Abstract
Background
Resistance to chemotherapy represents a significant obstacle in prostate cancer therapeutics. Novel mechanistic understandings in cancer cell chemotherapeutic sensitivity and resistance can optimize treatment and improve patient outcome. Molecular alterations in the metabolic pathways are associated with cancer development; however, the role of these alterations in chemotherapy efficacy is largely unknown.
Methods
In a bed-side to bench-side reverse translational approach, we used cDNA microarray and qRT-PCR to identify genes that are associated with biochemical relapse after chemotherapy. Further, we tested the function of these genes in cell proliferation, metabolism, and chemosensitivity in prostate cancer cell lines.
Results
We report that the gene encoding mitochondrial malate dehydrogenase 2 (MDH2) is overexpressed in clinical prostate cancer specimens. Patients with MDH2 overexpression had a significantly shorter period of relapse-free survival (RFS) after undergoing neoadjuvant chemotherapy. To understand the molecular mechanism underlying this clinical observation, we observed that MDH2 expression was elevated in prostate cancer cell lines compared to benign prostate epithelial cells. Stable knockdown of MDH2 via shRNA in prostate cancer cell lines decreased cell proliferation and increased docetaxel sensitivity. Further, MDH2 shRNA enhanced docetaxel-induced activations of JNK signaling and induced metabolic inefficiency.
Conclusion
Taken together, these data suggest a novel function for MDH2 in prostate cancer development and chemotherapy resistance, in which MDH2 regulates chemotherapy-induced signal transduction and oxidative metabolism.
Keywords: MDH2, chemotherapy response, docetaxel, JNK, metabolism
Introduction
Genetic and epigenetic alterations in the metabolic pathway have been identified in prostate cancer (1–4). Although these identifications enhance our understanding in how the proliferation and survival of cancer cells are interrelated with metabolism, it is still unclear whether and how these metabolic alterations mediate sensitivity or resistance to the anticancer therapy. Developing an understanding of these mechanisms is important because it will allow us to 1) deliver the most effective therapy to patients with a specific metabolic profile and 2) improve the therapy efficacy by targeting a specific metabolic alteration that is proven to confer resistance.
Docetaxel is a microtubule-targeting chemotherapeutic agent (5, 6). Following intracellular uptake, docetaxel causes microtubule (MT) damage by binding to tubulin and preventing tubulin de-polymerization (7, 8). The MT damage signals induce mitotic arrest in proliferating cells and activate the intrinsic, or mitochondrial, pathway of apoptosis (9). Specifically, the MT-damage signals activate the stress-sensing kinase such as SAPK/JNK, which in turn activates the BH3-only pro-apoptotic proteins such as Bad, Bax and Bak, and inactivate the anti-apoptotic protein such as BCL2, BCL-xL and MCL1 (10–12). Consequentially, Bak/Bax proteins concentrate on the outer membrane of mitochondria and cause an increase of mitochondrial outer membrane permeabilization (MOMP) (13–15). MOMP allows the release of multiple pro-apoptotic factors such as cytochrome c from the mitochondria to cytoplasm to induce the activation of apoptotic caspases and cell death (16–18). Mitochondria perform three interrelated functions: 1) cellular energy metabolism via oxidative phosphorylation, 2) cellular redox balance, and 3) intrinsic apoptosis regulation (17). Currently, it is not entirely clear how the mitochondrial energy and redox machinery interact with the apoptotic machinery, which can be activated by the chemotherapy.
The docetaxel-based chemotherapy is a standard treatment for castration resistant prostate cancer. However, the treatment efficacy is limited because only 50% of the patients initially respond to the treatment and most of them eventually develop resistance (19–22). In addition, docetaxel-based chemotherapy is tested adjuvantly and neoadjuvantly in high-risk local prostate cancer patients to prevent or delay the onset of metastatic relapse (23–25). Although the short-term treatment effect is encouraging, the long-term efficacy is yet to be established because it will take 5 – 10 years for the biochemical relapse to occur and not all patients respond to the treatment equally. One advantage of testing chemotherapy in the high-risk local cancer patients is the availability of tumor specific biospecimen, which can be analyzed to identify the patient specific gene and mRNA alteration (26–29). Subsequently, the molecular alteration can be correlated with the patient’s treatment outcome and disease status. This approach provides a unique opportunity to develop novel molecular understandings in why and how cancer cells are either sensitive or resistant to the chemotherapy. Ultimately, the indentified mechanism can improve chemotherapy outcome, provide reliable markers to predict efficacy and indentify patients who will benefit.
Materials and Methods
Cell lines and reagents
Human prostate cancer cell line LNCaP, C42B, and PC3 were cultured in RPMI media as described (30, 31). The benign prostate hyperplasia cell line, BPH1 (from Dr. Joshi Alumkal at OHSU), was cultured in DMEM media. Docetaxel and SP600125 were purchased from Sigma, reconstituted, and stored as frozen aliquots.
Stable MDH2 knock down cell lines
The pLKO.1-puro vector-based lentiviral transduction particles containing MDH2 shRNA or non-target control shRNA were purchased from Sigma. The stable cell lines were selected and maintained with puromycin.
Cell proliferation and viability assay
Cell proliferation and viability were measured as previously described (30, 31). For the proliferation experiment, cells were allowed to grow over a 3-day period. The total viable cell numbers by the end of experiments were adjusted to day-1, and the final results were presented as fold of proliferation. For viability experiments, equal amount of cells were treated with increasing doses of docetaxel for 48 hours. By the end of treatments, the number of viable cells in the absence of docetaxel (0 nM docetaxel) was considered as 100% viability, and cells treated with docetaxel were adjusted to the 0 nM docetaxel.
Western blotting
Cells were lysed with M-PER (Thermo scientific) lysis buffer. Protein concentrations were determined using BCA protein assay kit (Thermo). Equal amounts of lysates (30–40 μg of protein) were resolved with SDS-PAGE. Antibodies used were: MDH2 (Santa Cruz), β-tubulin (Sigma); and phospho-SEK1/MKK4 (Ser257), phospho-SAPK/JNK (Thr183/Tyr185), phospho-c-Jun (Ser63), phospho-ATF-2 (Thr71), Phospho-Bcl-2 (Thr56), Bcl-2, and PARP (Cell Signaling).
Caspase 3/7 activity assay
Caspase 3/7 activity was measured using Caspase 3/7-Glo assay kit (Promega) following the manufacturer’s instructions. Briefly, 1×104 cells per well were plated in 96 well plates and cultured for 24h. Cells were treated with docetaxel (0–20 nM) for 24h, and then 100 μl reagents were added to each well and incubated for 30 min at room temperature. Caspase 3/7 activity was measured using a luminometer. Luminescence values were normalized by cell numbers. The effect of docetaxel on caspase 3/7 activation was assessed as fold of DMSO-treated control cells.
Metabolic measurement
NAD/NADH, ADP/ATP contents were measured using kits from Biovision following the manufacturer’s instructions. Cell lysates were deproteinized using the perchloric acid kit from Biovision. Mitochondria were isolated using a mitochondria isolation kit (Thermo) following the manufacturer’s instructions. Briefly, 1×107 cells were harvested and homogenized by a dounce homogenizer. Nulei were removed by low speed centrifugation and mitochondria were further isolated by high speed centrifugation.
ROS production and oxygen consumption
Intracellular ROS production was determined by measuring O2− and H2O2 levels using H2DCFDA (Invitrogen) staining followed by flow cytometry analysis as described (30). Oxygen consumption was measured by a Clark-type electrode. 2×106 cells were used and oxygen levels were monitored for 15 minutes with 1 minute intervals. Data were normalized by viable cells numbers.
Data analysis
Results are expressed as mean ± SD. The statistical difference between multiple treatments and control was analyzed using one way ANOVA. Differences between two groups were analyzed by student’s t test. Experiments were performed in triplicates and were performed at least three times.
Results
High level of MDH2 was associated with poor relapse-free survival in prostate cancer patients
To identify mRNA alterations associated with chemotherapy outcomes in the clinical prostate cancer, we compared the gene expression of patients with high risk local disease, who enrolled in a neoadjuvant chemotherapy clinical trial (32). Procedures of laser capture microdissection of patient-matched cancer and adjacent benign epithelium from both pre- and post- chemotherapy, and the subsequent cDNA microarray analysis have been described by us previously (27, 28). In the pretreatment samples, we found that MDH2 gene was overexpressed in nearly 50% of the patients, and most of the upregulations occurred to patients with biochemical relapse (Figure S1). Subsequently, we used real-time PCR to confirm the upregulation. We found that 15/31 patients had more than 0.75 fold MDH2 upregulation in cancer epithelium compared to the adjacent benign epithelium (Figure 1A). On the other hand, the chemotherapy treatment did not significantly change the MDH2 transcript levels comparing patient-matched post-treatment samples to pre-treatment samples (Figure S2). When we correlated the MDH2 gene expression data with the patient disease status, we found that patients with pre-chemotherapy MDH2 overexpression had a significantly poorer relapse-free survival (Figure 1B). This correlation suggested to us that MDH2 plays a role in regulating prostate cancer cell response to docetaxel-based chemotherapy. In consistence with the clinical data, we also found that MDH2 mRNA and protein levels were upregulated in prostate cancer cell lines LNCaP, C42B and PC3 compared to the benign prostate cell line BPH1 (Figure 1C).
Figure 1.
High level of MDH2 is associated with low post-chemotherapy survival rate. A) Paired tumor and benign epithelium from 31 patients were used for qRT-PCR determination of MDH2 transcripts. Using a log2 value of 0.75 (caner/benign) as the cutoff, MDH2 mRNA levels in pretreatment prostate cancer patients can be divided into the normal (≤ 0.75, n = 15) and high (> 0.75, n = 16) groups. B) Kaplan-Meier analysis of biochemical (PSA) relapse free survival (RFS) stratified by the MDH2 level (time in month). P < 0.05, Log-rank test. C) MDH2 mRNA and protein levels in normal prostate cell line BPH1 and prostate cancer cell lines LNCaP, C42B and PC3 were examined by qRT-PCR and western blot, respectively. Results of qRT-PCR were expressed as fold change (Fc) compared to BPH1 as mean and standard deviation of three experiments. * P < 0.01, t-test. Tubulin was used as loading control for western blots. Representatives of three experiments were shown.
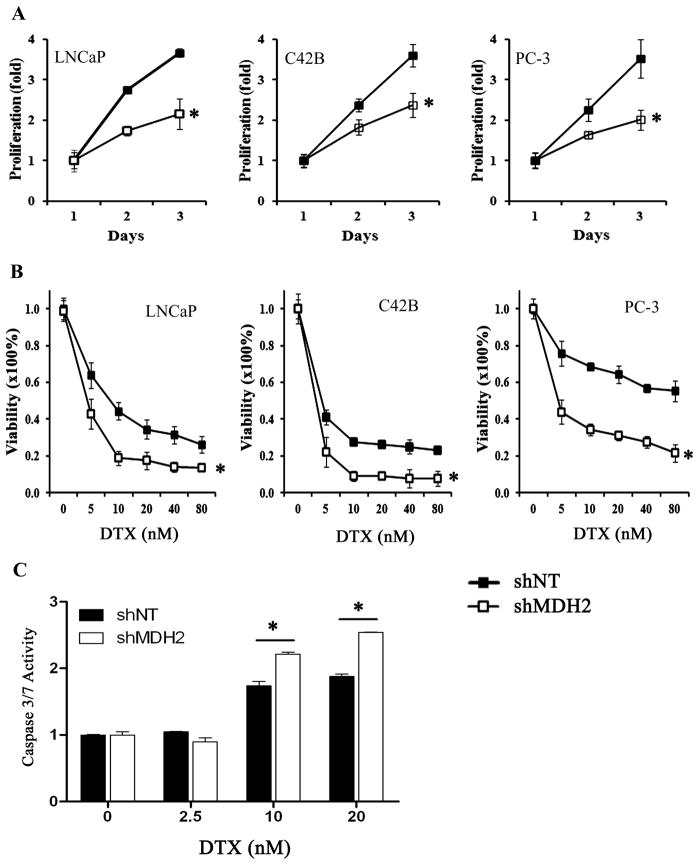
MDH2 shRNA knockdown increased docetaxel sensitivity in prostate cancer cell lines
To test the role of MDH2 in docetaxel chemotherapy, we established prostates cancer cell lines with MDH2 stable shRNA knockdown (Figure S3). We found that cell proliferation was significantly reduced in the MDH2 shRNA (shMDH2) cells compared to the non-targeting control shRNA (shNT) cells (Figure 2A). Next, we evaluated the role of MDH2 in docetaxel sensitivity. We treated the shNT and shMDH2 prostate cancer cell lines with different concentrations of docetaxel and measured the cell viability. We observed that prostate cancer cells became more sensitive to docetaxel with MDH2 shRNA as evidenced by a further decrease of cell viability in shMDH2 cells compared with shNT cells after docetaxel treatment (Figure 2B). We further examined whether MDH2 shRNA could increase docetaxel induced apoptosis by measuring the caspase 3/7 activity. The results showed that MDH2 shRNA did not change the basal caspase activities without chemotherapy; however, starting at 10 nM of docetaxel, MDH2 shRNA significantly increased the docetaxel induced caspase 3/7 activities compared to shNT cells (Figure 2C).
Figure 2.
The effect of MDH2 shRNA knockdown on prostate cancer cell proliferation and chemosensitivity. A) The proliferation of shNT and shMDH2 cells was measured over a 3-day period. The result was expressed as fold of proliferation over day 1. B) shNT and shMDH2 cells were treated with indicated concentrations of docetaxel (DTX). Cell viability at each dose was calculated by using solvent control (0 nM docetaxel) as 100% viability. C) The docetaxel-induced caspases 3/7 activation was measured in C42B shNT and shMDH2 cells. All measurements represent the mean and standard deviation of at least three measurements. * P < 0.01, t-test.
MDH2 shRNA enhanced docetaxel induced JNK signaling pathway activation
Docetaxel treatment can lead to the activation of JNK signaling pathway, which in turn mediates the cytotoxic effect (12, 33). We examined whether MDH2 shRNA affects the docetaxel-induced JNK signaling cascade activation. We treated C42B and PC3 cells with or without MDH2 shRNA with docetaxel for 24 hours. As expected, docetaxel treatment activated the JNK signaling cascade as seen in MKK4, JNK1/2, ATF2 and c-Jun phosphorylation in the non-targeting (NT) control shRNA cells (Figure 3A). Importantly, in MDH2 shRNA knockdown cells, the docetaxel-induced phosphorylations of JNK, ATF2 and c-Jun were further increased (Figure 3A). The activated JNK is able to phosphorylate and inactivate the anti-apoptotic protein Bcl2, which leads to the induction of mitochondria-based apoptosis (33–35). Consistent with the enhancement effect on JNK signaling, we observed that MDH2 shRNA further increased the docetaxel-induced phosphorylation of Bcl2, and the activation of PARP (Figure 3B). These results suggested that MDH2 shRNA knockdown enhanced docetaxel induced activation of JNK and the downstream apoptotic pathways.
Figure 3.
MDH2 shRNA enhances docetaxel-induced JNK and apoptosis signaling. A–B) shNT and shMDH2 cells were treated with solvent control (CT) or 10 nM docetaxel (DTX) for 24h. Cells were harvested and protein levels of MDH2, JNK pathway (A) and cell death pathway (B) were analyzed by western blots. Protein signals were quantified by NIH Image J software, adjusted by tubulin, and normalized to solvent treated shNT cells. C) shNT and shMDH2 cells were treated with solvent (CT) or 10 nM DTX for 24h. The drug containing medium was removed, and cells were cultured in growth medium for the indicated time before protein harvest and western analysis. The signals of phosphorylated proteins were quantified by NIH Image J software, adjusted by the total protein and normalized to the CT. D) PC3 (top) and C42B (bottom) shNT and shMDH2 cells were treated with JNK inhibitor SP600125 (5 μM), DTX (20 nM), or combination of both agents for 72h. The cell viability was measured. In PC3 (top) the percentage, by which JNK inhibitor rescued the cells from DTX-induced cytotoxicity, was calculated for shNT and shMDH2. In C42B (bottom), P values comparing shNT versus shMDH2 in DTX and combination treatments were calculated by t-test. In A) & B), the results were representative of at least three western blots. Results in D) represent the mean and standard deviation of three experiments.
To further investigate the enhancement of JNK signaling by MDH2 shRNA, we first treated PC3 cells with docetaxel for 24 hours, and then removed the drug-containing medium and measured the sustainability of JNK pathway activation by examining the phosphorylation of JNK pathway proteins. We observed that JNK, c-Jun and Bcl2 phosphorylations were gradually decreased after docetaxel withdraw in shNT cells. However, in shMDH2 cells, we observed a more sustained activation of these proteins after the docetaxel removal (Figure 3C). To test whether the JNK pathway enhancement plays a role in MDH2 shRNA enhanced docetaxel sensitivity, we treated PC3 shNT and shMDH2 cells with docetaxel in the presence of a JNK inhibitor SP600125. We observed that SP600125 rescued docetaxel induced cytotoxicity in both PC3 and C42B cells (Figure 3D). In PC3 cell lines, the JNK inhibitor provided a greater percentage of rescues in MDH2 shRNA cells than in the non-targeting control shRNA cells (Figure 3D top). In C42B cells, the JNK inhibitor abolished the enhanced docetaxel sensitivity due to MDH2 knockdown (Figure 3D bottom). Taken together, these data suggested that MDH2 negatively regulates docetaxel sensitivity. MDH2 knockdown enhances the docetaxel-induced JNK pathway activation, which leads to the increase of mitochondrial apoptosis signaling and chemosensitivity.
MDH2 shRNA induced inefficiency in oxidative phosphorylation
MDH2 is an important enzyme in the TCA cycle regulating the conversion of malate/NAD+ to oxaloacetate/NADH (36, 37). The NADH generated by MDH2 becomes the metabolic substrate for mitochondrial oxidative phosphorylation and ATP biosynthesis in the mitochondrial electron transport chain (ETC), which also requires cellular respiration by consuming molecular oxygen (16, 38). Disruption of the oxidative phosphorylation leads to less ATP production, with the consumed oxygen being converted to reactive oxygen species (ROS) instead of water (H2O) (39–42). Next, we measured the effect of MDH2 shRNA on prostate cancer cell mitochondrial energy metabolism. We found that mitochondrial NAD+ and NAD+/NADH ratio was significantly increased by MDH2 shRNA in PC3 cells (Figure 4A), suggesting that MDH2 knockdown disrupts the final step of TCA cycle. We also found that the ATP level was significantly decreased by MDH2 shRNA, and the ADP/ATP ratio was significantly increased in all the cell lines (Figure 4B) & (Figure S4). This suggested that MDH2 shRNA reduces the mitochondrial oxidative phosphorylation. However, this reduced ATP production was not associated with the reduced cellular respiration because we found that the oxygen consumption was not affected by MDH2 shRNA (Figure 4C, top) & (Figure S5). On the other hand, the ROS production was significantly increased by MDH2 shRNA in PC3 (Figure 4C, bottom) and in LNCaP (Figure S6) cells. Taken together, these results indicated that the MDH2 knockdown caused metabolic inefficiency in oxidative phosphorylation by decreasing ATP production and increasing ROS formation.
Figure 4.
MDH2 shRNA increased the inefficiency of oxidative phosphorylation. A) Mitochondria were isolated from PC3 shNT and shMDH2 cells and used to measure NAD+ and NADH levels. B) PC3 shNT and shMDH2 cells were used for ADP and ATP measurement. C) Top: The oxygen consumption in PC3 shNT and shMDH2 cells was measured by a Clark-type electrode. Bottom: The ROS levels in PC3 shNT and shMDH2 were measured by H2DCFDA staining and flow cytometry. The peak intensity shifting to the right suggests an increase of ROS. A representative histogram from more than three experiments was shown. D) Cell viabilities of PC3 cells were measured after DTX and oligomycin A treatments. Insert: PC3 cells were treated with vehicle (CT), 10nM DTX (D), 5 μM oligomycin A (O), and combination of DTX and oligomycin (DO). Phospho-JNK1/2 and JNK1/2 levels were measured by western blot. Results shown are representatives of three independent experiments. Results in all bar graphs are expressed as fold change over shNT cells. Mean and standard deviation was calculated from at least three experiments. * P < 0.05, *** P < 0.01, t-test.
Oxidative phosphorylation and ATP biosynthesis can be inhibited by the ETC inhibitor oligomycin. Therefore, we tested whether oligomycin, which is not a specific MDH2 inhibitor, can enhance docetaxel sensitivity in prostate cancer cells by mimicking the effect of MDH2 shRNA on mitochondria. We treated PC3 cell line with increasing concentrations of docetaxel and oligomycin individually and in combination. However, we did not observe any synergistic effect between docetaxel and oligomycin (Figure 4D). Interestingly, oligomycin treatment did not enhance docetaxel-induced JNK activation either (Figure 4D insert). This suggests that merely targeting mitochondrial oxidative phosphorylation and ATP biosynthesis cannot recapitulate the effect of MDH2 shRNA in enhancing chemotherapy sensitivity. Therefore, the enhanced docetaxel sensitivity due to MDH2 shRNA knockdown is mediated by the combination of JNK pathway enhancement and metabolic inefficiency. This also underscores the need to identify more specific MDH2 inhibitor, which can induce metabolic inefficiency and sensitizes cells to JNK activation, to enhance chemosensitivity.
Discussion
Mitochondrion provides crucial regulations for both apoptotic and necrotic cell death (17). Docetaxel causes cell death by damaging microtubules (MT) (14). Although the MT-damaging signals solicit cell death, the intrinsic cellular stress response machinery, which can be significantly enhanced in cancer cells, counters with survival signals (43–45). Together, they form the MT-damage response system. The net signal output between cell death and survival determines the cell fate. Both cytotoxic and survival signals inevitably converge on mitochondria, and the integration of these two conflicting signals by mitochondria plays a significant role in determining the level of cell death and the efficacy of docetaxel. Currently, how mitochondria integrate these signals and how resident mitochondrial proteins affect the integration process are largely unknown. In the current study, we found that the mitochondrial MDH2 plays a role of modifying the anti-tumor effect of the MT-damaging reagent docetaxel.
MDH2 regulates the final step of mitochondrial TCA cycle, which directly provides reducing equivalents for the oxidative phosphorylation, a process responsible for cellular ATP biosynthesis, oxygen consumption, and ROS generation (36, 37). In the current study, we found that MDH2 was overexpressed in prostate cancer cell lines and in patient specimens. Significantly, patients with MDH2 overexpression had shorter relapse free survival after neoadjuvant chemotherapy. This observation provided us an interesting hypothesis that cancer cells with MDH2 overexpression are more capable of surviving the chemotherapy – induced stress, and are less sensitive or more resistant to chemotherapy. Further, it also suggests that inhibiting MDH2 can increase the chemotherapy efficacy. Indeed, our bench-based in vitro mechanistic investigation supports the in vivo in patient results. The reduction of MDH2 in multiple prostate cancer cell lines reduced cellular proliferation, and increased the sensitivity toward docetaxel. On the molecular level, MDH2 knockdown significantly changed the metabolic phenotype of the prostate cancer cells with a decrease of ATP but increases of NAD+ and ROS. The oxygen consumption, ATP production and ROS generation are proportionally regulated during the oxidative phosphorylation. The change due to MDH2 knockdown versus control suggested to us that cancer cells consumed the same amount of oxygen but produced less ATP and more ROS as a byproduct, all of which are indications of metabolic inefficiency. Further, both NAD+ and ROS are sources of oxidant stress, which can augment the cytotoxic effect of chemotherapy. Because both NAD+ and ROS were increased by MDH2 shRNA, this can be a factor in mediating the enhanced docetaxel sensitivity.
The JNK signaling pathway is implicated in prostate cancer development (46). The biological effect of this pathway is dependent on the cellular context. The activation of JNK by oncogenic stress in normal cells can promote p53 mediated DNA damage response pathways (47). Constitutive activities of JNK in cancer cells can activate oncogenic AP-1 activity (48). The activation of JNK by MT-damaging chemotherapy is thought to mediate the cytotoxic response by activating pro-apoptotic proteins and inactivating anti-apoptotic proteins. There is no direct relationship between MDH2 and JNK. We unexpectedly observed that MDH2 shRNA knockdown led to a more sustained activation of JNK signaling pathway in the context of docetaxel treatment. Mechanistically, this can be another factor responsible for the increased docetaxel sensitivity by MDH2 shRNA inhibition. We further speculate that MDH2 can be connected to JNK signaling via metabolism. For example, the decrease of ATP due to MDH2 shRNA can activate the AMP-activated kinase (AMPK) pathway, which has been shown to cross-talk with the JNK pathway (49).
This study will have direct impact on the clinical treatment of prostate cancer. At the time of prostatectomy surgery, patients with high-risk and local prostate cancer may have already developed micrometastases outside the prostate (50–52). Therefore, surgery and local radiation represent undertreatment, and patients have a significantly higher risk of developing metastatic disease and dying of castration resistant prostate cancer (50). For these patients, early systemic chemotherapy can in principal kill the micrometastases and significantly inhibit and delay the onset of the metastatic disease (23–25). However, the patient response from neoadjuvant and adjuvant clinical trials is not unanimous, suggesting the existence of sensitivity and resistance mechanism. The clinical trial associated with the current study suggests that the neoadjuvant chemotherapy can be effective in a subset of these patients whose cancer MDH2 levels are not elevated at the time of chemotherapy. Personalized cancer therapy aims to deliver an appropriate therapy to the appropriate patient for more effective treatment and better outcome. The key is that we need to understand mechanisms of therapy sensitivity and resistance, and be able to measure these mechanisms in individual patients so that the patient population can be stratified and treated accordingly. The novel effect of MDH2 in this study can be highly translational and significantly impact the existing approach to docetaxel-based chemotherapy, especially in patients with high-risk and localized prostate cancer by providing a molecular basis for predicting docetaxel efficacy and setting the stage for more individualized chemotherapy. Patients with normal or low level of cancer MDH2 expression can be identified and selected for early chemotherapy to prevent or significantly delay the onset of disease recurrence. Patients with high levels of MDH2 can be considered for a combination treatment between chemotherapy and targeted therapy of anti-MDH2.
Supplementary Material
Acknowledgments
This work was supported by W81XWH-10-1-0142 from DOD Prostate Cancer Research Program, PNW Prostate Cancer SPORE Pilot Award, and Public Health Service grants – R01CA119125 and R01CA149253 from the National Cancer Institute.
Footnotes
The authors disclose no potential conflicts of interest
References
- 1.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold RS, Sun CQ, Richards JC, Grigoriev G, Coleman IM, Nelson PS, Hsieh CL, Lee JK, Xu Z, Rogatko A, Osunkoya AO, Zayzafoon M, Chung L, Petros JA. Mitochondrial DNA mutation stimulates prostate cancer growth in bone stromal environment. Prostate. 2009;69:1–11. doi: 10.1002/pros.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson PS. Predicting prostate cancer behavior using transcript profiles. J Urol. 2004;172:S28–32. doi: 10.1097/01.ju.0000142067.17181.68. discussion S33. [DOI] [PubMed] [Google Scholar]
- 5.Mackler NJ, Pienta KJ. Drug insight: Use of docetaxel in prostate and urothelial cancers. Nat Clin Pract Urol. 2005;2:92–100. doi: 10.1038/ncpuro0099. quiz 101 p following 112. [DOI] [PubMed] [Google Scholar]
- 6.Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: a new era begins. CA Cancer J Clin. 2005;55:300–318. doi: 10.3322/canjclin.55.5.300. quiz 323–305. [DOI] [PubMed] [Google Scholar]
- 7.Stein CA. Mechanisms of action of taxanes in prostate cancer. Semin Oncol. 1999;26:3–7. [PubMed] [Google Scholar]
- 8.Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18(Suppl 5):v3–8. doi: 10.1093/annonc/mdm172. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Vargas H, Palacios J, Moreno-Bueno G. Molecular profiling of docetaxel cytotoxicity in breast cancer cells: uncoupling of aberrant mitosis and apoptosis. Oncogene. 2007;26:2902–2913. doi: 10.1038/sj.onc.1210102. [DOI] [PubMed] [Google Scholar]
- 11.Wang LG, Liu XM, Kreis W, Budman DR. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review. Cancer Chemother Pharmacol. 1999;44:355–361. doi: 10.1007/s002800050989. [DOI] [PubMed] [Google Scholar]
- 12.Wang TH, Wang HS, Ichijo H, Giannakakou P, Foster JS, Fojo T, Wimalasena J. Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J Biol Chem. 1998;273:4928–4936. doi: 10.1074/jbc.273.9.4928. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria--specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Haldar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996;56:1253–1255. [PubMed] [Google Scholar]
- 15.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen PL. The cancer cell’s “power plants” as promising therapeutic targets: an overview. J Bioenerg Biomembr. 2007;39:1–12. doi: 10.1007/s10863-007-9070-5. [DOI] [PubMed] [Google Scholar]
- 17.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 18.Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 19.Beuzeboc P, Ropert S, Goldwasser F, Zerbib M. Management of metastatic castration-resistant prostate cancer following docetaxel. Bull Cancer. 2012 doi: 10.1684/bdc.2012.1560. [DOI] [PubMed] [Google Scholar]
- 20.Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011 doi: 10.1038/nrclinonc.2010.136. [DOI] [PubMed] [Google Scholar]
- 21.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 22.Beer TM, El-Geneidi M, Eilers KM. Docetaxel (taxotere) in the treatment of prostate cancer. Expert Rev Anticancer Ther. 2003;3:261–268. doi: 10.1586/14737140.3.3.261. [DOI] [PubMed] [Google Scholar]
- 23.Mazhar D, Waxman J. Early chemotherapy in prostate cancer. Nat Clin Pract Urol. 2008;5:486–493. doi: 10.1038/ncpuro1204. [DOI] [PubMed] [Google Scholar]
- 24.Gleave M, Kelly WK. High-risk localized prostate cancer: a case for early chemotherapy. J Clin Oncol. 2005;23:8186–8191. doi: 10.1200/JCO.2005.03.3068. [DOI] [PubMed] [Google Scholar]
- 25.Oh WK. High-risk localized prostate cancer: integrating chemotherapy. Oncologist. 2005;10(Suppl 2):18–22. doi: 10.1634/theoncologist.10-90002-18. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien C, True LD, Higano CS, Rademacher BL, Garzotto M, Beer TM. Histologic changes associated with neoadjuvant chemotherapy are predictive of nodal metastases in patients with high-risk prostate cancer. Am J Clin Pathol. 2010;133:654–661. doi: 10.1309/AJCP8EL5FTZSOBIH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian DZ, Huang CY, O’Brien CA, Coleman IM, Garzotto M, True LD, Higano CS, Vessella R, Lange PH, Nelson PS, Beer TM. Prostate cancer-associated gene expression alterations determined from needle biopsies. Clin Cancer Res. 2009;15:3135–3142. doi: 10.1158/1078-0432.CCR-08-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CY, Beer TM, Higano CS, True LD, Vessella R, Lange PH, Garzotto M, Nelson PS. Molecular alterations in prostate carcinomas that associate with in vivo exposure to chemotherapy: identification of a cytoprotective mechanism involving growth differentiation factor 15. Clin Cancer Res. 2007;13:5825–5833. doi: 10.1158/1078-0432.CCR-07-1037. [DOI] [PubMed] [Google Scholar]
- 29.Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S, Barnes AS, Tempany C, Catalona W, Kantoff PW, Oh WK. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–5240. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 30.Geng H, Rademacher BL, Pittsenbarger J, Huang CY, Harvey CT, Lafortune MC, Myrthue A, Garzotto M, Nelson PS, Beer TM, Qian DZ. ID1 enhances docetaxel cytotoxicity in prostate cancer cells through inhibition of p21. Cancer Res. 2010;70:3239–3248. doi: 10.1158/0008-5472.CAN-09-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian DZ, Rademacher BL, Pittsenbarger J, Huang CY, Myrthue A, Higano CS, Garzotto M, Nelson PS, Beer TM. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity. Prostate. 2010;70:433–442. doi: 10.1002/pros.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garzotto M, Higano CS, O’Brien C, Rademacher BL, Janeba N, Fazli L, Lange PH, Lieberman S, Beer TM. Phase 1/2 study of preoperative docetaxel and mitoxantrone for high-risk prostate cancer. Cancer. 2010;116:1699–1708. doi: 10.1002/cncr.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mhaidat NM, Zhang XD, Jiang CC, Hersey P. Docetaxel-induced apoptosis of human melanoma is mediated by activation of c-Jun NH2-terminal kinase and inhibited by the mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway. Clin Cancer Res. 2007;13:1308–1314. doi: 10.1158/1078-0432.CCR-06-2216. [DOI] [PubMed] [Google Scholar]
- 34.Wang TH, Popp DM, Wang HS, Saitoh M, Mural JG, Henley DC, Ichijo H, Wimalasena J. Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK)-dependent and -independent pathways in ovarian cancer cells. J Biol Chem. 1999;274:8208–8216. doi: 10.1074/jbc.274.12.8208. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goward CR, Nicholls DJ. Malate dehydrogenase: a model for structure, evolution, and catalysis. Protein Sci. 1994;3:1883–1888. doi: 10.1002/pro.5560031027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reisch AS, Elpeleg O. Biochemical assays for mitochondrial activity: assays of TCA cycle enzymes and PDHc. Methods Cell Biol. 2007;80:199–222. doi: 10.1016/S0091-679X(06)80010-5. [DOI] [PubMed] [Google Scholar]
- 38.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smeitink J, van den Heuvel L, DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat Rev Genet. 2001;2:342–352. doi: 10.1038/35072063. [DOI] [PubMed] [Google Scholar]
- 40.Higgins LH, Withers HG, Garbens A, Love HD, Magnoni L, Hayward SW, Moyes CD. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochim Biophys Acta. 2009;1787:1433–1443. doi: 10.1016/j.bbabio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Kirby DM, Thorburn DR, Turnbull DM, Taylor RW. Biochemical assays of respiratory chain complex activity. Methods Cell Biol. 2007;80:93–119. doi: 10.1016/S0091-679X(06)80004-X. [DOI] [PubMed] [Google Scholar]
- 43.Cosulich S, Clarke P. Apoptosis: does stress kill? Curr Biol. 1996;6:1586–1588. doi: 10.1016/s0960-9822(02)70779-3. [DOI] [PubMed] [Google Scholar]
- 44.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert LA, Hemann MT. Chemotherapeutic resistance: surviving stressful situations. Cancer Res. 2011;71:5062–5066. doi: 10.1158/0008-5472.CAN-11-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ, Jiao J, Rose J, Xie W, Loda M, Golub T, Mellinghoff IK, Davis RJ, Wu H, Sawyers CL. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 47.Haigis KM, Sweet-Cordero A. New insights into oncogenic stress. Nat Genet. 2011;43:177–178. doi: 10.1038/ng0311-177. [DOI] [PubMed] [Google Scholar]
- 48.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 49.Yun H, Kim HS, Lee S, Kang I, Kim SS, Choe W, Ha J. AMP kinase signaling determines whether c-Jun N-terminal kinase promotes survival or apoptosis during glucose deprivation. Carcinogenesis. 2009;30:529–537. doi: 10.1093/carcin/bgn259. [DOI] [PubMed] [Google Scholar]
- 50.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 51.Schwarzenbach H, Chun FK, Lange I, Carpenter S, Gottberg M, Erbersdobler A, Friedrich MG, Huland H, Pantel K. Detection of tumor-specific DNA in blood and bone marrow plasma from patients with prostate cancer. Int J Cancer. 2007;120:1465–1471. doi: 10.1002/ijc.22470. [DOI] [PubMed] [Google Scholar]
- 52.Weckermann D, Muller P, Wawroschek F, Krawczak G, Riethmuller G, Schlimok G. Micrometastases of bone marrow in localized prostate cancer: correlation with established risk factors. J Clin Oncol. 1999;17:3438–3443. doi: 10.1200/JCO.1999.17.11.3438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.