Abstract
Background
Endovascular interventions on peripheral arteries are limited by high rates of restenosis. Our hypothesis was that adventitial injection of rapamycin nanoparticles would be safe and reduce luminal stenosis in a porcine femoral artery balloon angioplasty model.
Methods and Results
Eighteen juvenile male crossbred swine were included. Single-injury (40%–60% femoral artery balloon overstretch injury; n=2) and double-injury models (endothelial denudation injury 2 weeks before a 20%–30% overstretch injury; n=2) were compared. The double-injury model produced significantly more luminal stenosis at 28 days, P=0.002, and no difference in medial fibrosis or inflammation. Four pigs were randomized to the double-injury model and adventitial injection of saline (n=2) or 500 μg of nanoparticle albumin-bound rapamycin (nab-rapamycin; n=2) with an endovascular microinfusion catheter. There was 100% procedural success and no difference in endothelial regeneration. At 28 days, nab-rapamycin led to significant reductions in luminal stenosis, 17% (interquartile range, 12%–35%) versus 10% (interquartile range, 8.3%–14%), P=0.001, medial cell proliferation, P<0.001, and fibrosis, P<0.001. There were significantly fewer adventitial leukocytes at 3 days, P<0.001, but no difference at 28 days. Pharmacokinetic analysis (single-injury model) found rapamycin concentrations 1500× higher in perivascular tissues than in blood at 1 hour. Perivascular rapamycin persisted ≥8 days and was not detectable at 28 days.
Conclusions
Adventitial nab-rapamycin injection was safe and significantly reduced luminal stenosis in a porcine femoral artery balloon angioplasty model. Observed reductions in early adventitial leukocyte infiltration and late medial cell proliferation and fibrosis suggest an immunosuppressive and antiproliferative mechanism. An intraluminal microinfusion catheter for adventitial injection represents an alternative to stent- or balloon-based local drug delivery.
Keywords: coronary restenosis, drug delivery systems, peripheral arterial disease, sirolimus
Endovascular interventions on the lower extremity arteries are limited by high rates of restenosis, leading to repeat procedures with high costs and diminished patient quality of life.1,2 Balloon angioplasty, the most cost effective treatment of infrainguinal atherosclerosis, has a binary restenosis rate as high as 40% to 60% at 1 year.3 Although some trials have demonstrated a short-term benefit with bare metal stents or covered stent-grafts, mid- to long-term results are mixed and the best results have consistently been demonstrated with short lesions.3–6 Adjunctive local drug delivery via drug-coated balloons and drug-eluting stents has been proposed to improve outcomes, and early clinical results seem promising.7–9 However, limitations unique to each of these drug delivery platforms may limit their clinical effectiveness in human superficial femoral arteries (SFA).
Rapamycin (sirolimus), a potent antiproliferative and immunosuppressive agent, has an established efficacy profile in coronary arteries, making it an attractive antirestenosis agent in the femoropopliteal arteries.10 However, stent-based, intimal delivery of rapamycin to the SFA has not been successful in clinical trials in part because of lesion length, overlapping stents, high incidence of stent fracture, and drug release kinetics.11,12 Hypersensitivity reactions, delinquent re-endothelialization, and late stent thrombosis documented in coronary arteries further tempers the enthusiasm for intimal-based paclitaxel or rapamycin delivery to the periphery.13 Nonstent-based platforms, such as drug-coated balloons, partially overcome these limitations by avoiding a permanent foreign body in the SFA. However, poor efficiency and unreliable release kinetics may limit their effectiveness.14
To address these challenges, we established an adventitial drug delivery program designed to deliver therapeutic agents to the target vessel injury site using a microinfusion catheter that has been used safely in human clinical trials.15 Theoretical advantages of this approach include the creation of an adventitial depot where drug is concentrated in the adventitia and media with relative sparing of the endothelial cells, direct targeting of potential neointimal progenitor cells in the outer media/adventitia interface and attenuation of adventitial inflammation.16–18 In this article, we propose that a novel albumin-bound rapamycin nanoparticle (nab-rapamycin) can be delivered safely to the SFA adventitia and reduce femoral artery luminal stenosis in a porcine balloon injury model.
Methods
Animals and Animal Care
Eighteen juvenile male Yorkshire cross pigs (mean weight, 34.7±3.3 kg) were included. For each procedure, pigs were anesthetized with ketamine (15 mg/kg IM) and atropine (0.04 mg/kg IM), intubated and kept under general anesthesia with isoflurane. After receiving antibiotics (cefazolin 25 mg/kg IV), open carotid artery access was secured, and heparin 5000 U IV was administered. After an angiogram, femoral artery injury, and adventitial injection (see below), the carotid artery access was repaired, and the animals were awoken from anesthesia. Postoperatively all animals received buprenorphine (0.03–0.05 mg/kg IM every 6–12 hours) for pain control, antibiotics (ceftiofur 3–5 mg/kg IM daily for 5 days), aspirin 81 mg daily, and normal pig chow. Blood samples were drawn preoperatively and postoperatively at 5 minutes, 20 minutes, 1 hour, 24 hours, and just before artery collection.
At the end of the study period, animals were anesthetized with ketamine (15 mg/kg IM) and atropine (0.04 mg/kg IM), intubated, and kept anesthetized with isoflurane. Open carotid artery access was secured followed by the administration of heparin 10 000 U IV and performance of a diagnostic angiogram to assess femoral artery patency. The animals were then euthanized with intravenous KCl solution (2 mEq/kg).
The animal care protocol was reviewed and approved by the Institutional Animal Care and Use Committee at ISIS Services LLC (San Carlos, CA).
Development of Porcine Femoral Artery Balloon Angioplasty Injury Models
As there are no established and validated peripheral porcine balloon injury models, we initially explored 2 techniques to create a reliable neointimal response. The first was a single-injury model using an angioplasty balloon to overstretch 4 cm of the proximal SFA 40% to 60% (3 inflations for 30 seconds each) to induce a high-level injury. The second was a double-injury model designed to create a diseased substrate before overstretch. In this model, an endothelial denudation injury was created with a compliant embolectomy balloon (Edwards Lifesciences, Irvine, CA) inflated to 2 atm and dragged 3× through the entire length of the target SFA. Two weeks later, balloon angioplasty was performed to overstretch 4 cm of the proximal and distal SFA (2 injury sites per femoral artery) by 20% to 30% (3 inflations for 30 seconds each).
Microinfusion Catheter
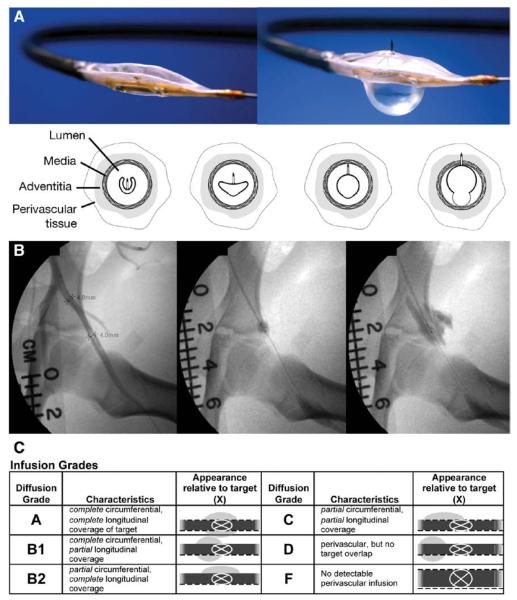
The Bullfrog Micro-Infusion Catheter (Mercator Medsystems, San Leandro, CA) is an US Food and Drug Administration (FDA) 510(k)-cleared, rapid-exchange, wire-guided catheter with a balloon-sheathed 0.9 mm long, 130 μm diameter (35 gauge) needle for delivering infusions to adventitial and perivascular tissues (Figure 1A). On inflation of the balloon, a pressure-valve apparatus on the catheter maintains a pressure of 2 atm to avoid barotraumas, and the needle is unsheathed. With the inflated balloon providing an opposing force, the needle passes through the artery wall into the adventitia. A mixture of infusate and contrast (4:1) is delivered to the adventitia under fluoroscopic guidance at a rate of 1 mL/min (Figure 1B). After injection, the balloon is deflated, thereby sheathing the needle and allowing the catheter to be withdrawn. Previous studies have demonstrated that a single circumferential adventitial injection (grade A, Figure 1C) can deliver a therapeutic dose of drug to the entire adventitia of a 5 cm length of artery, and additional injections can be performed when a >5 cm treatment length is needed.16
Figure 1.
Photographs and illustrations of the Bullfrog Micro-Infusion Catheter (A). Sequential angiogram images during microinfusion catheter deployment and adventitial injection in a pig femoral artery (B). Five-point grading scale for adventitial injection coverage and location (C). Images courtesy of Mercator MedSystems.
Nab-Rapamycin
Nab-rapamycin is a novel form of rapamycin complexed with albumin protein to create stable nanoparticles that are ≈100 nm in size. Unlike pure rapamycin, which is water insoluble, nab-rapamycin is freely dispersible in saline and easily injected. In preclinical studies with mouse xenograft cancer models, nab-rapamycin has shown an excellent efficacy and safety profile.19 In a phase I trial in patients with solid tumors, nab-rapamycin demonstrated activity in advanced metastatic disease and an excellent safety profile at its maximum tolerated dose of 100 mg/m2 (Neil Desai, PhD, unpublished data, 2012). In this report, doses of nab-rapamycin refer to the amount of rapamycin administered.
Femoral Artery Balloon Injury and Adventitial Injection
After anesthesia was induced and carotid access secured, the diameter of the femoral arteries was measured with digital subtraction angiography. A balloon angioplasty overstretch injury was performed according to the single- or double-injury model protocol. Immediately after the overstretch injury, 1.5 mL of a 4:1 mixture of infusate:contrast (IsoVue 370; Bracco Diagnostics, Princeton, NJ) was injected into the artery adventitia with a microinfusion catheter under fluoroscopy (Figure 1A and 1B). Adventitial infusions were graded on a 5-point scale for coverage and location (Figure 1C). After adventitial infusion, the microinfusion catheter was withdrawn, and a completion angiogram was performed. For cases of severe vasospasm from the angioplasty injury, intra-arterial nitroglycerin (100–200 μg) was injected.
The adventitial infusate consisted of sterile normal saline (control) or 500 μg of nab-rapamycin (Abraxis Bioscience, LLC, a wholly owned subsidiary of Celgene Corp, Summit, NJ) reconstituted in sterile normal saline. Preliminary in vivo dose finding experiments with adventitial nab-rapamycin injection of 5, 50, or 500 μg in a double-injury model determined the greatest efficacy with a 500 μg dose (data not shown).
Pharmacokinetic Analysis
The pharmacokinetic analysis was performed using a liquid chromatography/tandem mass spectroscopy (LC/MS/MS) technique (Bioanalytical Systems, McMinnville, OR) on whole blood samples, and femoral arteries treated with a single-injury model and an adventitial injection of nab-rapamycin 500 μg. For whole blood samples, an internal standard was added followed by a liquid–liquid extraction and evaporation of the organic layer to dryness using nitrogen. After reconstitution, samples were analyzed using high performance LC with gradient elution from the analytic column using a mobile phase starting of acetonitrile, 0.1% formic acid, and other additives. Detection by MS/MS incorporated an electrospray interface in positive ion mode. The range of the assay was 0.1 to 100 ng/mL of blood.
For the artery analysis, animals were euthanized, the femoral arteries flushed with 1 L of lactated Ringer solution and then 5 cm of femoral artery, and surrounding perivascular tissue was centered on the injection site was collected. Each artery was sectioned at 10 mm intervals with half of each section snap frozen at −70°C for the rapamycin analysis and half placed in 10% buffered formalin for histology. After thawing, the sample was minced and homogenized with a KH2PO4 buffer. A precipitation reagent containing the internal standard was added, and the supernatant was then analyzed with the LC/MS/MS technique used for whole blood. The range of the assay was 0.1 to 100ng/mL in tissue homogenate and is reported as ng/g of tissue.
Histomorphometry Analysis
All femoral arteries in the histomorphometry analysis were collected 28 days after a double-injury model and adventitial injection of saline or nab-rapamycin. Animals were euthanized, and the femoral arteries were flushed with 1 L Lactated Ringer solution followed by perfusion fixation with 10% buffered formalin at 120 mm Hg pressure for 10 minutes. The injection site was marked, and the femoral artery and its adventitia were sharply dissected 5 cm proximal and distal to the injection site. The artery was then sectioned and labeled at 0.5 cm intervals. Artery sections were embedded in paraffin, cut at 5-μm thickness onto slides and stained with hematoxylin and eosin or Masson trichrome.
Images of hematoxylin and eosin and Masson trichrome stained slides were captured with a Zeiss Axio ImagerA1 microscope and Axio MRc5 camera (Carl Zeiss Microscopy, Jena, Germany). Quantitative image analysis for artery histomorphometry was performed with National Institutes of Health ImageJ software (version 1.43). Measured parameters included the circumference and area of the artery lumen, internal elastic lamina, external elastic lamina, and adventitia as well as the maximum neointimal hyperplasia thickness and medial thickness. The intima/media ratio and percent lumen area stenosis [(1–lumen area/internal elastic lamina area)×100] were calculated from measured values.
Histology Analysis
Femoral artery sections treated with a single-injury model and adventitial injection of saline or nab-rapamycin were collected at 3 and 8 days after injury. Arteries were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 0.5 cm intervals and stained with hematoxylin and eosin or Masson trichrome. Arteries from the 28-day histomorphometry analysis also underwent histology analysis. To focus on the effect of nab-rapamycin on artery segments with demonstrable balloon angioplasty injury, 3 cm of contiguous artery sections centered on the injury/injection site were analyzed. Two femoral artery injury/injection sites were analyzed for each pig. In the double injury model, the 2 injury/injection sites (left proximal/distal and right proximal/distal SFA) with the highest injury scores were analyzed.
A semiquantitative analysis of artery wall injury, inflammation, fibrin/thrombus, hemorrhage, and medial fibrosis was performed on both hematoxylin and eosin and Masson trichrome stained sections using published 4-point scales.20 To assess cellular proliferation, Ki-67 immunohistochemistry staining (mouse monoclonal M7240, Dako, Denmark) was performed, and a proliferation index was calculated as the number of Ki-67–positive cells /500 cells per high-power field (400×). Endothelial cell coverage was assessed using a 4-point semiquantitative scale of the extent and intensity of factor VIII immunohistochemistry staining (rabbit polyclonal A0082, Dako, Denmark). The number of adventitial microvessels was counted in each arterial section. Because of the eccentric distribution of adventitial leukocytes, each arterial section was divided into 4 quadrants, and the number of adventitial leukocytes per high-power field per quadrant was counted by hand. The number of adventitial leukocytes per arterial section was calculated as the mean of the 4 quadrants.
Statistical Analysis
Data were assumed to be nonparametric and described as median with interquartile range (IQR). Because multiple artery sections were analyzed from each animal, linear mixed-effects models with maximum likelihood estimates were used to allow for adjustment of variance to account for within-animal and between-animal variability (see online-only Data Supplement). All statistical tests were performed with STATA/SE version 11 (StataCorp, College Station, TX).
Results
Femoral Artery Balloon Angioplasty Injury Models
A comparison of the single-injury (n=2 pigs) and double-injury (n=2) models was performed at 28 days after balloon angioplasty injury. All animals survived to 28 days after femoral artery injury, with equivalent weight gain and no significant adverse events. As expected, after 28 days femoral arteries injured with a double-injury model demonstrated significantly more luminal stenosis. However, at 28 days, there was no difference in the degree of medial fibrosis or inflammation (Table 1). To investigate the effect of adventitial nab-rapamycin infusion on luminal stenosis, the double-injury model was chosen for a 28-day histomorphometry analysis. Because the single-injury model created less neointimal hyperplasia but similar levels of medial fibrosis at 28 days, while avoiding an extra surgical procedure, this model was chosen for pharmacokinetic studies and early time-point histology analysis.
Table 1.
Femoral Artery Balloon Angioplasty Injury Models
| Single Injury | Double Injury | PValue | |
|---|---|---|---|
| Quantitative analysis | |||
| Percentage luminal stenosis | 5.5% (2.5–9.7) | 11% (8.0–17) | 0.002 |
| Neointimal thickness, mm | 0.18 (0.08–0.32) | 0.13 (0.09–0.24) | 0.9 |
| Medial thickness, mm | 0.35 (0.31–0.40) | 0.51 (0.46–0.54) | <0.001 |
| Intima/media ratio | 0.08 (0.04–0.16) | 0.10 (0.07–0.15) | 0.5 |
| Adventitial microvessels | 25 (10–83) | 11 (9–15) | 0.2 |
| Semiquantitative analysis | |||
| Injury | 2 (0–3) | 0 (0–1) | 0.4 |
| Inflammation | 1 (1–1) | 1 (1–1) | 0.3 |
| Medial fibrosis | 1.5 (0–2) | 0.5 (0–1.5) | 0.4 |
| Fibrin/thrombus | 0 | 0 | 1 |
| Hemorrhage | 0 | 0 | 1 |
| Pigs (femoral injury sites), n | 2 (4) | 2 (4) | … |
All values are median (IQR). Pvalues are from mixed-effects linear models (see the online-only Data Supplement).
Injection Parameters and Procedure Safety
Eighteen pigs underwent bilateral femoral artery balloon angioplasty injury and adventitial injection of either saline (control) or 500 μg of nab-rapamycin (Table 2). There was 100% procedural success with 32 injection sites, graded immediately after infusion as 28 A, 2 B1, and 2 B2 distributions. Ultimately, distributions that began as B1 or B2 continued to diffuse longitudinally and circumferentially, resulting in grade A distributions within 10 minutes in every case. The average injection time was 90 seconds. There were no dissections, early or late thrombosis, hemorrhagic foci or arteriovenous fistulas of the injected femoral arteries. There were no animal deaths, major infections or systemic signs of rapamycin toxicity. There was no difference in animal weight gain between treatment groups.
Table 2.
Study Design by Treatment Group and Injury Models
| Control (Animals/Arteries/Injections) | Nab-Rapamycin (Animals/Arteries/Injections) | |
|---|---|---|
| Single-injury model | ||
| 1 h | … | 2/4/4 |
| 3 d | 2/4/0 | 2/4/4 |
| 8 d | 2/4/0 | 2/4/4 |
| 28 d | 2/4/0 | 2/4/4 |
| Double-injury model | ||
| 28 d | 2/4/8 | 2/4/8 |
| Total number of pigs | 8 | 10 |
Animals: pigs per group. Arteries: total number of treated femoral arteries per group. Injections: total number of injection sites per group.
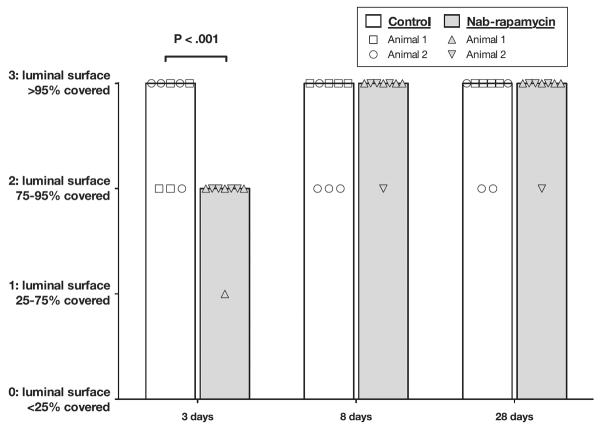
Semiquantitative histological analysis demonstrated significantly less endothelial coverage at 3 days after balloon angioplasty injury and nab-rapamycin treatment, but showed no difference in endothelial coverage between control and nab-rapamycin treated arteries at 8 and 28 days (Figure 2).
Figure 2.
Femoral artery endothelialization after balloon angioplasty injury (bar is median, 2 pigs per treatment per time point).
Histomorphometry and Histology Analysis
Femoral arteries from 4 pigs treated with the double-injury model and adventitial injection (2 control and 2 nab-rapamycin) were assessed at 28 days. By 28 days, adventitial nab-rapamycin treatment was associated with a 42% reduction in lumen area stenosis, 17% (IQR, 12%–35%) versus 10% (IQR, 2.5%–9.7%), P=0.001. Total femoral artery vessel area and medial area were similar between control and treated arteries, but control arteries demonstrated significantly greater intimal area and intimal thickness than treated arteries. There were no differences in the artery injury, inflammation, fibrin/thrombus, or hemorrhage on semiquantitative analysis. However, there was a significant reduction in the extent of medial fibrosis observed with nab-rapamycin treatment, P<0.001 (Table 3; Figure 3).
Table 3.
Histomorphometry and Histology Results at 28 Days
| Control | Nab-Rapamycin | PValue | |
|---|---|---|---|
| Quantitative analysis | |||
| Lumen area, mm2 | 1.9 (0.85–2.6) | 2.6 (1.8–3.3) | 0.04 |
| Percent luminal stenosis | 17%(12–35) | 10% (8.3–14) | 0.001 |
| Neointimal area, mm2 | 0.38 (0.19–0.57) | 0.26 (0.22–0.32) | 0.04 |
| Neointimal thickness, mm | 0.16 (0.096–0.37) | 0.096 (0.081–0.19) | 0.02 |
| Medial thickness, mm | 0.51 (0.41–0.53) | 0.45 (0.37–0.53) | 0.63 |
| Intima/media ratio | 0.13 (0.087–0.21) | 0.099 (0.088–0.14) | 0.05 |
| Total vessel area, mm2 | 6.8 (5.0–7.3) | 6.6 (5.7–8.0) | 0.1 |
| Adventitial microvessels | 15 (9–22) | 7 (3–10) | 0.001 |
| Semiquantitative analysis | |||
| Injury | 1 (0–1.5) | 1 (0–1) | 1 |
| Inflammation | 1 (1–1) | 1 (1–1) | 0.6 |
| Medial fibrosis | 1 (1–2) | 0.5 (0–1) | <0.001 |
| Fibrin/thrombus | 0 | 0 | 0.6 |
| Hemorrhage | 0 | 0 | 1 |
| Pigs (femoral injury sites), n | 2 (4) | 2 (4) | … |
All values are median (IQR). Pvalues are from mixed-effects linear models (see the online-only Data Supplement).
Figure 3.
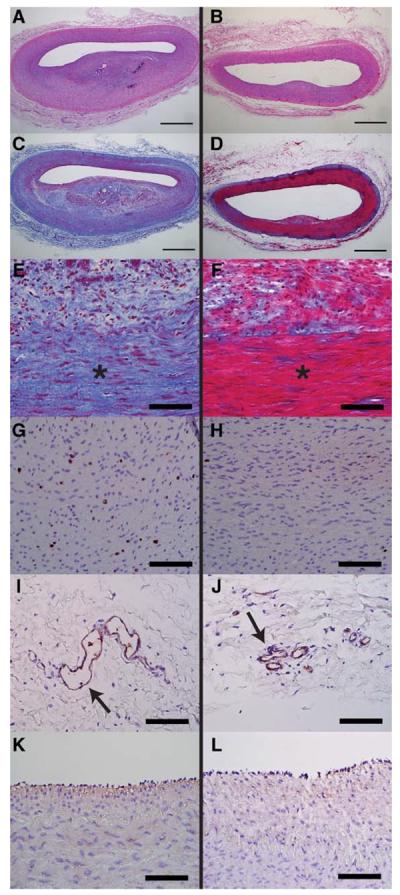
Representative histology images of pig femoral arteries 28 days after balloon angioplasty injury and adventitial injection in control (left) or nab-rapamycin (right) groups. Control arteries (A) had increased neointimal hyperplasia and luminal stenosis on hematoxylin and eosin staining compared with nab-rapamycin–treated arteries (B). Low- and high-power views of Masson trichrome stained control (C and E) and nab-rapamycin–treated arteries (D and F) demonstrate increased medial fibrosis (blue staining) in control arteries. Control arteries demonstrated more medial cell proliferation (Ki-67–stained cells in brown; G) than nab-rapamycin–treated arteries (H). Control arteries (I) had significantly more adventitial microvessels (arrow, Factor VIII staining) than nab-rapamycin–treated arteries (J). There was no significant difference endothelialization between control (K) and nab-rapamycin–treated (L) arteries (Factor VIII staining). *Marks media. Bar, 1 mm in A–D. Bar, 100 μm in E–L.
Adventitial Leukocyte Infiltration and Medial Cell Proliferation of the Femoral Arteries
It is known that the injury incurred from balloon angioplasty produces an intense early inflammatory cell infiltrate, which has been correlated with the rate of restenosis.17 In control femoral arteries, there was a substantial early inflammatory cell infiltrate. Arteries treated with adventitial nab-rapamycin, however, had significantly fewer adventitial leukocytes at 3 days, 130 (IQR, 123–148) versus 92 (IQR, 88–115), P<0.001 (Figure 4). At 8 days, the number of adventitial leukocytes had decreased slightly in both control and treated arteries, and there was no longer a significant difference between groups. By 28 days, the adventitial leukocyte counts had fallen 60% and 48% in the control and nab-rapamycin groups, respectively.
Figure 4.

Time course of adventitial leukocyte infiltration in control (2 pigs per time point) and nab-rapamycin-treated (2 pigs per time point) femoral arteries. HPF indicates high-power field.
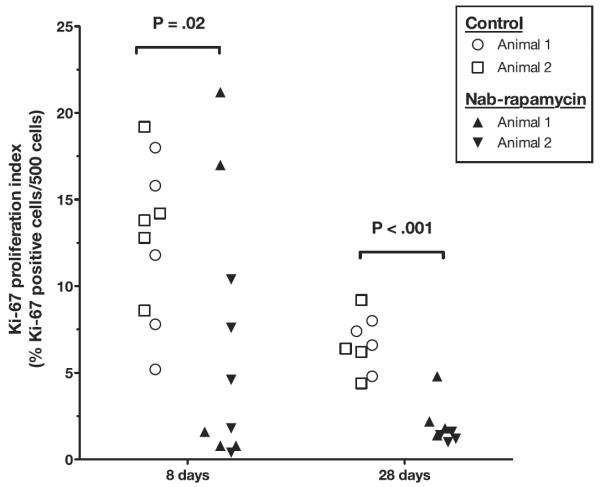
Although there was no difference in adventitial leukocyte count at 28 days, arteries treated with nab-rapamycin had significantly fewer adventitial microvessels and medial fibrosis, suggesting reduced artery wall proliferation and inflammation (Table 3). In addition, medial cell proliferation measured by Ki-67 immunohistochemistry staining showed that the proliferation rate in control and nab-rapamycin–treated arteries fell between 8 and 28 days. By 28 days, the proliferation index was significantly lower in nab-rapamycin–treated arteries, 6.5% (IQR, 5.5–7.7) versus 1.5% (IQR, 1.3–2), P<0.001 (Figure 5).
Figure 5.
Time course of medial cell proliferation (Ki-67 index) in control (2 pigs per time point) and nab-rapamycin-treated (2 pigs per time point) femoral arteries.
Pharmacokinetic Assay
Eight pigs (2 per time point) were treated with a single-injury model for a pharmacokinetic analysis of nab-rapamycin (Table 2). Each pig received bilateral femoral artery injuries and adventitial injections of nab-rapamycin 500 μg (1 mg total nab-rapamycin per pig). At 1 hour after injection, the rapamycin concentration in the femoral artery/perivascular tissues was 1500× higher than the concentration of rapamycin in blood (Figure 6). The maximum plasma level of rapamycin was 43 ng/mL (IQR, 30–51 ng/mL) at 1 hour falling to 5.4 ng/mL (IQR, 4.8–5.8 ng/mL) at 24 hours. The tissue concentration initially fell but was stable from 3 to 8 days. At 28 days after injection, rapamycin was no longer measurable in the blood or artery/perivascular tissues.
Figure 6.
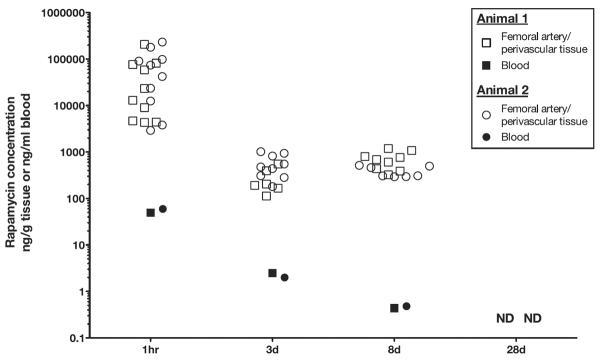
Rapamycin concentrations in blood and femoral artery/perivascular tissues after a femoral artery injury and adventitial injection of nab-rapamycin (2 pigs per time point). ND indicates not detectable.
Discussion
Adventitial delivery of nab-rapamycin after balloon angioplasty injury of a pig femoral artery was safe, efficient, and associated with a significant decrease in percentage luminal stenosis at 28 days, thus establishing the adventitia as a viable reservoir for local drug delivery and alternative to intimal delivery of nab-rapamycin.
By 2 hours after balloon angioplasty, there is an acute adventitial leukocyte infiltration that peaks at 72 hours and represents a major source of the progenitor cells responsible for intimal hyperplasia during the subsequent proliferative and remodeling wound healing phases.17,18 In this study, nab-rapamycin was associated with a significant reduction in adventitial leukocytes at 3 days, indicating an attenuation of the early inflammatory response. Barotrauma stimulates the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway, leading to cell growth, proliferation, and migration, particularly by neutrophils, monocytes, and vascular smooth muscle cells (VSMC). Rapamycin inhibits mammalian target of rapamycin and has been shown to reduce the innate immune response through a direct inhibitory effect on neutrophil proliferation and migration.21,22 In addition, rapamycin reduces the expression of inflammation- and adhesion-related genes by VSMC and reduces vascular endothelial growth factor expression and vascular endothelial growth factor–driven angiogenesis.23,24 Nab-rapamycin treatment was also associated with a significant reduction in adventitial microvessels, which are thought to be induced by vascular endothelial growth factor and have been linked to neointimal formation and restenosis after vascular injury.25
One of the most striking histological observations was the reduction in medial fibrosis associated with nab-rapamycin treatment compared with control vessels. To our knowledge, this is the first report of a reduction of vascular fibrosis, the end product of the injury response, with the use of rapamycin. Fibrosis has been associated with late lumen loss after balloon angioplasty attributable to constrictive remodeling, which is likely the result of inflammation induced fibroblast and VSMC activation.26,27 Rapamycin has been shown to arrest cell cycle progression and maintain VSMCs in a differentiated phenotype.28 Interestingly, the profibrotic transforming growth factor-β cell signaling pathway can also stimulate the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway, leading to increased levels of connective tissue growth factor, a factor associated with fibroblast proliferation, extracellular matrix production, and tissue fibrosis, which can be inhibited by rapamycin.29–31 Along with the early anti-inflammatory effects, the direct effect of nab-rapamycin on fibroblasts and VSMC proliferation and migration are likely responsible for the significant reduction in medial fibrosis, neointimal growth, and late luminal stenosis.
A single adventitial dose of 500 μg of nab-rapamycin had beneficial effects after balloon angioplasty with little crossover into the systemic circulation and without evidence of systemic toxicity. Blood concentrations of rapamycin ranged from 43 ng/mL (IQR, 30–51 ng/mL) at 1 hour to 5.4 ng/mL (IQR, 4.8–5.8 ng/mL) at 24 hours, modestly higher than the recommended trough levels of 5 to 15 ng/mL for therapeutic sirolimus therapy after kidney transplant.32 Simultaneously, the femoral artery/perivascular tissue concentration was >1500× higher than in blood at 1 hour, demonstrating the high efficiency of the microinfusion catheter as a local drug delivery platform. Direct injection of nab-rapamycin into the adventitia delivers the drug into an environment where the highly hydrophobic rapamycin molecules can partition into lipid-rich tissues and set up an adventitial drug depot with relative sparing of the endothelium. In addition, binding of the albumin–rapamycin complex to cell surface albumin-binding proteins may increase the intracellular delivery of rapamycin.33 Remarkably, rapamycin levels in the femoral artery were stable between 3 and 8 days after injection when the adventitial leukocyte count and cell proliferation rate were the highest. At 28 days, rapamycin was not detectable, but nab-rapamycin–treated arteries continued to have a significantly lower cell proliferation rate. The durability of this effect is unknown, and these results support the need for further studies.
Artery characteristics, such as the presence of thrombus, have been shown to reduce intimal-based drug delivery to the vessel wall.34 Injection of nab-rapamycin directly into the adventitia and perivascular space at the site of injury has the advantage of predictable drug delivery and scalable treatment allowing for optimal titration, depending on the extent of disease using a purely transcatheter technique. Similar to previously published reports,15,16 this study found that the advantages of adventitial drug delivery did not seem to come at expense of safety: there was no evidence of local toxicity, thrombosis, dissection, hemorrhage, or arteriovenous fistula with adventitial injection. Importantly, delayed endothelialization was not observed with nab-rapamycin injection. This highlights a fundamental difference between adventitial and intimal drug delivery: both approaches target the medial and adventitial cells responsible for intimal hyperplasia, but placing a drug-eluting stents delivers the highest dose of drug directly to the intima, inhibiting endothelialization of the stent struts and increasing the risk of late stent thrombosis.13,22
Existing animal models of femoral artery stenosis have been limited by either animal size or the appropriateness of the biological response. Pigs are ideally suited for studies of local drug delivery in the femoral artery because they have large arteries that are similar in diameter to human arteries. In addition, extensive work in coronary arteries has demonstrated that pigs exhibit a healing response similar to human arteries.35 However, the injury response of porcine femoral arteries to balloon angioplasty is not as robust as other animals. We attempted to increase neointimal formation by adding an endothelial denudation injury and were able to develop a model of SFA balloon angioplasty injury in juvenile cross pigs that demonstrates a modest but significant neointimal response, but the effect remains much less than mouse or rabbit models. In addition, the small library of commercially available pig antibodies limits immunohistochemistry staining to investigate the mechanisms of healing and neointimal formation. Nonetheless, the similarities between pig and human artery size and vessel injury response support the continued development of the preclinical porcine model for investigating novel therapeutics and nonstent-based drug delivery methods to treat femoropopliteal arteries.
This study has several limitations. There is a small sample size, and the stenosis induced by the balloon angioplasty injury with or without endothelial denudation injury was modest. Although pigs with familial or induced hypercholesterolemia have been used to increase stenosis after coronary artery injuries, it is unknown whether these models differ in the stenosis provoked by femoral artery angioplasty. In addition, our results lack time points between 8 and 28 days, which could help further characterize the pharmacokinetic behavior of nab-rapamycin in the adventitia and perivascular tissue as well as the initiation of the intimal hyperplasia and medial fibrotic response. Although the 28-day end point is commonly used and recommended for studying the safety, feasibility, and efficacy of local drug delivery,20,35 later time points may also help describe the longevity of the observed effect.
In conclusion, we have demonstrated that local delivery of nab-rapamycin to the adventitia of femoral arteries after balloon angioplasty injury is safe and decreases luminal stenosis. The mechanism of this effect seems to be mediated through decreased inflammation because there were significant decreases in early adventitial leukocytes, adventitial microvessel formation, and medial fibrosis associated with the treatment.
Supplementary Material
WhAT IS KNOWN
Endovascular interventions in the femoropopliteal arteries for peripheral artery disease are limited by high rates of restenosis.
Balloon angioplasty induces inflammation in the artery adventitia, which is associated with the development of intimal hyperplasia and restenosis.
Rapamycin is known to reduce restenosis, but drug-eluting stents in the femoropopliteal arteries have not demonstrated a benefit.
WhAT ThE STuDy ADDS
A novel, saline-dispersible form of rapamycin, nanoparticle albumin-bound rapamycin (nab-rapamycin), is safe when injected in the femoral artery adventitia using an endovascular microinfusion catheter in a porcine animal model.
Adventitial injection of nab-rapamycin 500 μg at a femoral artery balloon angioplasty injury site in a pig model is associated with a significant reduction in luminal stenosis and medial fibrosis at 28 days.
Acknowledgments
Nab-rapamycin was a gift from Abraxis Bioscience, LLC, a wholly owned subsidiary of Celgene Corp, Summit, NJ. We thank Barry Puget and the University of California, Davis Veterinary Medical Teaching Hospital histopathology laboratory for their assistance with immunohistochemistry staining.
Sources of Funding This project was supported by the National Heart, Lung, and Blood Institute award numbers R43HL102998 (Drs Owens and Seward) and K23HL92163 (Dr Owens) and a Lifeline award from the American Vascular Association (Dr Owens).
Footnotes
Disclosures Dr Seward is President and Chief Technology Officer for Mercator MedSystems, Inc, San Leandro, CA. The other authors report no conflicts.
The online-only Data Supplement is available at http://circinterventions.ahajournals.org/lookup/suppl/doi:10.1161/CIRCINTERVENTIONS.113.000195/-/DC1.
References
- 1.Stoner MC, Defreitas DJ, Manwaring MM, Carter JJ, Parker FM, Powell CS. Cost per day of patency: understanding the impact of patency and reintervention in a sustainable model of healthcare. J Vasc Surg. 2008;48:1489–1496. doi: 10.1016/j.jvs.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Sabeti S, Czerwenka-Wenkstetten A, Dick P, Schlager O, Amighi J, Mlekusch I, Mlekusch W, Loewe C, Cejna M, Lammer J, Minar E, Schillinger M. Quality of life after balloon angioplasty versus stent implantation in the superficial femoral artery: findings from a randomized controlled trial. J Endovasc Ther. 2007;14:431–437. doi: 10.1177/152660280701400401. [DOI] [PubMed] [Google Scholar]
- 3.Rocha-Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G, VIVA Physicians, Inc Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69:910–919. doi: 10.1002/ccd.21104. [DOI] [PubMed] [Google Scholar]
- 4.Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, Loewe C, Cejna M, Lammer J, Minar E. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–2749. doi: 10.1161/CIRCULATIONAHA.107.688341. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen BN, Conrad MF, Guest JM, Hackney L, Patel VI, Kwolek CJ, Cambria RP. Late outcomes of balloon angioplasty and angioplasty with selective stenting for superficial femoral-popliteal disease are equivalent. J Vasc Surg. 2011;54:1051–1057.e1. doi: 10.1016/j.jvs.2011.03.283. [DOI] [PubMed] [Google Scholar]
- 6.Johnston PC, Vartanian SM, Runge SJ, Hiramoto JS, Eichler CM, Owens CD, Schneider DB, Conte MS. Risk factors for clinical failure after stent graft treatment for femoropopliteal occlusive disease. J Vasc Surg. 2012;56:998–1006. 1007.e1. doi: 10.1016/j.jvs.2012.03.010. discussion 1006. [DOI] [PubMed] [Google Scholar]
- 7.Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, Claussen CD, Oldenburg A, Scheller B, Speck U. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–699. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 8.Werk M, Albrecht T, Meyer DR, Ahmed MN, Behne A, Dietz U, Eschenbach G, Hartmann H, Lange C, Schnorr B, Stiepani H, Zoccai GB, Hänninen EL. Paclitaxel-coated balloons reduce restenosis after femoropopliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5:831–840. doi: 10.1161/CIRCINTERVENTIONS.112.971630. [DOI] [PubMed] [Google Scholar]
- 9.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS. Zilver PTX Investigators. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 10.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 11.Duda SH, Pusich B, Richter G, Landwehr P, Oliva VL, Tielbeek A, Wiesinger B, Hak, Tielemans H, Ziemer G, Cristea E, Lansky A, Bérégi JP. Sirolimus-eluting stents for the treatment of obstructive superficial fem-oral artery disease: six-month results. Circulation. 2002;106:1505–1509. doi: 10.1161/01.cir.0000029746.10018.36. [DOI] [PubMed] [Google Scholar]
- 12.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Tielbeek A, Anderson J, Wiesinger B, Tepe G, Lansky A, Mudde C, Tielemans H, Bérégi JP. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J Vasc Interv Radiol. 2005;16:331–338. doi: 10.1097/01.RVI.0000151260.74519.CA. [DOI] [PubMed] [Google Scholar]
- 13.Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011;57:390–398. doi: 10.1016/j.jacc.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 14.Speck U, Cremers B, Kelsch B, Biedermann M, Clever YP, Schaffner S, Mahnkopf D, Hanisch U, Böhm M, Scheller B. Do pharmacokinetics explain persistent restenosis inhibition by a single dose of paclitaxel? Circ Cardiovasc Interv. 2012;5:392–400. doi: 10.1161/CIRCINTERVENTIONS.111.967794. [DOI] [PubMed] [Google Scholar]
- 15.Penn MS, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn FO, Strasser D, Ting AE, Sherman W. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ Res. 2012;110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 16.Karanian JW, Peregoy JA, Chiesa OA, Murray TL, Ahn C, Pritchard WF. Efficiency of drug delivery to the coronary arteries in swine is dependent on the route of administration: assessment of luminal, intimal, and adventitial coronary artery and venous delivery methods. J Vasc Interv Radiol. 2010;21:1555–1564. doi: 10.1016/j.jvir.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 19.Cirstea D, Hideshima T, Rodig S, Santo L, Pozzi S, Vallet S, Ikeda H, Perrone G, Gorgun G, Patel K, Desai N, Sportelli P, Kapoor S, Vali S, Mukherjee S, Munshi NC, Anderson KC, Raje N. Dual inhibition of Akt/mammalian target of rapamycin pathway by nanoparticle albumin-bound-rapamycin and perifosine induces antitumor activity in multiple myeloma. Mol Cancer Ther. 2010;9:963–975. doi: 10.1158/1535-7163.MCT-09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz RS, Edelman E, Virmani R, Carter A, Granada JF, Kaluza GL, Chronos NA, Robinson KA, Waksman R, Weinberger J, Wilson GJ, Wilensky RL. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008;1:143–153. doi: 10.1161/CIRCINTERVENTIONS.108.789974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Cambronero J. Rapamycin inhibits GM-CSF-induced neutrophil migration. FEBS Lett. 2003;550:94–100. doi: 10.1016/s0014-5793(03)00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessely R, Blaich B, Belaiba RS, Merl S, Görlach A, Kastrati A, Schömig A. Comparative characterization of cellular and molecular anti-restenotic profiles of paclitaxel and sirolimus. Implications for local drug delivery. Thromb Haemost. 2007;97:1003–1012. [PubMed] [Google Scholar]
- 23.Nührenberg TG, Voisard R, Fahlisch F, Rudelius M, Braun J, Gschwend J, Kountides M, Herter T, Baur R, Hombach V, Baeuerle PA, Zohlnhöfer D. Rapamycin attenuates vascular wall inflammation and progenitor cell promoters after angioplasty. FASEB J. 2005;19:246–248. doi: 10.1096/fj.04-2431fje. [DOI] [PubMed] [Google Scholar]
- 24.Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, Benjamin LE. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler Thromb Vasc Biol. 2009;29:1172–1178. doi: 10.1161/ATVBAHA.109.185918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khurana R, Zhuang Z, Bhardwaj S, Murakami M, De Muinck E, Yla-Herttuala S, Ferrara N, Martin JF, Zachary I, Simons M. Angiogenesis-dependent and independent phases of intimal hyperplasia. Circulation. 2004;110:2436–2443. doi: 10.1161/01.CIR.0000145138.25577.F1. [DOI] [PubMed] [Google Scholar]
- 26.Sierevogel MJ, Velema E, van der Meer FJ, Nijhuis MO, Smeets M, de Kleijn DP, Borst C, Pasterkamp G. Matrix metalloproteinase inhibition reduces adventitial thickening and collagen accumulation following balloon dilation. Cardiovasc Res. 2002;55:864–869. doi: 10.1016/s0008-6363(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt MR, Maeng M, Kristiansen SB, Andersen HR, Falk E. The natural history of collagen and alpha-actin expression after coronary angioplasty. Cardiovasc Pathol. 2004;13:260–267. doi: 10.1016/j.carpath.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–C517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 29.Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoo YT, Ong CT, Mukhopadhyay A, Han HC, Do DV, Lim IJ, Phan TT. Upregulation of secretory connective tissue growth factor (CTGF) in keratinocyte-fibroblast coculture contributes to keloid pathogenesis. J Cell Physiol. 2006;208:336–343. doi: 10.1002/jcp.20668. [DOI] [PubMed] [Google Scholar]
- 31.Pantou MP, Manginas A, Alivizatos PA, Degiannis D. Connective tissue growth factor (CTGF/CCN2): a protagonist in cardiac allograft vasculopathy development? J Heart Lung Transplant. 2012;31:881–887. doi: 10.1016/j.healun.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald A, Scarola J, Burke JT, Zimmerman JJ. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther. 2000;22(suppl B):B101–B121. doi: 10.1016/s0149-2918(00)89027-x. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 34.Hwang CW, Levin AD, Jonas M, Li PH, Edelman ER. Thrombosis modulates arterial drug distribution for drug-eluting stents. Circulation. 2005;111:1619–1626. doi: 10.1161/01.CIR.0000160363.30639.37. [DOI] [PubMed] [Google Scholar]
- 35.Wilson GJ, Nakazawa G, Schwartz RS, Huibregtse B, Poff B, Herbst TJ, Baim DS, Virmani R. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation. 2009;120:141–149. doi: 10.1161/CIRCULATIONAHA.107.730010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.