl C, Siems W, Eckl PM. Cytotoxicity of beta-carotene cleavage products and its prevention by antioxidants. Acta Biochim Pol. 2010;57(2):217–21. [PubMed] [Google Scholar]
- 32.Russell RM. The enigma of beta-carotene in carcinogenesis: what can be learned from animal studies. J Nutr. 2004 Jan;134(1):262S–8. doi: 10.1093/jn/134.1.262S. [DOI] [PubMed] [Google Scholar]
- 33.Dietary Supplement Fact Sheet: Vitamin A and Carotenoids. [Accessed August 22, 2011]. http://ods.od.nih.gov/factsheets/vitamina/
- 34.Milunsky A, Jick H, Jick SS, et al. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA. 1989 Nov 24;262(20):2847–52. doi: 10.1001/jama.262.20.2847. [DOI] [PubMed] [Google Scholar]
- 35.Talaulikar VS, Arulkumaran S. Folic Acid in obstetric practice: a review. Obstet Gynecol Surv. 2011 Apr;66(4):240–7. doi: 10.1097/OGX.0b013e318223614c. [DOI] [PubMed] [Google Scholar]
- 36.Daley CA, Abbott A, Doyle PS, Nader GA, Larson S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr J. 2010;9:10. doi: 10.1186/1475-2891-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Top 10 Foods Highest in Vitamin A. [Accessed August 24, 2011]. www.healthaliciousness.com/articles/food-sources-of-vitamin-A.php.
- 38.Black RE. Micronutrients in pregnancy. Br J Nutr. 2001 May;85( Suppl 2):S193–7. doi: 10.1079/bjn2000314. [DOI] [PubMed] [Google Scholar]
- 39.Meram I, Bozkurt AI, Kilincer S, Ozcirpici B, Ozgur S. Vitamin A and beta-carotene levels during pregnancy in Gaziantep, Turkey. Acta Medica (Hradec Kralove) 2004;47(3):189–93. [PubMed] [Google Scholar]
- 40.Berti C, Biesalski HK, Gartner R, et al. Micronutrients in pregnancy: Current knowledge and unresolved questions Clin Nutr August 25, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Scaife AR, McNeill G, Campbell DM, Martindale S, Devereux G, Seaton A. Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal plasma levels at delivery. Br J Nutr. 2006 Apr;95(4):771–8. doi: 10.1079/bjn20051718. [DOI] [PubMed] [Google Scholar]
- 42.Strobel M, Tinz J, Biesalski HK. The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur J Nutr. 2007 Jul;46( Suppl 1):I1–20. doi: 10.1007/s00394-007-1001-z. [DOI] [PubMed] [Google Scholar]
- 43.Thurnham DI, Northrop-Clewes CA. Optimal nutrition: vitamin A and the carotenoids. Proc Nutr Soc. 1999 May;58(2):449–57. doi: 10.1017/s0029665199000592. [DOI] [PubMed] [Google Scholar]
- 44.West CE. Meeting requirements for vitamin A. Nutr Rev. 2000 Nov;58(11):341–5. doi: 10.1111/j.1753-4887.2000.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 45.Underwood BA. Teratogenicity of vitamin A. Int J Vitam Nutr Res Suppl. 1989;30:42–55. [PubMed] [Google Scholar]
- 46.Underwood BA. Maternal vitamin A status and its importance in infancy and early childhood. Am J Clin Nutr. 1994 Feb;59(Suppl 2):17S–22. doi: 10.1093/ajcn/59.2.517S. discussion 522S–4. [DOI] [PubMed] [Google Scholar]
- 47.Mathews-Roth MM. Amenorrhea associated with carotenemia. JAMA. 1983 Aug 12;250(6):731. [PubMed] [Google Scholar]
- 48.Nishimura T. A correlation between carotenemia and biliary dyskinesia. J Dermatol. 1993 May;20(5):287–92. doi: 10.1111/j.1346-8138.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 49.Bendich A. The safety of beta-carotene. Nutr Cancer. 1988;11(4):207–14. doi: 10.1080/01635588809513989. [DOI] [PubMed] [Google Scholar]