Abstract
Background
Polycystic ovary syndrome (PCOS) is one of the most common endocrinopathies affecting women in the reproductive age group, and is one of the most common causes of hyperandrogenic anovulatory infertility. The aromatase inhibitor, letrozole, has been used for induction of ovulation. The purpose of this study was to compare the effects of letrozole and clomiphene citrate in induction of ovulation among patients with PCOS undergoing intrauterine insemination.
Methods
In a double-blind randomized study, 60 infertile patients with PCOS received standard doses of either clomiphene citrate or letrozole as an induction protocol prior to intrauterine insemination. A hormonal profile, pelvic ultrasound, hysterosalpingogram, and/ or laparoscopy were done for all patients. The patients were monitored for ovulation by translational ultrasonographic folliculometry, with measurement of number and size of the follicles, as well as endometrial thickness. Human chorionic gonadotrophin (HCG) was injected intramuscularly when at least one mature follicle ≥18 mm diameter was detected, and intrauterine insemination was performed 32–36 hours later. Transvaginal ultrasound and β-HCG measurement were performed for confirmation of pregnancy.
Results
Letrozole and clomiphene citrate achieved follicle maturation within a mean ± standard deviation (SD) of 13.2 ± 1.53 and 14.1 ± 1.35 days, respectively, showing no significant difference (P > 0.05). The mean number of follicles reaching ≥18 mm on the day of HCG administration was significantly higher in patients who received clomiphene citrate (2.9 ± 1.77) than in those receiving letrozole (1.2 ± 0.9). Letrozole had a significantly greater effect than clomiphene citrate on endometrial thickness (9.16 ± 1.36 versus 4.46 ± 1.71). The number of pregnancies achieved in the letrozole group was significantly (P < 0.05) greater than in the clomiphene group.
Conclusion
Letrozole in patients with PCOS is as effective as clomiphene citrate in inducing ovulation, and although the number of follicles produced by induction with letrozole were less than those produced by clomiphene, letrozole had a significantly greater effect on endometrial thickness than clomiphene citrate, and the incidence of pregnancy after intrauterine insemination was significantly higher, with a lower incidence of multiple pregnancy.
Keywords: polycystic ovary syndrome, letrozole, clomiphene citrate, intrauterine insemination
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women of childbearing age. Although it is a major cause of infertility, its etiology remains unknown and its treatment is difficult. However, most symptoms of PCOS can be adequately controlled or eliminated with proper diagnosis and treatment. Therefore, treatment modalities and ovulation induction protocols must be balanced for optimal results.1
To date, clomiphene citrate has been the first-line treatment for induction of ovulation in PCOS, although aromatase inhibitors are an effective and safe alternative in this indication. Letrozole, a third-generation aromatase inhibitor, has the potential to be used for ovulation induction. It acts by reversibly inhibiting the enzyme responsible for estrogen biosynthesis. By decreasing estrogen levels in the body, letrozole releases the hypothalamus and/or pituitary gland from the negative feedback of estrogen. This results in increased levels of endogenous follicle-stimulating hormone and luteinizing hormone, which stimulates the development of ovarian follicles.2
Intrauterine insemination is a useful method of assisted reproduction, with better pregnancy rates now achieved by good patient selection and ovarian stimulation.3 Intrauterine insemination, together with ovarian stimulation, is a less expensive and less invasive treatment in comparison with other assisted reproductive techniques, and has been widely used for the treatment of infertile couples, and has a variety of indications, including unexplained infertility, male subfertility, cervical mucus hostility, and ovulatory disturbances.4 The aim of this study was to compare the effects of letrozole and clomiphene citrate in induction of ovulation in patients with PCOS undergoing intrauterine insemination.
Methods
This double-blind randomized study was conducted in 60 primary infertile patients (defined as one year of unprotected coitus without conception in patients who have never conceived before)5 with PCOS, aged younger than 35 years, and attending the infertility outpatient clinic at Ain Shams University Hospital and/or a local private outpatient setting. Diagnosis of PCOS was based on the Rotterdam criteria (2003 ESHRE/ ASRM consensus),6 whereby patients diagnosed with PCOS require the presence of two of three criteria, ie, oligomenorrhea and/or anovulation, clinical and/ or biochemical signs of hyperandrogenism, and/or polycystic ovaries on ultrasound. All patients had a history of failed induction of ovulation with appropriately timed intercourse at least 4–6 times. Informed consent was obtained from all participants, and the study was approved by the medical ethics committee of Ain Shams University Hospital.
All patients were subjected to full history-taking and a thorough clinical examination, including a pelvic examination. All patients had an infertility workup including:
A hormonal profile, including follicle-stimulating hormone, luteinizing hormone, prolactin, thyroid-stimulating hormone, estradiol, and free testosterone (day 2–3 of menstrual cycle)
A pelvic ultrasound for confirmation of polycystic changes in the ovary (ovaries with at least 10 subcapsular cysts 2–10 mm in diameter and hyperacrogenic stoma) and exclusion of any other pelvic anomalies
Hysterosalpingography and/or laparoscopy to determine tubal patency and any other pelvic pathology
Semen analysis in the patient’s partner to rule out male factor.
Patients with infertility due to uterine and tubal pathologies or male factor were excluded from the study. They were then randomized into two groups using a computer generated program. Group 1 included 30 women who were given the aromatase inhibitor, letrozole (Femara®, Novartis, Basel, Switzerland) orally at a dose of 2.5 mg once daily on days 3–7 of the menstrual cycle. Group 2 included 30 women who were given clomiphene citrate ( Clomid®, Sanofi Aventis, France) 50 mg orally twice daily on days 3–7 of the menstrual cycle.
Transvaginal ultrasonographic folliculometry using a microconvex ultrasound endocavity probe 6CV1 at 5–8 mHz (Mindray DC-3, China) was performed by the same operator starting from day 10 of the menstrual cycle and every other day, with measurement of number and size of follicles and endometrial thickness.
Human chorionic gonadotrophin 10,000 IU (Choriomon IBSA, Switzerland) was given intramuscularly to trigger ovulation when at least one mature follicle ≥18 mm diameter was detected. Endometrial thickness was recorded on the day of human chorionic gonadotrophin administration. Intrauterine insemination was performed 32–36 hours after administration of human chorionic gonadotrophin.
A single insemination was performed. Semen specimens were produced by masturbation and collected for insemination within half an hour of production. The semen specimen was diluted using 5 mL of Ham’s F-10 medium with L-glutamine (Cellgro®, Mediatech, Inc, Inc, 9345 Discovery Blvd., Manassas, VA 20109, (800) Cellgro ( 235–5476)). After centrifugation at 600–900 × g for 20 minutes, the pellet was resuspended and washed twice with Ham’s F-10 medium with L-glutamine (Mediatech Inc). The sperm suspension was kept at room temperature for 45 minutes until transfer into the uterine cavity using a Wallace intrauterine insemination catheter (LabIVF®, LEC Instruments, Scoresby, Victoria, Australia). The time interval between semen production and transfer did not exceed 2.5 hours. Luteal phase support was done using progesterone vaginal pessaries (Cyclogest®, Noristan Ltd, Waltloo, Pretoria) 400 mg once daily for 15 days.
The beta-human chorionic gonadotrophin level was measured two weeks after the intrauterine insemination procedure, and a level >5 mIU/mL was considered to be a positive pregnancy. Transvaginal ultrasound was performed 2–4 weeks later to confirm clinical pregnancy.
Statistical methods
The data were transferred to IBM cards using an IBM personal computer, and analyzed with the Statistical Program for Social Sciences V11.0 (SPSS Inc, Chicago, IL). Descriptive statistics comprised the mean, standard deviation (SD), minimum and maximum values, and number and percentage of qualitative data. Analytical statistics comprised the Student’s-test to make comparisons between independent quantitative means, and the Chi-square test to make comparisons between the different groups with regard to qualitative data. For all tests, a P value > 0.05 was deemed insignificant, P < 0.05 was significant, and P < 0.001 was highly significant. Data were presented using Microsoft Office Word 2007.
Results
Group 1 included 30 women aged 21–34 (mean ± SD, 27.2 ± 5.18) years, with a body mass index of 24–31 (26.2 ± 1.8). Group 2 included 30 women aged 20–33 (25.21 ± 5.18) years, with a body mass index of 23–32 (29.1 ± 2.3). There was no significant difference between the groups for body mass index (P > 0.05, data not shown). Comparison of hormonal profiles between the groups was also insignificant (Table 1).
Table 1.
Comparison of hormonal profiles for Groups 1 and 2.
| Group 1 (n = 30) Letrozole |
Group 2 (n = 30) Clomiphene citrate |
P | Sig. | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Range | Mean ± SD | Range | Mean ± SD | |||
| FSH mIU/mL | 3.1–5.6 | 4.23 ± 0.56 | 3.5–5.5 | 4.51 ± 0.5 | >0.05 | NS |
| LH mIU/mL | 6.7–11.5 | 8.25 ± 1.2 | 7.1–12.2 | 9.1 ± 0.9 | >0.05 | NS |
| TSH uIU/mL | 1.5–2.0 | 1.8 ± 0.1 | 1.3–2.4 | 1.9 ± 0.2 | >0.05 | NS |
| PRL ng/mL | 5–15 | 11.2 ± 2.2 | 9–16 | 12.4 ± 1.9 | >0.05 | NS |
| Serum E2 pg/mL | 46–65 | 50.56 ± 7.8 | 52–68 | 51.1 ± 6.9 | >0.05 | NS |
| Free testosterone pg/mL | 3.2–4.3 | 3.6 ± 0.2 | 3.1–4.4 | 3.5 ± 0.2 | >0.05 | NS |
Abbreviations: FSH, follicle stimulating hormone; LH, luteinizing hormone; NS, not statistically significant; TSH, thyroid-stimulating hormone; PRL, prolactin; Sig., significance level.
During folliculometry, two patients in Group 1 and four patients in Group 2 showed no follicular response and were excluded from the study. Comparison of the mean duration of days until human chorionic gonadotrophin injection between both groups showed no statistical significance (P > 0.05), although follicular maturity was achieved in a shorter period for women in Group 1 than in Group 2 (Table 2).
Table 2.
Comparison of the mean duration of days until human chorionic gonadotropin injection between Groups 1 and 2.
| Group 1 (n = 28) Letrozole |
Group 2 (n = 26) Clomiphene citrate |
P | Sig. | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Range | Mean ± SD | Range | Mean ± SD | |||
| Days of cycle until HCG injection | 11–16 | 13.2 ± 1.53 | 12–17 | 14.1 ± 1.35 | >0.05 | NS |
Abbreviations: HCG, human chorionic gonadotropin; NS, not significant; Sig., significant.
The number of follicles on the day of human chorionic gonadotrophin administration showed a statistically significant (P < 0.05) difference between the groups (1.2 ± 0.9 for Group 1 versus 2.9 ± 1.77 in Group 2). Endometrial thickness on the day of human chorionic gonadotrophin administration also showed a statistically significant (P < 0.05) difference (9.16 ± 1.36 for Group 1 versus 4.46 ± 1.71 for Group 2, Table 3).
Table 3.
Comparison of number of mature follicles (> 18 mm) and endometrial thickness between Groups 1 and 2 on day of human chorionic gonadotropin administration.
| Group 1 (n = 28) Letrozole |
Group 2 (n = 26) Clomiphene citrate |
P | Sig. | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Range | Mean ± SD | Range | Mean ± SD | |||
| Number of follicles >18 (mm) | 1–3 | 1.2 ± 0.9 | 2–7 | 2.9 ± 1.77 | <0.05 | S |
| Endometrial thickness (mm) | 9–12 | 9.16 ± 1.36 | 4–7 | 4.46 ± 1.71 | <0.05 | S |
Abbreviation: SD, standard deviation.
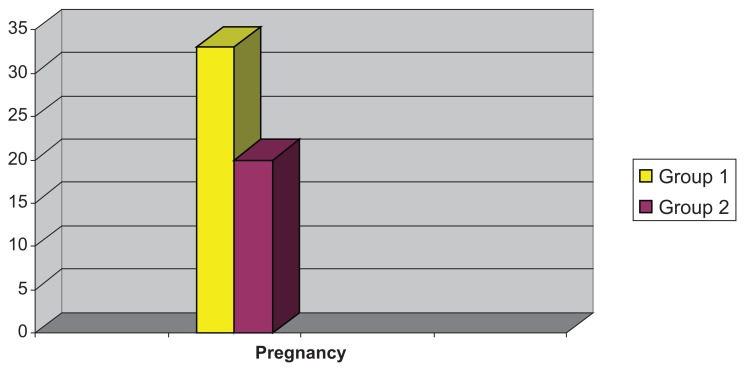
Comparison of the number of pregnancies achieved in the groups showed a significant statistical difference (P < 0.05), being higher in Group 1 (33%) than in Group 2 (20%, see Fig. 1). Two twin pregnancies were recorded in Group 2, while no multiple pregnancies occurred in Group 1.
Figure 1.
Comparison of the number of pregnancies in each group.
Discussion
PCOS is a common endocrine disorder. It is associated with chronic anovulation, and causes infertility in 4%–6% of women in the reproductive age group. Despite the wide acceptance of clomiphene citrate as the first-line drug for ovulation induction in women with PCOS, a significant proportion of women do not respond to this treatment.2 Recent studies have suggested that letrozole, a third-generation aromatase inhibitor, can be used for ovulation induction, and it is associated with a higher chance of uncomplicated pregnancy and a lower risk of multiple pregnancy than clomiphene citrate.7
The results of this prospective randomized study showed that letrozole was as effective as clomiphene citrate for induction of ovulation, and both letrozole and clomiphene achieved follicle maturation within a similar time frame (13.2 ± 1.53 and 14.1 ± 1.35 days, respectively).
Although the number of follicles reaching ≥18 mm on the day of human chorionic gonadotrophin administration was significantly higher in patients who received clomiphene than in those receiving letrozole, the number of pregnancies achieved after intrauterine insemination in Group 1 was significantly higher than in Group 2 (P < 0.05). This statistically significant difference in the number of pregnancies could be attributed to the finding that letrozole had a significantly (P < 0.05) better effect on endometrial thickness than clomiphene.
Similar studies comparing letrozole with clomiphene citrate for ovulation induction in PCOS detected significantly fewer mature follicles but a greater endometrial thickness in the letrozole group.2 Letrozole was found to be as effective as clomiphene citrate in inducing ovulation, and with a slightly higher pregnancy rate.1
In addition, a clinical trial comparing the efficacy of letrozole and clomiphene for ovulation induction in 107 infertile patients with PCOS with induction protocols similar to those used in this study, showed no significant difference in the number and size of mature follicles, but a higher pregnancy rate in the letrozole group was observed.8
In contrast, other research has shown no superiority of letrozole over clomiphene citrate for inducing ovulation prior to intrauterine insemination in women with unexplained infertility.9 This could be related to other factors, such as differences in the study populations, drug doses, or intrauterine insemination technique, or to the fact that the study groups showed no significant difference in endometrial thickness at the time of human chorionic gonadotrophin administration.
In general, use of clomiphene citrate is known to have several disadvantages, including a discrepancy between ovulation and conception rates.10 Clomiphene citrate initiates ovulation by blocking the negative feedback of endogenous estrogen at the level of the hypothalamus and pituitary gland, and promoting an increase in the pulsatile release of luteinizing hormone and follicle-stimulating hormone. However, clomiphene citrate has an antagonistic effect on the endometrium and may reduce endometrial thickness and receptivity, which could contribute to this discrepancy.
Letrozole, on the other hand, acts by decreasing the conversion of androstenedione and testosterone to estrogen in the ovary. This decrease in circulating estrogen increases gonadotropin secretion. Multiple developing follicles appear from day 7, but because letrozole does not deplete estrogen receptors, normal negative feedback occurs centrally as the dominant follicle grows and the estrogen level increases. This results in follicle-stimulating hormone suppression and atresia of smaller follicles, and midcycle monoovulation occurs in most patients.11
Furthermore, letrozole has been shown to be effective in inducing ovulation and pregnancies in women with anovulatory PCOS and in patients with clomiphene citrate resistance or failure.12 Letrozole has fewer side effects, a shorter half life than clomiphene citrate, and has no adverse effects on endometrial receptivity. Also, its safety is superior to that of clomiphene citrate. Using bioequivalent doses, letrozole pregnancy rates are equal or superior to those achieved with clomiphene.13
Previous studies have demonstrated that monoovulation is one of the major advantages of using the aromatase inhibitors for induction of ovulation.14 Because patients with PCOS are often hyperresponsive to gonadotropins, a drug that consistently results in a single ovulation would be particularly desirable. In this study, letrozole achieved a significantly higher number of pregnancies than clomiphene citrate (33% versus 20%, P < 0.05), and twin pregnancies were only recorded in the clomiphene group, further supporting the notion that letrozole could be superior to clomiphene citrate in induction of ovulation in PCOS.
Despite the fact that gonadotropin therapy may be superior in achieving higher pregnancy rates than clomiphene citrate or letrozole, it is expensive, and there is a significantly higher risk of ovarian hyperstimulation and multiple pregnancies.1 Studies have shown that ovarian stimulation with letrozole is associated with more acceptable pregnancy rates than in(s) gonadotropin therapy, and at significantly less cost, less risk, and more patient convenience.15 Furthermore, combination protocols are less costly and equally effective, with potentially fewer multiple births than with gonadotropins alone. Letrozole may be more effective than clomiphene and tamoxifen in a combination protocol.16
In conclusion, letrozole in patients with PCOS is as effective as clomiphene citrate in inducing ovulation, and although fewer follicles were produced by induction with letrozole than with clomiphene, letrozole had a significantly better effect on endometrial thickness than clomiphene citrate, and the incidence of pregnancy after intrauterine insemination was significantly higher, with a lower incidence of multiple pregnancy.
Letrozole could replace clomiphene citrate as the primary medication for chronic anovulation in PCOS. Furthermore, letrozole may be advantageous in the intrauterine insemination procedure, being less liable to result in multiple pregnancies. It could augment or even obviate the use of gonadotropins in the treatment of women who have been unsuccessful in achieving pregnancy with clomiphene citrate. Further studies are needed to determine optimal dosing and the long-term safety for women treated with the drug.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Rajashekar L, Krishna D, Patil M. Polycystic ovaries and infertility: Our experience. J Hum Reprod Sci. 2008;1:65–72. doi: 10.4103/0974-1208.44113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atay V, Cam C, Muhcu M, Cam M, Karateke A. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Int Med Res. 2006;34:73–6. doi: 10.1177/147323000603400109. [DOI] [PubMed] [Google Scholar]
- 3.Karuppaswamy J, Smedley M, Carter L. Intrauterine insemination: Pregnancy rate in relation to number, size of preovulatory follicle and day of insemination. J Indian Med Assoc. 2009;107:141–7. [PubMed] [Google Scholar]
- 4.Zadehmodarres S, Oladi B, Saeedi S, Jahed F, Ashraf H. Intrauterine insemination with husband semen: An evaluation of pregnancy rate and factors affecting outcome. J Assist Reprod Genet. 2009;2:7–11. doi: 10.1007/s10815-008-9273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosher WD, Pratt WF. Fecundity and infertility in the United States: Incidence and trends. Fertil Steril. 1991;56:192–3. [PubMed] [Google Scholar]
- 6.The Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long term health risks related to PCOS. Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 7.Ekerhovd E. Ovulation induction by means of letrozole. Tidssker nor Laegeforen. 2009;26:129. doi: 10.4045/tidsskr.08.0129. :412–5. [DOI] [PubMed] [Google Scholar]
- 8.Zeinalzadeh M, Basirat Z, Esmailpour M. Efficacy of letrozole in ovulation induction compared to that of clomiphene citrate in patients with polycystic ovarian syndrome. J Reprod Med. 2010;55:36–40. [PubMed] [Google Scholar]
- 9.Badawy A, Elnashar A, Totongy M. Clomiphene citrate or aromatase inhibitors for superovulation in women with unexplained infertility undergoing intrauterine insemination: A prospective randomized trial. Fertil Steril. 2009;92:1355–9. doi: 10.1016/j.fertnstert.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y, Ono M, Youshida Y, Sugino N, Ueda K, Kato H. Effects of clomiphene citrate on the endometrial thickness and echogenic pattern of the endometrium. Fertil Steril. 1997;67:256–60. doi: 10.1016/S0015-0282(97)81907-3. [DOI] [PubMed] [Google Scholar]
- 11.Ohno Y, Fujimoto Y. Endometrial estrogen and progesterone receptors and their relationship to sonographic appearance of the endometrium. Hum Reprod Update. 1998;4:560–4. doi: 10.1093/humupd/4.5.560. [DOI] [PubMed] [Google Scholar]
- 12.Mitwally MF, Casper RF. Use of aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305–9. doi: 10.1016/s0015-0282(00)01705-2. [DOI] [PubMed] [Google Scholar]
- 13.Pritts EA. Letrozole for ovulation induction and controlled ovarian hyperstimulation. Curr Opin Obstet Gynecol. 2010;22:289–94. doi: 10.1097/GCO.0b013e32833beebf. [DOI] [PubMed] [Google Scholar]
- 14.Casper RF. Letrozole: Ovulation or superovulation? Fertil Steril. 2003;80:1335–7. doi: 10.1016/j.fertnstert.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Gregorious O, Vlahos NF, Konidaris S, Papadias K, Botsis D, Creatsas GK. Randomized controlled trial comparing superovulation with letrozole versus recombinant follicle-stimulating hormone combined with intrauterine insemination for couples with unexplained infertility who had failed clomiphene citrate stimulation and intrauterine insemination. Fertil Steril. 2008;90:678–83. doi: 10.1016/j.fertnstert.2007.06.099. [DOI] [PubMed] [Google Scholar]
- 16.Ryan GL, Moss V, Davis WA, Sparks AE, Dokras A, Van Voorhis BJ. Oral ovulation induction agents combined with low-dose gonadotrophin injections and intrauterine insemination: Cost and clinical effectiveness. J Reprod Med. 2005;50:943–50. [PubMed] [Google Scholar]