Abstract
A 2-year-old patient with a neuroblastoma developed haemolytic uraemic syndrome (HUS) following treatment with cisplatin and carboplatin. Following treatment with eculizumab, there was a substantial improvement in renal function with the recovery of the platelet count and the cessation of haemolysis. Subsequent investigations showed a novel, heterozygous CD46 splice site mutation with reduced peripheral blood neutrophil CD46 expression. Withdrawal of eculizumab was followed by the recurrence of disease activity, which resolved with re-introduction of therapy. Abnormal regulation of complement may be associated with other cases of cisplatin-induced HUS and treatment with eculizumab may be appropriate for other affected individuals.
Keywords: CD46, cisplatin, eculizumab, haemolytic uraemic syndrome
Background
Haemolytic uraemic syndrome (HUS) is most frequently caused by infection with Shiga toxin-producing strains of Escherichia coli or Shigella dysenteriae type 1. Other causes include inherited and acquired abnormalities affecting the alternative pathway of complement, other infections and certain drugs [1], including cisplatin [2] and carboplatin [3]. Recently eculizumab, a humanized monoclonal antibody directed against C5 that prevents the activation of the terminal complement pathway, has been shown to be an effective treatment for patients with atypical HUS (aHUS) [4].
Thrombotic microangiopathy has long been recognized as a complication of disseminated cancer [5] and cisplatin therapy [6]. There are two patterns of presentation of cisplatin-associated HUS. Some patients present acutely, days after receiving the last dose of chemotherapy. Others present more insidiously with a latent period of up to 7 months [2]. The acute form has a high mortality rate, although successful treatment with plasma exchange has been described [2, 7, 8].
Case report
A previously well 27-month-old boy with no family history of kidney disease presented with a cervical mass unresponsive to antibiotics. The plasma lactate dehydrogenase (LDH) was elevated at 1600 iu/L (normal 266–500). Biopsy of the mass confirmed neuroblastoma with MYCN gene amplification. Bone marrow examination and metaiodobenzylguanidine (MIBG) scan showed no evidence of distant metastases. Pre-treatment, the plasma creatinine concentration was 21 μmol/L, and the glomerular filtration rate (GFR; measured by plasma disappearance of Technetium (Tc)-99m labelled Diethylene Triamine Pentacaetic Acid (DTPA) was 107 mL/min/1.73 m2 body surface area. Therapy was started with a combination of vincristine, etoposide, carboplatin, cisplatin and cyclophosphamide.
On Day 45 of treatment (35 days after the first cisplatin dose, 15 days after the second), the GFR had fallen to 60 mL/min/1.73 m2. This was presumed to be due to acute cisplatin nephrotoxicity. Two days later, the patient became fluid overloaded and hypertensive. He was profoundly pancytopaenic, which was attributed to chemotherapy-induced myelosuppression. The hypertension became increasingly severe (maximum value recorded 160 mmHg systolic) and he required five agents to control the blood pressure.
He required seven infusions of platelets and four of red blood cells between Days 50 and 59, suggesting that the pancytopaenia was not solely due to myelosuppression and the plasma creatinine concentration rose from 39 to 140 μmol/L. A diagnosis of HUS was considered. A blood film confirmed the presence of schistocytes, the LDH had risen to 2438 iu/L and the plasma haptoglobin concentration was <0.06 g/L (normal 0.5–2.0 g/L). Serum complement C3 (1.06 g/L) and C4 (0.20 g/L) concentrations were normal. Renal biopsy was considered unsafe at this time, but the results above were considered compatible with a diagnosis of HUS, likely due to cisplatin. Stool and blood cultures were negative, as were serological tests for HIV1&2. ADAMTS13 (von Willebrand factor-cleaving protease) levels were 80% of control values.
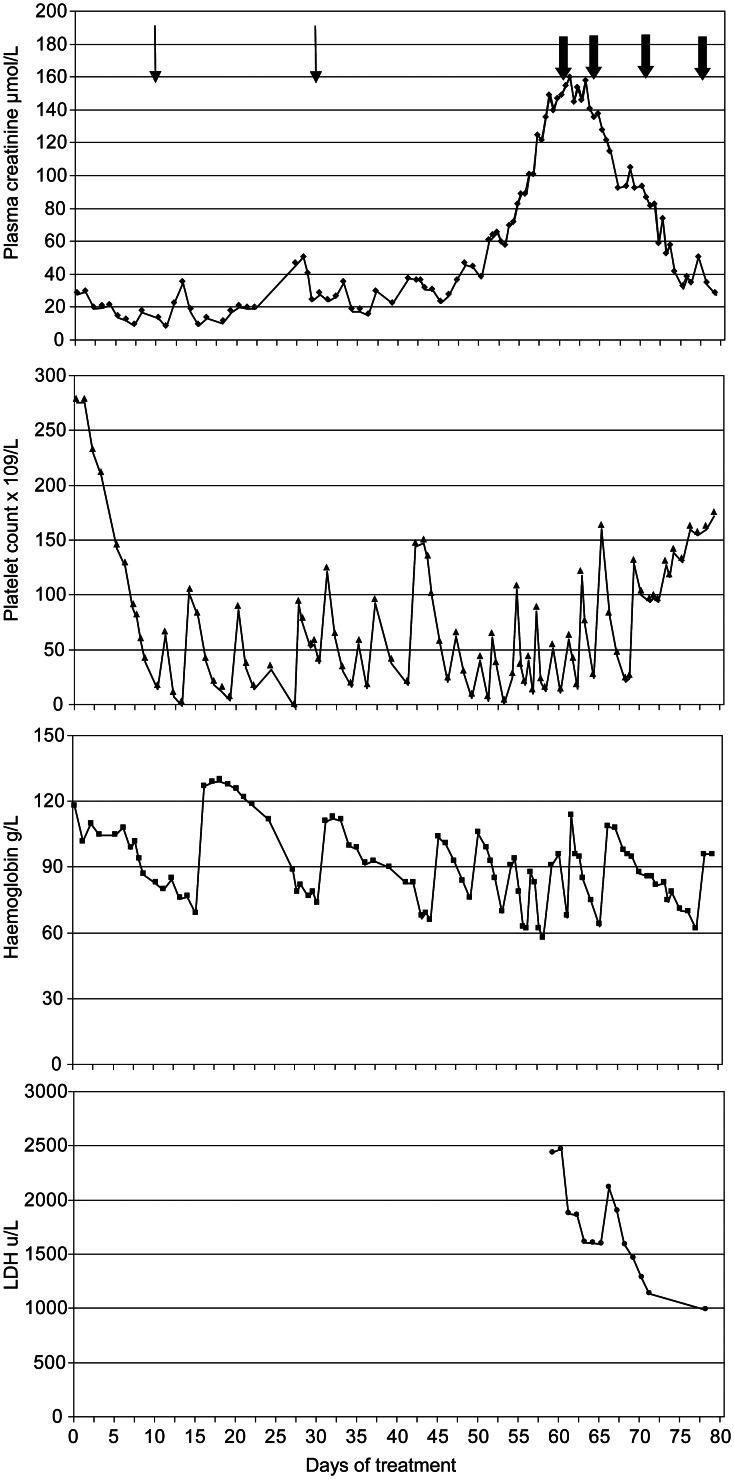
Despite the dearth of information concerning the pathogenesis of cisplatin/carboplatin-associated chemotherapy, we opted for therapy with eculizumab partly because it was felt that his severe hypertension, profound thrombocytopaenia and small size made plasma exchange hazardous. He received the first dose of 600 mg the following day when his plasma creatinine was 155 μmol/L. The next day the plasma creatinine peaked at 160 μmol/L and it steadily decreased thereafter (Figure 1). He also required progressively less blood product support. After a further dose of 300 mg a week later, he was maintained on fortnightly doses of 300 mg according to the manufacturer's dosing guidelines. Five weeks after administration of the first dose of eculizumab, the plasma creatinine concentration had reduced to 34 μmol/L, the plasma LDH was 532 iu/L and the GFR was 67 mL/min/1.73 m2. A decision was made to avoid further exposure to platinum-containing agents, and therapy was changed to a combination of vincristine, topotecan and doxorubicin. There was little further change in tumour size and, 126 days after starting chemotherapy, the tumour was surgically removed with clear excision margins and eculizumab was discontinued. At this time, the platelet count was 181 × 109/L and the plasma creatinine concentration was 34 µmol/L.
Fig. 1.
Graph summarizing key laboratory results. Thin arrows indicate doses of cisplatin; bold arrows indicate doses of eculizumab.
The platelet count fell to 138 four weeks after the last eculizumab dose and remained in the range of 98–160 × 109/L for the next 2 months. The plasma creatinine remained stable for 6 weeks, and then rose to 44 µmol/L. Two months after discontinuing the eculizumab, the plasma LDH had risen to 807 iu/L. The serum haptoglobin concentration was mildly reduced at 0.4 g/L and a blood film showed scanty red cell fragments. The plasma concentration of the terminal complement complex (sC5b-9) was elevated at 137 ng/mL (normal range <80). A renal biopsy was therefore performed.
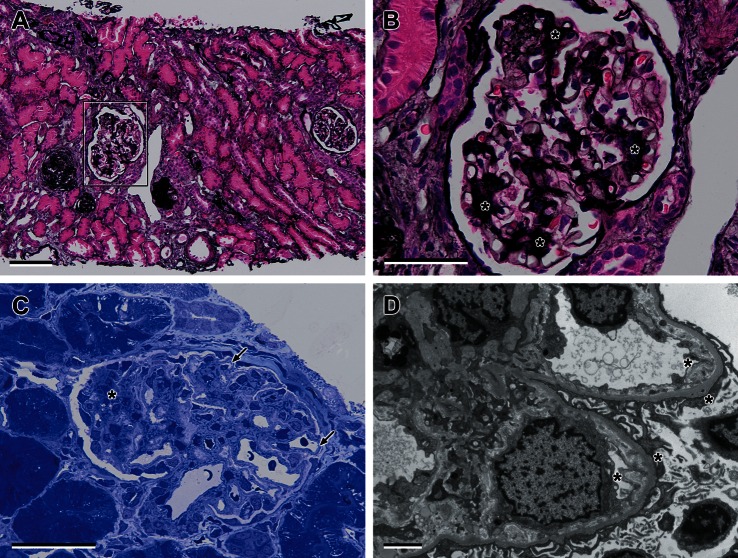
Of the 20 glomeruli in the light microscopy sections, five were globally sclerosed, and two showed periglomerular fibrosis. The patent glomeruli showed an increase in mesangial cells and matrix with segmental sclerosis and capsular adhesions. There were no platelet thrombi and extraglomerular vessels appeared normal. Immunostains for IgA, IgG, IgM and C3 were negative. There was focal tubular atrophy and interstitial fibrosis around damaged glomeruli. The toludine blue preparation and electron microscopy (EM) showed thickened glomerular capillary loops (double contour appearance), and areas of widening of the sub-endothelial space were seen. This space was filled with electron-lucent ‘fluffy’ material. This marked widening of the sub-endothelial space and fibrillar/particulate material is characteristic of HUS (Figure 2).
Fig. 2.
Light and electron microscopic findings in HUS (A). Light microscopy showing sclerosed glomeruli (to the left of the figure) and a glomerulus showing increased mesangial matrix expansion and segmental sclerosis (boxed area). There is also focal tubular atrophy around damaged glomeruli (Jones Silver histochemical stain, scale bar = 10 µm). (B) High power light microscopy showing glomerulus with mesangial matrix expansion and segmental sclerosis (asterisks) (Jones Silver histochemical stain, scale bar = 5 µm. (C) Toludine blue semithin preparation showing increased mesangial matrix expansion and segmental sclerosis (asterisks) and thickened capillary loop (arrow) mimicking membranoproliferative glomerulonephritis, scale bar = 5 µm. (D) EM showing fluffy-like material within a markedly widened sub-endothelial space consistent with HUS scale bar = 2 µm.
The haematological, biochemical and histopathological features suggested active HUS, and eculizumab was therefore recommenced with an initial dose of 600 mg given 105 days after the last dose. Before restarting the eculizumab, the GFR was measured at 54 mL/min/1.73 m2, the serum complement C3 concentration was 0.9 g/L and the C4 was 0.25 g/L. The total haemolytic complement (CH50) was 111%, but the alternative pathway haemolytic complement (AP50) was low at 34% (normal 80–200). The haptoglobin was low at 0.4 g/L and rose to 0.96 g/L a week after the first dose. After 7 months of eculizumab therapy, the patient had normal haemoglobin (122 g/L), normal platelets (248 × 109/L) and a plasma creatinine concentration of 32 µmol/L. The GFR had risen to 71 mL/min/1.73 m2. The plasma concentration of sC5b-9 remained elevated at 133 ng/mL, casting doubt on the suitability of this assay for assessing activity and response to treatment.
DNA extracted from a buccal smear at the time of diagnosis of HUS was amplified by PCR for all coding exons ± 10 bases of the CFH (factor H), CFI (factor I), C3 (complement component 3), CFB (factor B) and CD46 (membrane cofactor protein, MCP) genes. Amplified products were subjected to bi-directional Sanger sequencing (ABI), as previously described [9]. CD46 expression on cell surfaces was assessed on peripheral blood neutrophils after recovery from myelosuppression by flow cytometry using CD46 fluorescein isothiocyanate-conjugated antibody (BD Pharmingen, 555949).
An intronic sequence variant (c.1027+5G>T) was identified in CD46. This variant had not been previously reported nor was it detected in DNA from 188 normal control subjects. In silico splicing prediction analysis undertaken using Alamut (Interactive Bioscience Software, Seine Biopolis, 70 route de Lyons-la-Foret, 76 000 Rouen, France; http://www.interactive-biosoftware.com) suggests that this variant may cause aberrant splicing. Flow cytometry analysis showed reduced CD46 expression on neutrophils compatible with haploinsufficiency. After discussion with the parents, a decision was made not to test other family members, because the result was felt to be of limited clinical utility, outweighed by potential anxiety induced in a carrier.
Discussion
There is evidence that cisplatin causes widespread endothelial damage [10]. Within the glomerulus, endothelial cells, glomerular basement membrane and podocytes all show ultrastructural evidence of damage after cisplatin exposure [11]. Opsonization of damaged or apoptotic cells with C3b plays an important role in the efficient removal of such cells by phagocytes [12], and there is evidence of complement activation in cisplatin-induced AKI. On host cell surfaces, deposited C3b is usually rapidly inactivated by several regulatory proteins including CD46 (MCP) and factor H, both of which act as cofactors for factor I. Deficiency or reduced activity of these complement regulatory proteins would potentially lead to amplification of the endothelial cell damage induced by cisplatin. This in turn would potentiate and maintain the thrombotic microangiopathy. aHUS is most frequently associated with impaired regulation of the alternate pathway of complement regulation on the surface of host cells with normal regulation in the fluid phase, and plasma C3 levels are therefore frequently normal [13].
Because endothelial damage is associated with complement activation [14] and C5 plays a pivotal role in the development of aHUS [15], it seemed appropriate to initiate treatment with eculizumab. The clinical condition of our patient improved rapidly after starting eculizumab. The finding of a novel mutation causing decreased expression of CD46 suggests that dysregulated complement activation played an important role in pathogenesis. The response to eculizumab, deterioration on withdrawal of eculizumab and improvement after restarting treatment all support this hypothesis. Our patient is the first to have genetic investigation of the factors involved in the regulation of the alternative pathway of complement regulation and the first to be treated with eculizumab. The possibility that dysregulated complement activation underlies the thrombotic microangiopathy in other patients with cisplatin-induced HUS should be considered, and testing for abnormalities of complement regulation should be performed in future patients. Eculizumab therapy may be useful in other patients with cisplatin-induced HUS. The optimal duration of eculizumab therapy remains unclear; 4 months of therapy was clearly inadequate in our patient but we hope to be able to withdraw treatment in the future.
Conflict of interest statement
R.D.G. and T.H.G. have received fees for speaking at events sponsored by Alexion. R.D.G. received a travel grant to attend the ERA-EDTA conference in Paris in 2012. This manuscript has not been published in any format elsewhere.
Acknowledgements
We thank Lisa Strain of the Northern Molecular Genetics Laboratory, Newcastle-upon-Tyne and Deborah Dockey of the Department of Immunology, Newcastle-upon-Tyne for laboratory analysis and advice. Dr Shuman Haq of the Regional Paediatric Nephro-Urology Unit and Dr Juliet Gray of the Paediatric Oncology Unit, University Hospital Southampton for clinical input.
Footnotes
A version has been published to make this article Open Access.
References
- 1.Besbas N, Karpman D, Landau D, et al. A classification of hemolytic uremic syndrome and throbotic thrombocytopaenia and related disorders. Kidney Int. 2006;70:423–431. doi: 10.1038/sj.ki.5001581. [DOI] [PubMed] [Google Scholar]
- 2.Canpolat C, Pearson P, Jaffe N. Cisplatin-associated hemolytic uremic syndrome. Cancer. 1994;74:3059–3062. doi: 10.1002/1097-0142(19941201)74:11<3059::aid-cncr2820741125>3.0.co;2-z. doi:10.1002/1097-0142(19941201)74:11<3059::AID-CNCR2820741125>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Walker RW, Rosenblum MK, Kempin SJ, et al. Carboplatin-associated thrombotic microangiopathic hemolytic anaemia. Cancer. 1989;64:1017–1020. doi: 10.1002/1097-0142(19890901)64:5<1017::aid-cncr2820640508>3.0.co;2-n. doi:10.1002/1097-0142(19890901)64:5<1017::AID-CNCR2820640508>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 4.Loirat C, Frémeaux-Bacchi V. Atypical haemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60. doi:10.1186/1750-1172-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon LI, Kwaan HC. Cancer and drug-associated thrombotic thromocytopaenic purpure and haemolytic uremic syndrome. Semin Hematol. 1997;34:140–147. [PubMed] [Google Scholar]
- 6.Jackson AM, Rose BD, Graff LG, et al. Thrombotic angiopathy and renal failure associated with antineoplastic chemotherapy. Ann Int Med. 1994;101:41–44. doi: 10.7326/0003-4819-101-1-41. [DOI] [PubMed] [Google Scholar]
- 7.Mathur RV, Kumar S, Aparicio S, et al. Haemolytic uraemic syndrome following chemotherapy for an unusual germ-cell tumour. Nephrol Dial Transplant. 1998;14:1786–1788. doi: 10.1093/ndt/14.7.1786. doi:10.1093/ndt/14.7.1786. [DOI] [PubMed] [Google Scholar]
- 8.Thurner D, Kletzmayr J, Formanek M, et al. Chemotherapy-related haemolytic-uremic syndrome following treatment of a carcinoma of the nasopharynx. Oncology. 2001;61:143–146. doi: 10.1159/000055365. doi:10.1159/000055365. [DOI] [PubMed] [Google Scholar]
- 9.Moore I, Strain L, Pappworth I, et al. Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4 and with mutations in CFH, CFI, CD46, and C3 in patients with atypical haemolytic uraemic syndrome. Blood. 2010;115:379–387. doi: 10.1182/blood-2009-05-221549. doi:10.1182/blood-2009-05-221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dieckmann K-P, Struss WJ, Budde U. Evidence for acute vascular toxicity of cisplatin-based chemotherapy in patients with germ cell tumour. Anticancer Res. 2011;31:4501–4506. [PubMed] [Google Scholar]
- 11.Kohn S, Fradis M, Ben-David J, et al. Nephrotoxicity of combined treatment with cisplatin and gentamicin in the guinea-pig: glomerular injury findings. Ultrastruct Pathol. 2002;26:371–382. doi: 10.1080/01913120290104683. doi:10.1080/01913120290104683. [DOI] [PubMed] [Google Scholar]
- 12.Mevorach D, Mascarenhas JO, Gershov D, et al. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. doi:10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frémeaux-Bacchi V, Fakhouri F, Garnier A, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide french series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. doi:10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banz Y, Rieben R. Role of complement and perspectives for intervention in ischaemia-reperfusion damage. Ann Med. 2012;44:205–217. doi: 10.3109/07853890.2010.535556. doi:10.3109/07853890.2010.535556. [DOI] [PubMed] [Google Scholar]
- 15.De Jorge EG, Macor P, Paixão-Cavalcante D, et al. The development of atypical haemolytic uremic syndrome depends on complement C5. J Am Soc Nephrol. 2011;22:137–145. doi: 10.1681/ASN.2010050451. doi:10.1681/ASN.2010050451. [DOI] [PMC free article] [PubMed] [Google Scholar]