Abstract
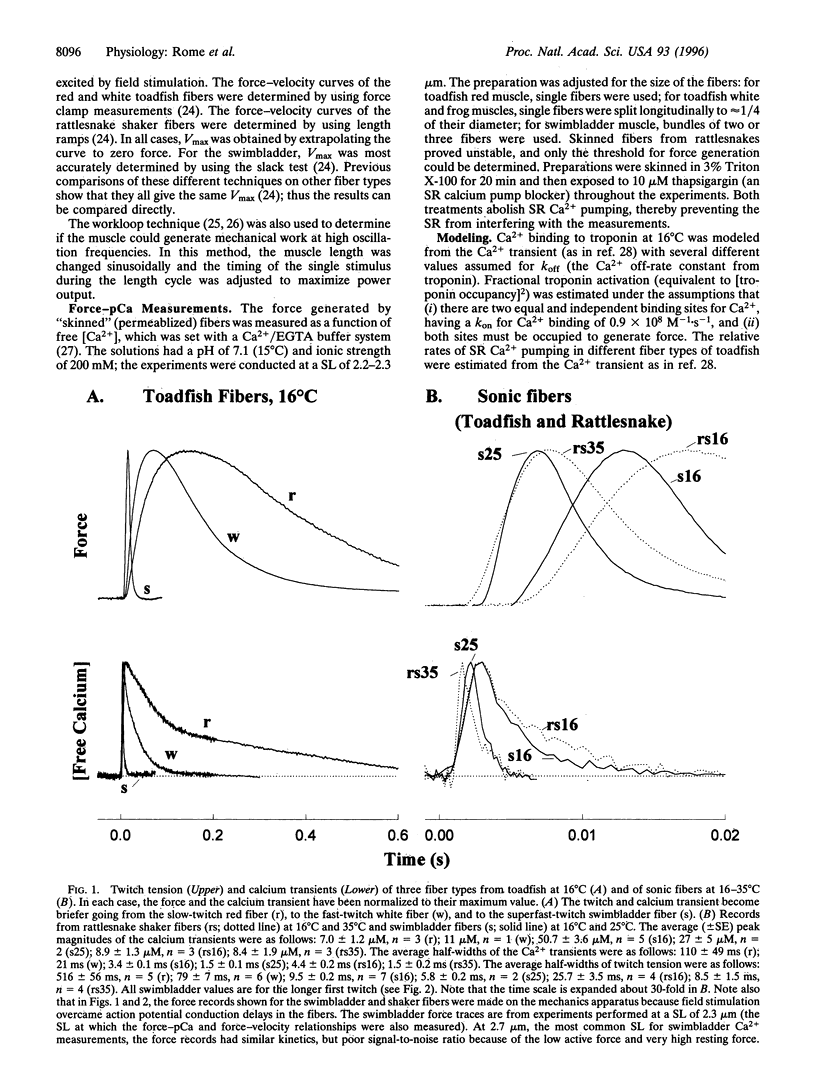
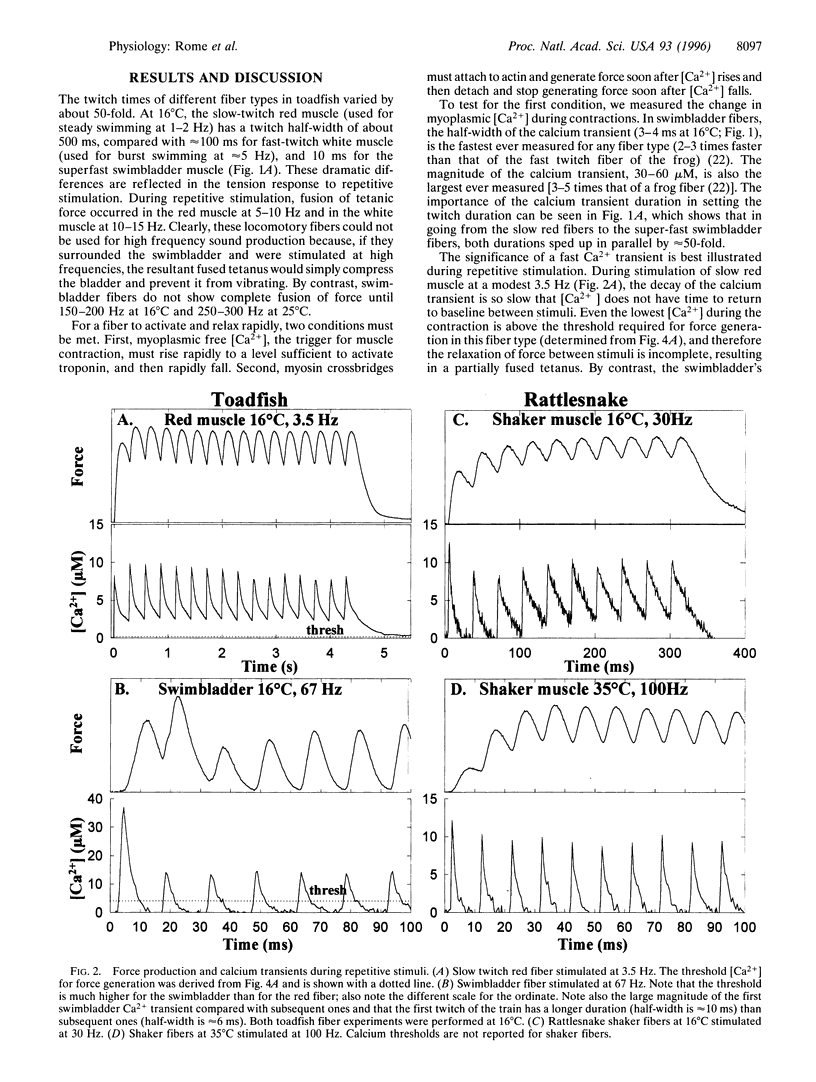
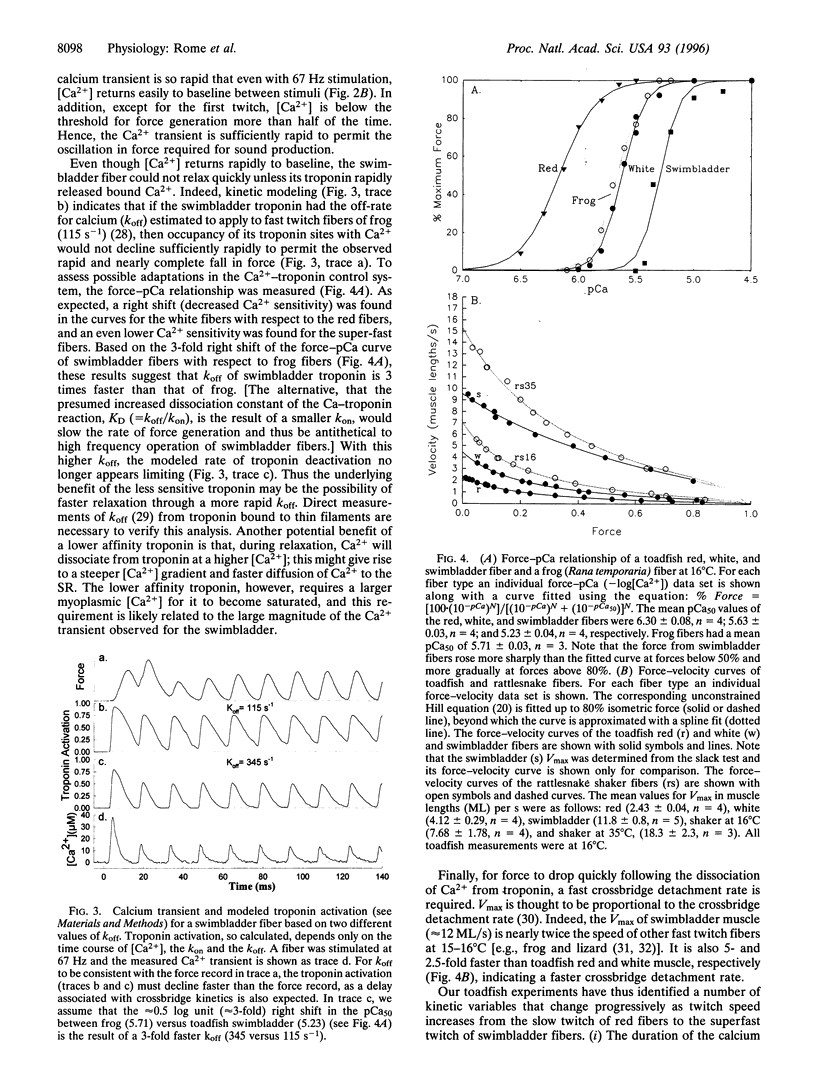
Vertebrate sound producing muscles often operate at frequencies exceeding 100 Hz, making them the fastest vertebrate muscles. Like other vertebrate muscle, these sonic muscles are "synchronous," necessitating that calcium be released and resequestered by the sarcoplasmic reticulum during each contraction cycle. Thus to operate at such high frequencies, vertebrate sonic muscles require extreme adaptations. We have found that to generate the "boatwhistle" mating call (approximately 200 Hz), the swimbladder muscle fibers of toadfish have evolved (i) a large and very fast calcium transient, (ii) a fast crossbridge detachment rate, and (iii) probably a fast kinetic off-rate of Ca2+ from troponin. The fibers of the shaker muscle of rattlesnakes have independently evolved similar traits, permitting tail rattling at approximately 90 Hz.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelt D., Shen V., Franzini-Armstrong C. Quantitation of Ca ATPase, feet and mitochondria in superfast muscle fibres from the toadfish, Opsanus tau. J Muscle Res Cell Motil. 1991 Dec;12(6):543–552. doi: 10.1007/BF01738442. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B. A. Thermogenesis in muscle. Annu Rev Physiol. 1994;56:535–577. doi: 10.1146/annurev.ph.56.030194.002535. [DOI] [PubMed] [Google Scholar]
- Bloomfield V. Editorial. Biophys J. 1993 Jul;65(1):1–1. doi: 10.1016/S0006-3495(93)81020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHADWICK L. E., RAHN H. Temperature dependence of rattling frequency in the rattlesnake, Crotalus v. viridis. Science. 1954 Apr 2;119(3092):442–443. doi: 10.1126/science.119.3092.442. [DOI] [PubMed] [Google Scholar]
- Diao Y. C., So K. F. Dendritic morphology of visual callosal neurons in the golden hamster. Brain Behav Evol. 1991;37(1):1–9. doi: 10.1159/000114342. [DOI] [PubMed] [Google Scholar]
- Ferguson D. G., Franzini-Armstrong C. The Ca2+ ATPase content of slow and fast twitch fibers of guinea pig. Muscle Nerve. 1988 Jun;11(6):561–570. doi: 10.1002/mus.880110607. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Heizmann C. W., Berchtold M. W., Rowlerson A. M. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. S., Altringham J. D., Goldspink D. F. The mechanical properties of fast and slow skeletal muscles of the mouse in relation to their locomotory function. J Exp Biol. 1995 Feb;198(Pt 2):491–502. doi: 10.1242/jeb.198.2.491. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Nakkula R. J., Vasulka C., Smillie L. B. Modulation of Ca2+ exchange with the Ca(2+)-specific regulatory sites of troponin C. J Biol Chem. 1994 Mar 25;269(12):8919–8923. [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The influence of free calcium on the maximum speed of shortening in skinned frog muscle fibres. J Physiol. 1986 Nov;380:257–273. doi: 10.1113/jphysiol.1986.sp016284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Secrist D., Coby R., Lucas S. Development of difference between red and white muscles in sensitivity to Ca2+ in the rabbit from embryo to adult. Nature. 1976 Apr 1;260(5550):440–441. doi: 10.1038/260440a0. [DOI] [PubMed] [Google Scholar]
- Konishi M., Hollingworth S., Harkins A. B., Baylor S. M. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991 Feb;97(2):271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Burk S. E., Shull G. E., MacLennan D. H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992 Jul 15;267(20):14483–14489. [PubMed] [Google Scholar]
- Marsh R. L., Bennett A. F. Thermal dependence of contractile properties of skeletal muscle from the lizard Sceloporus occidentalis with comments on methods for fitting and comparing force-velocity curves. J Exp Biol. 1986 Nov;126:63–77. doi: 10.1242/jeb.126.1.63. [DOI] [PubMed] [Google Scholar]
- Martin J. H., Bagby R. M. Properties of rattlesnake shaker muscle. J Exp Zool. 1973 Sep;185(3):293–300. doi: 10.1002/jez.1401850303. [DOI] [PubMed] [Google Scholar]
- Pringle J. W. The excitation and contraction of the flight muscles of insects. J Physiol. 1949 Mar 15;108(2):226–232. doi: 10.1113/jphysiol.1949.sp004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Rome L. C., Swank D., Corda D. How fish power swimming. Science. 1993 Jul 16;261(5119):340–343. doi: 10.1126/science.8332898. [DOI] [PubMed] [Google Scholar]
- Schaeffer P, Conley K, Lindstedt S. Structural correlates of speed and endurance in skeletal muscle: the rattlesnake tailshaker muscle. J Exp Biol. 1996;199(Pt 2):351–358. doi: 10.1242/jeb.199.2.351. [DOI] [PubMed] [Google Scholar]
- Sparrow J. C. Muscle. Flight and phosphorylation. Nature. 1995 Apr 13;374(6523):592–593. doi: 10.1038/374592a0. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Young D., Josephson R. K. 100 Hz is not the upper limit of synchronous muscle contraction. Nature. 1984 May 17;309(5965):286–287. doi: 10.1038/309286b0. [DOI] [PubMed] [Google Scholar]
- Zhao M., Hollingworth S., Baylor S. M. Properties of tri- and tetracarboxylate Ca2+ indicators in frog skeletal muscle fibers. Biophys J. 1996 Feb;70(2):896–916. doi: 10.1016/S0006-3495(96)79633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


