Abstract
Objective
To compare the clinical results of four different protocols of COH for IVF-ICSI in normovulatory women, using in all cases pituitary suppression with GnRH antagonists.
Materials/methods
A single center, open label, parallel-controlled, prospective, post-authorization study under the approved conditions for use where 305 normal responders women who were candidates to COH were assigned to r-FSH +hp-hMG (n = 51, Group I), hp-hMG (n = 61, Group II), fixed-dose r-FSH (n = 118, Group III), and r-FSH with potential dose adjustment (n = 75, Group IV) to subsequently undergo IVF-ICSI.
Results
During stimulation, Group IV needed significantly more days of stimulation as compared to Group II [8.09 ± 1.25 vs. 7.62 ± 1.17; P < 0.05], but was the group in which more oocytes were recovered [Group I: 9.43 ± 4.99 vs. Group II: 8.96 ± 4.82 vs. Group III: 8.78 ± 3.72 vs. Group IV: 11.62 ± 5.80; P < 0.05]. No significant differences were seen between the groups in terms of clinical and ongoing pregnancy, but among patients in whom two embryos with similar quality parameters (ASEBIR) were transferred, the group treated with hp-hMG alone achieved a significantly greater clinical pregnancy rate as compared to all other groups [Group I: 31.6%, Group II: 56.4%, Group III: 28.7%, Group IV: 32.7%; P < 0.05].
Conclusions
Although randomized clinical trials should be conducted to achieve a more reliable conclusion, these observations support the concept that stimulation with hp-hMG could be beneficial in normal responders women undergoing pituitary suppression with GnRH antagonists.
Keywords: GnRH antagonist, highly purified menotropin, recombinant-FSH, controlled ovulation hyperstimulation, embryo quality, IVF/ICSI
Introduction
GnRH antagonists induce a rapid decrease in LH and FSH levels, preventing spontaneous LH surges. Their properties do not require a desensitization period, which allows for their use in the last phase of the follicular cycle. GnRH antagonists are being increasingly used, and may eventually replace GnRH agonists because of their lower adverse event rate.1
Although some authors have reported that use of GnRH antagonists may result in lower ongoing pregnancy rates as compared to GnRH agonists,2 recent findings have associated treatment with these drugs to an increased mitochondrial activity of oocytes that could be to the detriment of non- dominant oocytes.3 Moreover, use of GnRH antagonists may be safer for patients because it is associated to a lower rate of occurrence of the ovarian hyperstimulation syndrome (OHS) as compared to protocols using GnRH agonists (OR: 0.47; 95% CI: 0.18–1.25).2 Finally, this type of drugs may decrease total cycle duration,4 as well as relieve the emotional stress experienced by patients undergoing cycles of controlled ovarian hyperstimulation (COH).5
In assisted reproduction cycles, not only the drug used to achieve pituitary suppression is important, but also the type of gonadotropin used for stimulation.6
In fact, many studies have focused on identification of which factors may be more significant for predicting ovarian response and pregnancy. As regards ovarian response, factors mentioned include age, ovarian volume, number of antral follicles, blood flow in ovarian stroma, serums levels of FSH, LH, estradiol and inhibin B, smoking, and body mass index. Pregnancy predictors identified include age, serum levels of FSH, LH, estradiol and inhibin B, endometrial thickness, smoking, and body mass index, and parity.7
Most of these studies included one or only a few predictors of ovarian response or pregnancy, but virtually none included the COH protocol in the multivariate analysis,7 despite the fact that many differences have been reported depending on the type of gonadotropin used.
The most commonly used gonadotropins are highly purified hMG (hp-hMG) and recombinant FSH (r-FSH). This is why most studies conducted to date in normally responding women have focused on comparing the two abovementioned preparations in women undergoing suppression with a GnRH agonist. The most recent publication on this subject include a meta-analysis where treatment with hp-hMG in IVF-ICSI was found to be associated to a 4% increase in the rate of live newborns as compared to r-FSH (RR = 1.18; 95% CI: 1.02–1.38).8 This result was supported by a study published by Platteau et al where treatment with hp-hMG provided a greater chance of achieving a live newborn in women undergoing IVF (OR = 1.36; 95% CI: 1.01–1.83).9 These improved result may be explained by the fact that treatment with highly purified urinary gonadotropins has been associated to a better patient response, as demonstrated by a reduction in treatment duration and gonadotropin dosage,10 differences in serum endocrine and follicular profile,11 an increased number of high quality embryos,12 and an improved endometrial receptivity.13
Studies comparing data from patients treated with r-FSH or hp-hMG following pituitary suppression with an antagonist are much less common. One such study found no significant differences between both patient groups in terms of ongoing pregnancy (RR: 1.09; 95% CI: 0.78–1.51), but did report a trend to higher number of live newborns. The number of oocytes recovered was significantly greater in the group treated with r-FSH. Authors also noted hormonal differences, as on the day of ovulation induction the group treated with hp-hMG had higher estradiol levels, while the group treated with r-FSH showed significantly higher progesterone levels.14
Another similar study where a third combined stimulation protocol (r-FSH up to day S5, replaced by hp-hMG until the end of treatment) was also added did not also find significant differences in the ongoing pregnancy rate, but reported a greater number of high quality embryos and improved laboratory parameters for the combined protocol.15
Based on the foregoing, it was considered adequate to further analyze the behavior of different gonadotropin stimulation protocols for controlled ovarian hyperstimulation of normally responding women in cycles with GnRH antagonist in a clinical care setting.
Materials and Methods
Three hundred and five female patients who were to undergo IVF/ICSI cycles at our center were assigned to any of the four treatment protocols with GnRH antagonist: Group I (n = 51; r-FSH + hp-hMG), Group II (n = 61; hp-hMG), Group III (n = 118; r-FSH at fixed doses), Group IV (n = 75; r-FSH with dose adjustment). The study was conducted from October 2007 to February 2008 in compliance with Good Clinical Practice. It was therefore submitted to an authorized ethics committee for evaluation, and approved by the relevant regulatory authorities.
Patients were enrolled if they met all of the following inclusion criteria: i) partner with sterility treatable by IVF or ICSI; ii) age ranging from 18–38 years; iii) BMI ranging from 18–30; iv) serum levels of FSH, LH, PRL, and testosterone within the normal laboratory ranges during the early follicular phase (days 2–4 of cycle); v) infertility attributable to tubal factors, mild current endometriosis (Grade I-II/ American Fertility Society), or male factors, or from an unknown cause; vi) no more than 3 prior assisted reproduction cycles (IVF/ICSI, etc.); vii) normal responders women; viii) no use of clomiphene citrate or gonadotropins within one month of study start; ix) evidence, as documented (in the past three years) by HSC/HSG/laparoscopy, of a normal uterine cavity, normal endometrium, presence of both ovaries; x) spermiogram performed in the past 6 months meeting the following requirements: if IVF is performed using the standard techniques, with partner or donor sperm, spermiogram should be normal according to World Health Organization criteria. Otherwise, fertilization using ICSI may be used. In this case, the couple must meet the minimum requirements for performing this technique. Exclusion criteria were as follows: i) Sperm samples not suitable for IVF-ICSI (according to World Health Organization criteria). Signs of significant bacterial infection within the past 6 months in the spermiogram of the partner; ii) data suggesting ovarian failure, even “hidden”, according to center criteria; iii) history of severe ovarian hyperstimulation syndrome; iv) significant systemic disease; v) pregnancy or contraindication to pregnancy; vi) treatment protocol refusal; vii) simultaneous participation in another study that could interfere with the results of this study; viii) contraindications of the study drug; ix) inability to comply with the rules established in this protocol for any reason.
Study design
This was a single center, open label, prospective, parallel cohort study comparing the clinical results of hp-hMG (Menopur®, Ferring Pharmaceuticals, Copenhagen, Denmark) and r-FSH (Gonal-F®, Merck Serono International, Geneva, Switzerland) in four groups of patients undergoing pituitary suppression with a GnRH antagonist (Ganirelix®, Orgalutran, Schering-Plough-Organon, Oss, The Netherlands) for IVF or ICSI. Patients who met the inclusion criteria were assigned to any treatment protocol based on the investigator decision. Patients received a maximum of one treatment cycle during the study.
The primary endpoint of the study was the ongoing pregnancy rate per started cycle. Study analysis was performed on the intent-to-treat (ITT) population, consisting of patients who had received at least one dose of gonadotropins. Ongoing pregnancy was defined as the presence of at least one viable fetus 10–11 weeks after embryo transfer, as documented by transvaginal ultrasound. Secondary efficacy endpoints included the clinical pregnancy rate (defined as the presence of a gestational sac with heartbeat two weeks after a biochemical diagnosis of pregnancy), implantation rate, stimulation days and dose, follicle number and size, plasma estradiol and progesterone levels on the days of transfer and ovulation induction, endometrial thickness on the transfer day, oocyte recovery, degree of embryo fragmentation, cancellation rate, miscarriage rate, and rates of other adverse events.
Protocols
All patients recruited into the study received oral contraceptives (Yasmin®, Química Farmacéutica Bayer, Barcelona, Spain) for 14–25 days before the start of stimulation, which was started on days 2 and 3 post-menstruation and was performed as follows:
Group I: Stimulation with r-FSH, started with 150–300 IU/day, until ultrasound control on day S6. From the second stimulation phase and until the end, r-FSH was replaced by hp-hMG, with potential dose adjustment if considered necessary by the investigator. The GnRH antagonist was added on day S6 at a dose of 0.25 mg and continued until the end of stimulation.
Group II: Stimulation with hp-hMG, started with 150–300 IU/day, until ultrasound control on day S6, when dose could be adjusted if considered necessary by the investigator. The GnRH antagonist was also added on day S6 at a dose of 0.25 mg and continued until the end of stimulation.
Group III: Stimulation with r-FSH at constant doses ranging from 150–300 IU/day on all stimulation days. The physician did not consider appropriate a dose adjustment at the day S6 control. The GnRH antagonist was also added on day S6 at a dose of 0.25 mg and continued until the end of stimulation.
Group IV: Stimulation with r-FSH, started with 150–300 IU/day, until ultrasound control on day S6, and the physician considered appropriate a dose increase (+75–150 IU) at such control. The GnRH antagonist was also added on day S6 at a dose of 0.25 mg and continued until the end of stimulation.
Once adequate follicular size was achieved, ie, three or more follicles with a mean diameter of 17 mm or greater as documented by transvaginal ultrasound, ovulation was induced by administering 250 μg of r-hCG (Ovitrelle®, Merck Serono International, Geneva, Switzerland). Follicular puncture and embryo transfer were performed according to the standard center criteria. A maximum of 3 embryos were transferred on day 3, according to the applicable Spanish regulations on assisted human reproduction techniques.
Luteal phase support consisted of 400–600 mg/day of micronized progesterone by the vaginal route.
To determine the miscarriage rate, a miscarriage was defined as a pregnancy documented by transvaginal ultrasound two weeks after a biochemical diagnosis of pregnancy but not confirmed 10–11 weeks after embryo transfer (ongoing pregnancy).
Statistical analysis
Sample size was calculated using an 80.0% power for detecting differences when the null hypothesis H0: p1 = p2 is tested using a one-sided Chi-square test for independent samples, considering a 5% significance level and assuming a 20% difference (25%–45%). Seventy experimental units were required in each treatment group, ie, a total of 280 patients in the study. Assuming 5% losses in each group, a total of 294 patients were considered to be required for the study.
Quantitative variables were displayed as mean (standard deviation) or median (25th percentile, 75th percentile) depending on whether or not they were normally distributed (Kolmogorov-Smirnov test). Qualitative variables were displayed by their frequency distribution.
For multiple between-group comparisons, an analysis of variance (ANOVA) was performed for quantitative variables that met the criteria for normality and homogeneity of variance (Levene test). A Kruskal-Wallis test or a median test was used for all other comparisons.
Multiple comparisons between proportions were performed using a Fisher’s exact test. Finally, a logistic regression model was used to control for the effect of confounding variables.
All analyses (except as otherwise stated) were performed using the intention-to-treat (ITT) method.
A value of P < 0.05 was considered statistically significant.
Results
Baseline data
Table 1 shows the demographic characteristics and baseline hormonal data of patients participating in the study. As may be seen, there were no relevant differences in any of the parameters reported in the table. Most patients received their first COH cycle on study entry. As regards infertility causes, approximately 70% of patients had a normal reproductive capacity, with a homogeneous distribution between the groups (Table 2).
Table 1.
Demographic data and baseline hormonal characteristics.
| r-FSH +hp-hMG (Group I) | hp-hMG (Group II) | Fixed-dose r-FSH (Group III) | r-FSH dose-adjusted (Group IV) | |
|---|---|---|---|---|
| Patient age (years) | 31.27 ± 2.83 | 32.39 ± 2.70 | 33.72 ± 3.33 | 33.19 ± 3.17 |
| Partner age (years) | 33.72 ± 4.54 | 35.94 ± 4.27 | 36.23 ± 4.68 | 35.74 ± 5.09 |
| Years of sterility | 4.69 ± 1.45 | 5.04 ± 2.14 | 5.75 ± 2.94 | 5.49 ± 2.54 |
| BMI (kg/m2) | 21.87 ± 2.32 | 22.34 ± 2.62 | 22.61 ± 2.66 | 23.45 ± 2.83 |
| First cycle | 42 (84%) | 43 (70.5%) | 72 (61%) | 54 (72%) |
| Baseline FSH (mIU/mL) | 6.12 ± 1.30 | 5.92 ± 1.47 | 6.42 ± 1.53 | 6.17 ± 1.21 |
| Baseline LH (mIU/mL) | 4.56 ± 1.73 | 5.28 ± 2.74 | 5.20 ± 2.22 | 4.76 ± 1.85 |
| Prolactin (ng/mL) | 21.16 ± 9.73 | 21.76 ± 12.44 | 20.09 ± 11.42 | 17.98 ± 7.86 |
| Baseline testosterone (ng/mL) | 0.52 ± 0.23 | 0.49 ± 0.22 | 0.48 ± 0.14 | 0.62 ± 0.39 |
| Baseline progesterone (pg/mL) | 7.10 (4.40 ± 11.20) | 5.85 (4.35 ± 8.40) | 8.30 (5.70 ± 13.00) | 9.60 (7.80 ± 17.00) |
Note: Data are given as the mean (standard deviation) or frequency (%).
Abbreviations: r-FSH, recombinant FSH; hp-hMG, highly purified hMG.
Table 2.
Primary cause of infertility.
| r-FSH +hp-hMG (Group I) | hp-Hmg (Group II) | Fixed-dose r-FSH (Group III) | r-FSH dose-adjusted (Group IV) | |
|---|---|---|---|---|
| Male factor (%) | 72.5 | 70.5 | 64.4 | 68.0 |
| Tubal lesion (%) | 17.7 | 8.2 | 13.6 | 9.4 |
| Other (%) | 9.8 | 21.3 | 22.0 | 22.6 |
Abbreviations: r-FSH, recombinant FSH; hp-hMG, highly purified hMG.
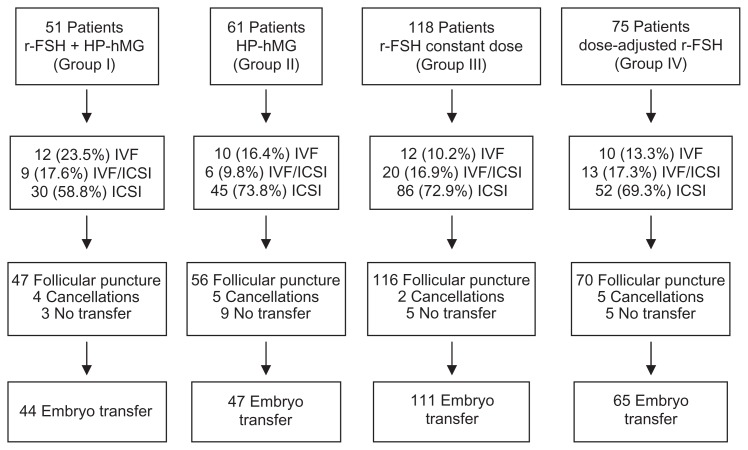
As discussed, the decision to include each patient in each of the treatment groups was taken beforehand by the investigator. As a result, 51 patients were assigned to Group I, 61 to Group II, 118 to Group III, and 75 to Group IV (Fig. 1). There were no statistical differences in the proportions of patients undergoing embryo transfer: 44 (86%) in Group I, 47 (77%) in Group II, 111 (94%) in Group III, and 65 (87%) in Group IV.
Figure 1.
Flow chart.
Controlled ovarian hyperstimulation
The mean number of days of gonadotropin therapy received by patients was 7.71 ± 1.19 in Group I, 7.62 ± 1.17 in Group II, 7.87 ± 1.31 in Group III, and 8.09 ± 1.25 in Group IV. Differences between Groups II and IV were statistically significant.
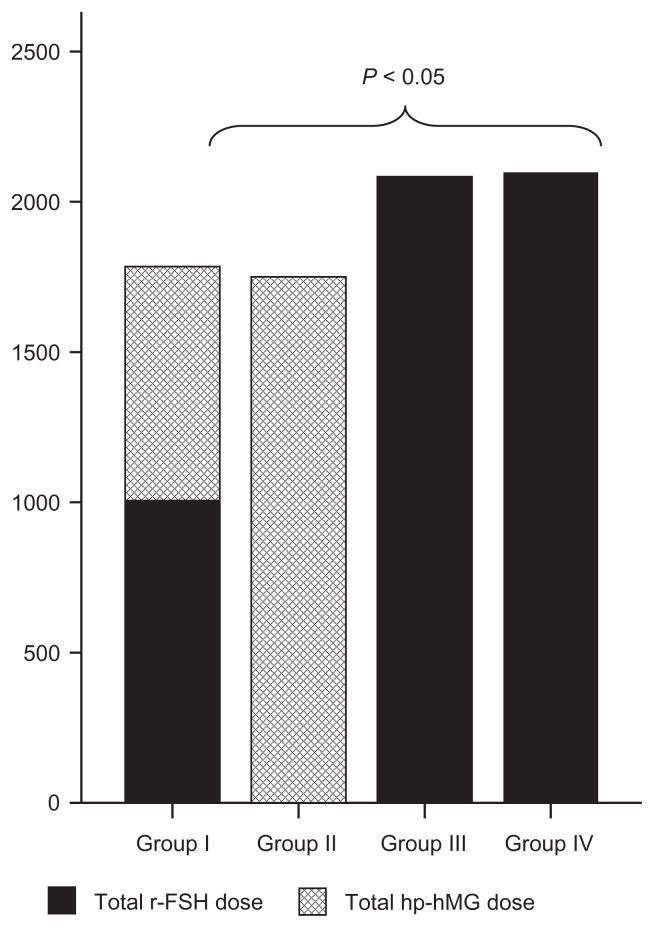
As regards the gonadotropin dose used for stimulation, the groups treated with r-FSH at constant and adjusted doses were found to require a higher total amount as compared to the other two groups (Fig. 2).
Figure 2.
Mean gonadotropin doses used in each stimulation protocol (international units). Significant differences in Groups I and II as compared to Groups III and IV. Kruskal-Wallis test.
A statistically higher total number of follicles were obtained in the group treated with r-FSH with potential dose adjustment as compared to the group given r-FSH at constant doses (Table 3). As regards follicle size, groups treated with hp-hMG and with dose-adjusted r-FSH were seen to achieve a higher number of follicles 10–14 mm in size than the other two groups, while the group treated with dose-adjusted r-FSH achieved a significantly higher number of follicles greater than 16 mm as compared to all other groups (Table 3).
Table 3.
Follicular, endometrial, and hormonal development during stimulation.
| r-FSH +hp-hMG (Group I) | hp-hMG (Group II) | Fixed-dose r-FSH (Group III) | r-FSH dose-adjusted (Group IV) | P | |
|---|---|---|---|---|---|
| Total follicles | 15.22 ± 7.13 | 14.00 ± 6.88 | 12.91 ± 5.96 | 15.70 ± 7.44 | <0.05a |
| Follicles < 10 mm | 4.64 ± 3.52 | 4.26 ± 3.14 | 3.49 ± 2.95 | 3.92 ± 3.15 | ns |
| Follicles 10–14 mm | 4.51 ± 2.85 | 5.29 ± 3.82 | 3.59 ± 2.11 | 5.02 ± 2.77 | <0.05b |
| Follicles 14–16 mm | 4.51 ± 3.52 | 3.75 ± 1.92 | 3.48 ± 2.19 | 4.19 ± 2.31 | ns |
| Follicles > 16 mm | 6.35 ± 2.37 | 6.81 ± 2.70 | 6.89 ± 2.84 | 8.70 ± 2.31 | <0.05c |
| Follicle diameter on hCG day | 20.35 ± 1.43 | 20.47 ± 1.38 | 20.41 ± 1.51 | 20.54 ± 2.02 | ns |
| Estradiol on hCG day (pg/mL) | 1680.11 ± 701.25 | 2022.67 ± 809.94 | 1516.36 ± 515.93 | 1914.14 ± 759.45 | <0.05b |
| Endometrial thickness on hCG day (mm) | 11.41 ± 1.67 | 11.72 ± 1.90 | 11.42 ± 2.15 | 11.65 ± 2.02 | ns |
| Progesterone on hCG day (pg/mL) | 41.10 (29.10. 60.0) | 45.00 (30.00. 65.00) | 46.50 (37.40. 61.00) | 51.30 (34.90. 92.00) | ns |
| Estradiol on transfer day | 750.72 ± 404.07 | 785.28 ± 325.89 | 719.51 ± 384.84 | 827.79 ± 340.40 | ns |
| Number of oocytes recovered | 9.43 ± 4.99 | 8.96 ± 4.82 | 8.78 ± 3.72 | 11.62 ± 5.80 | <0.05d |
| Number of oocytes transferred | 1.88 ± 0.32 | 1.83 ± 0.52 | 1.88 ± 0.45 | 1.81 ± 0.49 | ns |
Notes:
Group III vs. Group IV;
Groups II and IV vs. Group III;
Group IV vs. all other groups;
Group IV vs. Groups II and III. Kruskal-Wallis test.
All treatment groups achieved a similar endometrial development, approximately 11.5 cm. The small differences found were not significant (Table 3). No differences were also found between the groups in progesterone and estradiol levels on the transfer day (Table 3). However, groups treated with hp-hMG and dose-adjusted r-FSH alone achieved significantly higher estradiol levels than the group treated with r-FSH at constant doses (Table 3).
A significantly higher number of oocytes recovered were seen in the group treated with r-FSH with potential dose adjustment as compared to the group treated with hp-hMG and the group treated with r-FSH at constant doses. On the other hand, the number of embryos transferred was similar in all groups (Table 3).
Pregnancy-related parameters
As shown in Table 4, the group treated with hp-hMG alone achieved higher clinical and ongoing pregnancy rates than the other three groups, but the difference was not significant. If the results are stratified by the number of embryos transferred, significant differences were found favoring the above mentioned group because a 56.4% clinical pregnancy rate was achieved in this group if two embryos were transferred (Table 4). No significant differences were found between the treatment groups in the implantation rate, but a trend to a better result was seen in the group treated with hp-hMG alone (Table 4).
Table 4.
Pregnancy-related variables.
| r-FSH +hp-hMG (Group I) | hp-hMG (Group II) | Fixed-dose r-FSH (Group III) | r-FSH dose-adjusted (Group IV) | P | |
|---|---|---|---|---|---|
| Clinical pregnancy | 13/51 (25.5%) | 25/61 (41.0%) | 29/118 (24.6%) | 20/75 (26.7%) | ns |
| Ongoing pregnancy | 12/51 (23.5%) | 22/61 (36.1%) | 27/118 (22.9%) | 20/75 (26.7%) | ns |
| Implantation rate | 12% | 21% | 11% | 14% | ns |
| Clinical pregnancy* | 12/38 (31.6%) | 22/39 (56.4%) | 27/94 (28.7%) | 17/52 (32.7%) | <0.05a |
| Miscarriage rate | 1/51 (2.0%) | 3/61 (4.9%) | 2/118 (1.7%) | 0 (0%) | ns |
Notes:
Clinical pregnancy in patients with double-embryo transfer.
Statistically significant differences in the hp-hMG group as compared to all other groups. Pearson’s Chi-square test.
Finally, no differences were found between the treatment groups in the miscarriage rate (Table 4).
Safety
No differences were seen in the proportion of cycles cancelled in each group. The most common reasons for cancellation were decreased estradiol levels before the day of hCG and lack of ovarian response in Group I (r-FSH +hp-hMG), lack of ovarian response in Group II (hp-hMG), an accidental cause and lack of ovarian response in Group III (r-FSH at constant dose), and lack of ovarian response and risk of hyperstimulation in Group IV (r-FSH with dose adjustment) (Table 5).
Table 5.
Safety-related variables.
| r-FSH +hp-hMG (Group I) | hp-hMG (Group II) | Fixed-dose r-FSH (Group III) | r-FSH dose-adjusted (Group IV) | |
|---|---|---|---|---|
| Accidental | 0 (0%) | 1 (1.6%) | 1 (0.8%) | 0 (0%) |
| Estradiol drop before hCG | 2 (3.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Follicle dysfunction | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.3%) |
| Lack of ovarian response | 2 (3.9%) | 3 (4.9%) | 1 (0.8%) | 2 (2.7%) |
| Risk of hyperstimulation | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2.7%) |
| Other reasons | 0 (0%) | 1 (1.6%) | 0 (0%) | 0 (0%) |
| Total cancellations | 7.8% | 8.1% | 1.6% | 6.7% |
Discussion
This was the first study to compare four different protocols of controlled ovarian hyperstimulation in patients undergoing pituitary suppression with GnRH antagonists. Although no global differences were found between the protocols with regard to the primary objective of our study, if patients transferred two embryos are considered it can be seen that the clinical pregnancy rate was significantly higher in the group treated with hp-hMG as compared to all other groups.
This is a particularly significant finding considering that double-embryo transfer is the procedure most commonly used in Spain (60% of cycles) (SEF Registry) and Europe (56.1% of cycles).17
Our findings agree with the trend seen in a recent clinical trial showing an RR of 1.09 (95% CI: 0.78–1.51) favoring hp-HMG as compared to r-FSH in protocols with GnRH antagonists.14 Our data also agree with those collected in a study performed with three stimulation protocols with GnRH antagonists15 which, in addition to groups treated with r-FSH and hp-hMG, included a third protocol by which women were treated with f-FSH until day 5 of stimulation and with hp-hMG alone thereafter. Clinical pregnancy rates were 37.1% in the group treated with hp-hMG, 32.4% in the group treated with r-FSH, and 44.7% in the group given combined treatment. These differences were not statistically significant, but a significant difference favoring the combined protocol was found in the high quality embryo/oocyte recovery ratio.15
Both gonadotropin preparations have been compared many times, but in most cases the drug used for pituitary suppression was a GnRH agonist. In the most recent studies, use of hp-hMG was associated to a higher live newborn rate (RR: 1.18; 95% CI: 1.02–1.38)8 in women subject to IVF-ICSI procedures or to IVF alone (OR: 1.36; 95% CI: 1.01–1.83).9
In our study, patients treated with hp-hMG needed less stimulation days and a lower gonadotropin dose as compared to patients treated with r-FSH. This improved treatment efficacy has previously been reported by other authors.10 In addition, the group treated with r-FSH with potential dose adjustment achieved a greater follicle development than all other groups.
These results agree with those obtained in the study conducted by Platteau et al in 2006, where patients receiving hp-hMG were found to have a significantly lower number of follicles than those treated with r-FSH,18 and also with those from the Merit study13 and the abovementioned study by Bosch.14 This could be explained by the potential atretic effect on non-dominant follicles attributed to the LH/hCG effect.18
Patients treated with hp-hMG have also been reported to have a greater expression of antiapoptotic proteins in granulosa cells. This, combined with the higher progesterone levels found in patients treated with r-FSH,14 could have implications for oocyte development and competence,19 and hp-hMG therefore appears to be a potential modulator of follicle development.
All of these data, combined with the fact that treatment with GnRH antagonists may increase apoptosis of non-dominant oocytes,3 could be the explanation for the high pregnancy rates achieved in our study, particularly in the group treated with hp-hMG (41%), taking as reference the 25.6% transfer pregnancy rate in Spain recorded in the last ESHRE registry for IVF/ICSI cycles.17
In conclusion, women undergoing pituitary suppression with GnRH antagonists appear to have better results when COH with hp-hMG is administered. Such improvements would be a shorter duration of stimulation, lower gonadotropin doses, and improved pregnancy rates when two embryos are transferred.
A weakness of the study is that patients who met the inclusion criteria were assigned to any treatment protocol based on the investigator decision, ie, the decision to include each patient in each of the treatment groups was taken beforehand by the investigator. As a result, groups are misbalanced: 51 patients were assigned to Group I, 61 to Group II, 118 to Group III, and 75 to Group IV. The risk of bias due to the non randomization could not be excluded and it is recommend carrying out larger randomized controlled studies to confirm our findings.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The study was supported by Ferring Spain. The investigators received an economic compensation that corresponds to the inclusion and collection of data in accordance with the study that was carried out. The peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Olivennes F, Cunha-Filho JS, Fanchin R, Bouchard P, Frydman R. The use of GnRH antagonists in ovarian stimulation. Hum Reprod Update. 2002;8(3):279–90. doi: 10.1093/humupd/8.3.279. [DOI] [PubMed] [Google Scholar]
- 2.Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev. 2006;19(3):CD001750. doi: 10.1002/14651858.CD001750.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Dell’Aquila ME, Ambruosi B, de Santis T, Cho YS. Mitochondrial distribution and activity in human mature oocytes: gonadotropin-releasing hormone agonist versus antagonist for pituitary down-regulation. Fertil Steril. 2009;91(1):249–55. doi: 10.1016/j.fertnstert.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 4.Hayden C. GnRH analogues: applications in assisted reproductive techniques. Eur J Endocrinol. 2008;159(Suppl 1):S17–25E. doi: 10.1530/EJE-08-0354. [DOI] [PubMed] [Google Scholar]
- 5.Devroey P, Aboulghar M, Garcia-Velasco J, et al. Improving the patient’s experience of IVF/ICSI: a proposal for an ovarian stimulation protocol with GnRH antagonist co-treatment. Hum Reprod. 2009;24(4):764–74. doi: 10.1093/humrep/den468. [DOI] [PubMed] [Google Scholar]
- 6.Westergaard LG, Erb K, Laursen SB, Rex S, Rasmussen PE. Human menopausal gonadotropin versus recombinant follicle-stimulating hormone in normogonadotropic women down-regulated with a gonadotropin-releasing hormone agonist who were undergoing in vitro fertilization and intracytoplasmic sperm injection: a prospective randomized study. Fertil Steril. 2001;76(3):543–9. doi: 10.1016/s0015-0282(01)01973-2. [DOI] [PubMed] [Google Scholar]
- 7.Pinto F, Oliveira C, Cardoso MF, et al. Impact of GnRH ovarian stimulation protocols on intracytoplasmic sperm injection outcomes. Reprod Biol Endocrinol. 2009;15(7):5. doi: 10.1186/1477-7827-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coomarasamy A, Afnan M, Cheema D, van der Veen F, Bossuyt PM, van Wely M. Urinary hMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down-regulation protocol in IVF or ICSI treatment: a systematic review and meta-analysis. Hum Reprod. 2008;23(2):310–5. doi: 10.1093/humrep/dem305. [DOI] [PubMed] [Google Scholar]
- 9.Platteau P, Nyboe Andersen A, Loft A, Smitz J, Danglas P, Devroey P. Highly purified HMG versus recombinant FSH for ovarian stimulation in IVF cycles. Reprod Biomed Online. 2008;17(2):190–8. doi: 10.1016/s1472-6483(10)60194-0. [DOI] [PubMed] [Google Scholar]
- 10.Kilani Z, Dakkak A, Ghunaim S, et al. A prospective, randomized, controlled trial comparing highly purified hMG with recombinant FSH in women undergoing ICSI: ovarian response and clinical outcomes. Hum Reprod. 2003;18(6):1194–9. doi: 10.1093/humrep/deg252. [DOI] [PubMed] [Google Scholar]
- 11.Smitz J, Andersen AN, Devroey P, Arce JC MERIT Group. Endocrine profile in serum and follicular fluid differs after ovarian stimulation with HP-hMG or recombinant FSH in IVF patients. Hum Reprod. 2007;22(3):676–87. doi: 10.1093/humrep/del445. [DOI] [PubMed] [Google Scholar]
- 12.Ziebe S, Lundin K, Janssens R, Helmgaard L, Arce JC MERIT ( Menotrophin vs. Recombinant FSH in vitro Fertilisation Trial) Group. Influence of ovarian stimulation with HP-hMG or recombinant FSH on embryo quality parameters in patients undergoing IVF. Hum Reprod. 2007;22(9):2404–13. doi: 10.1093/humrep/dem221. [DOI] [PubMed] [Google Scholar]
- 13.Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21(12):3217–27. doi: 10.1093/humrep/del284. [DOI] [PubMed] [Google Scholar]
- 14.Bosch E, Vidal C, Labarta E, Simon C, Remohi J, Pellicer A. Highly purified hMG versus recombinant FSH in ovarian hyperstimulation with GnRH antagonists—a randomized study. Hum Reprod. 2008;23(10):2346–51. doi: 10.1093/humrep/den220. [DOI] [PubMed] [Google Scholar]
- 15.Requena A. Clinical efficacy and laboratory parameters for three controlled ovarian hyperstimulation (COH) protocols in IVF/ICSI following gonadotropin supression with GnRH antagonists in normoresponder women. Fertil Steril. 2008;90(Suppl 1):S478. [Google Scholar]
- 16.Sociedad Española de Fertilidad. Registro FIV-IAC/IAD de la Sociedad Española de Fertilidad 2006. Revista Iberoamericana de Fertilidad. 2009;26(Suppl 2):3–53. [Google Scholar]
- 17.Andersen A, Goossens V, Bhattacharya S, Ferraretti AP, Kupka MS, de Mouzon J, et al. European IVF-monitoring (EIM) Consortium, for the European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology and intrauterine inseminations in Europe, 2005: results generated from European registers by ESHRE: ESHRE. The European IVF Monitoring Programme (EIM), for the European Society of Human Reproduction and Embryology (ESHRE) Hum Reprod. 2009;24(6):1267–87. doi: 10.1093/humrep/dep035. [DOI] [PubMed] [Google Scholar]
- 18.Platteau P, Andersen AN, Balen A, et al. Menopur Ovulation Induction (MOI) Study Group. Similar ovulation rates, but different follicular development with highly purified menotrophin compared with recombinant FSH in WHO Group II anovulatory infertility: a randomized controlled study. Hum Reprod. 2006;21(7):1798–804. doi: 10.1093/humrep/del085. [DOI] [PubMed] [Google Scholar]
- 19.Grøndahl ML, Borup R, Lee YB, Myrhøj V, Meinertz H, Sørensen S. Differences in gene expression of granulosa cells from women undergoing controlled ovarian hyperstimulation with either recombinant follicle-stimulating hormone or highly purified human menopausal gonadotropin. Fertil Steril. 2009;91(5):1820–30. doi: 10.1016/j.fertnstert.2008.02.137. [DOI] [PubMed] [Google Scholar]