Abstract
Th17 cells, a recently discovered inflammatory T cell subtype, have been implicated with autoimmune disorders. However, mechanism of generation or functions of intratumoral Th17 cells are still unclear. We have been investigating the mechanism of induction and role of Th17 cells in malignant gliomas using primary tumor as well as cell lines. We report here that: (1) a higher frequency of Th17 cells in gliomas were associated with higher number of myeloid (CD11b) cells as well as the expression of TGF-β1 or IL-6; (2) conditioned medium from glioma cells (Gl CM) induced Th17 cell differentiation, which was inhibited by anti-TGF-β1 and anti-IL-6; (3) glioma-associated monocytes secreted Th17-promoting cytokines IL-1β and IL-23; (4) CM from glioma and monocyte co-culture (Gl+Mo CM) induced high frequency of Th17 cells in naïve T cell culture, which was abrogated by anti-IL-1β and anti-IL-23 antibodies; (5) In vitro Gl+Mo CM-mediated Th17 generation was associated with a decrease in IFN-γ and a concomitant increase in IL-10 secretion. Anti-TGF-β1, but not anti-IL-6, significantly reversed this cytokine profile. These results demonstrate prevalence of Th17 cells in gliomas and implicate the cytokines derived from the tumor as well as infiltrating myeloid cells in the induction of Th17 cells in glioma microenvironment. Moreover, the data also suggest that glioma-associated Th17 cells may contribute to immune-suppression via TGF-β1-induced IL-10 secretion. Further studies on the mechanism of tumor-infiltration, developmental pathways, and pro-/anti-tumor functions of Th17 cells will provide rationale for developing novel adjuvant immunotherapeutic strategies for malignant gliomas.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1312-7) contains supplementary material, which is available to authorized users.
Keywords: Th17 cells, Glioma, Myeloid cells, TGF-β, IL-1β, IL-6
Introduction
Malignant gliomas are one of the most lethal tumors with 22,000 new cases and 13,000 deaths per year in the United States [1, 2]. In spite of aggressive surgery and radio-chemotherapeutic regimens, the median survival for malignant gliomas remains around 15 months [3]. An increasing body of evidence derived from studies in extra-cranial tumors points to a critical role of inflammation in cancer progression and recurrence [4, 5]. It is therefore important to understand the mechanism of this link in order to find ways to intervene and improve survival. The Th17 cells, a recently discovered inflammatory T cell subtype [6], have well described roles in auto-immune diseases, tumor immunology and may be a target for cancer therapy [7]. Th17 cells have been reported in several extra-cranial tumors, where they have been implicated with either pro- or anti-tumor activity, depending on the tumor type [8]. A mechanistic insight into regulation of Th17-mediated inflammation and its role in glioma progression would provide novel avenues for developing more effective therapies for this deadly disease.
The T helper type 17 cells (Th17) were recently identified as an independent subtype of inflammatory T cells with a distinct cytokine (IL-17) and transcription factor (RORγt) profile [6]. Naive CD4+ T cells (nT) can be induced to differentiate towards Th1, Th2, Th17 and regulatory T cell (Treg) phenotypes according to the local cytokine milieu. Each phenotype is characterized by unique signaling pathways and expression of specific transcription factors, notably T-bet for Th1, GATA-3 for Th2, forkhead box P3 (FoxP3) for Tregs, and retinoid-related orphan receptor (ROR)α and RORγt for Th17 cells [9]. Gliomas have been known to secrete TGF-β1 and IL-6 [10–13] and in mouse models, Tregs and Th17 cells have been demonstrated to arise from common precursors in a reciprocal manner based on exposure to transforming growth factor (TGF)-β or TGF-β plus IL-6, respectively [6, 14]. A number of groups have now described conversion of Treg to the Th17 phenotype or vice versa, induced by appropriate inflammatory stimuli [15]. Unlike the rapid advances in understanding their role in inflammation and autoimmunity, there are few studies on the activity of Th17 cells in cancer, and the results are controversial [8].
Myeloid (CD11b+) cells are predominant among immune cell that infiltrate malignant gliomas, constituting more than 1 % of total cells and are often the first ones to be recruited [16–18]. IL-1β and IL-23 are critical in the induction of Th17 phenotype in humans [19–22]. Tumor-associated monocytes have been shown to induce and expand Th17 cells via secretion of IL-1β and IL-23 in ovarian carcinoma and hepatocellular carcinoma [23, 24]. Tumor associated macrophages expressed higher levels of IL-1β than normal tissue macrophages and normal-monocyte derived macrophages. Thus, molecular mechanisms involved in inducing Th17 cell in patients with tumors can be different from those in patients with auto-immune diseases [7]. Therefore, it is pertinent to study the role of glioma-associated myeloid cells in the generation or expansion of tumor-promoting Th17 cells in the tumor microenvironment.
We report here that factors derived from glioma and glioma-activated myeloid cells mediate the generation of intratumoral Th17 cells. Moreover, glioma-associated Th17 cells are potentially non-cytotoxic and may also contribute to immune suppression via secretion of IL-10.
Materials and methods
Glioma tissues, primary tumor culture (pGL) and cell lines
Tumor tissues were obtained from patients with glioblastoma immediately after surgical resection, with informed consent under a protocol (#111610M1E), approved by the WSU IRB. One half of the tumor tissue was immediately frozen in O.C.T. solution (Sakura Finetek USA, Torrance, CA) for immunohistochemical analysis. The other half of the tumor was enzymatically digested to prepare pGL cell culture, as described elsewhere [25]. Briefly, tumor tissue was cut into fine pieces and incubated at 37 °C for 30 min in the presence of 14 U/ml of Liberase Blendzyme II reagent containing collagenase and dispase (Roche Applied Science, Indianapolis, IN). The dissociated tumor cells were cultured in poly-l-lysine coated tissue culture flasks in DMEM containing 10 % FBS to derive pGL. U87-MG cells were obtained from American type culture collection (ATCC, Manassas, VA) and maintained in DMEM containing 10 % FBS.
Preparation of peripheral blood leukocytes (PBL), isolation of CD14+ monocytes and CD4+ naïve T cells
A published protocol [26] was followed with some modifications. Briefly, CD14+ cells were isolated by positive selection using CD14 magnetic beads (Miltenyi Biotec, Auburn, CA). CD4+ naïve T cells were then isolated by negative selection using a kit (Miltenyi Biotec) following the manufacturer’s instructions.
Preparation of conditioned media (CM) from glioma and monocyte (co-)cultures
U87-MG cells were either cultured alone at 1 × 106 cells/ml or were co-cultured with monocytes (U87+Mo) at 1:1 ratio. The U87-MG CM or U87+Mo CM were harvested after 72 h. The CM were frozen at −80 °C in 2 ml aliquots until use.
In vitro Th17 (IL-17) induction model
In order to determine the effect of glioma and monocyte-derived factors on the induction of Th17 cells, an in vitro model was developed by modifying, combining and appropriating two protocols, [27, 28]. Briefly, PBL or MACS-isolated CD4+ naïve T (nT) cells were cultured 1 × 106 cells/well, in serum-free X-VIVO 20 medium (Lonza, Walkersville, MD), in a 6-well plate with anti-CD2/CD3-loaded MACS microbeads (Miltenyi Biotec, Auburn, CA) and low dose IL-2 (50 IU/ml). In order to induce Th17 phenotype (IL-17), conditioned medium from glioma and monocyte cultures (as described above) or a combination of IL-6 (10 ng/ml), IL-1β (1 ng/ml), IL-23 (1 ng/ml) and TGF-β1 (10 ng/ml) were added as indicated. The human recombinant cytokines and respective neutralizing antibodies were obtained from PeproTech, Inc (Rocky Hills, NJ). The culture supernatants and the Th17 or control cells, harvested after 7 days of culture, were analyzed for cytokine secretion or phenotype, as described below. All experiments were performed at least three times using PBL obtained from three different donors.
Cytokine analysis
Cytokines were measured in the culture supernatants using a 25-plex human cytokine Luminex Array (Invitrogen, Carlsbad, CA) and Bio-Plex system (Bio-Rad Lab., Hercules, CA). The multiplex panel includes interleukin (IL)-1β, IL-6, IL-10, IL-17 and IFN-γ. The limit of detection for these assays is <10 pg/mL based on detectable signal of > twofold above background (Bio-Rad). Cytokine concentration was automatically calculated from a standard curve by the BioPlex Manager Software (Bio-Rad). TGF-β1 and IL-23 were detected using respective ELISA kits (BD Biosciences, San Jose, CA and R&D Systems, Minneapolis, MN). In order to include the latent TGF-β in the measurement, the culture supernatants were acid-treated as per the manufacturer’s protocol.
Immunohistochemistry
A published protocol [29] was followed with some modifications. Briefly, the frozen or paraffinized tumor specimens were cut into 5 μm tissue sections, fixed in acetone for 5 min and stored at −80 °C until staining time. For staining, slides were brought to room temperature, blocked and hydrated in staining buffer (PBS with 5 % goat serum) and appropriate primary antibodies (anti-CD4 from B.D. Biosciences; anti-TGF-β1, anti-IL-6 and anti-IL-17 from Santa Cruz Biotechnology; anti-CD11b and anti-IL-1β from eBioscience, San Diego, CA) were applied, followed by washing and incubation with the appropriate biotinylated secondary antibody (Vector Labs, Burlingame, CA) for 30 min at room temperature. Detection was performed with diaminobenzidine (DAB) and counterstaining with Mayer hematoxylin followed by dehydration and mounting. For co-localization, appropriate secondary antibodies conjugated with FITC or PE (Santa Cruz Biotechnology) were utilized. The nucleus was stained with DAPI. Negative staining was performed with appropriate isotype control antibodies (eBioscience, San Diego, CA) instead of the specific primary antibody. The sections were then analyzed under a fluorescent microscope equipped with a digital camera (Olympus BX51) and micrographed at 200× or 400× magnification. The number of cells with respective immunostaining were recorded after counting 100 cells with distinct nuclear staining in at least three consecutive high power fields in each slide. Necrotic or thick areas and severely overlapping tumor cells were excluded. The infiltrating immune cells were calculated as percentage of the total number of nuclear-stained cells counted. Tumor tissues obtained from five patients with malignant gliomas were studied, as described below in the Results section
Flow cytometry
Fluorescein-conjugated human antigen-specific or isotype control antibodies were purchased from SantaCruz Biotechnology, Santa Cruz, CA (rabbit anti-human IL-17, goat anti-rabbit APC) and B.D. Biosciences, San Jose, CA (CD3 FITC, CD4 FITC, IFN-γ PE, and IL-10 PE). A published protocol was followed for surface and intracellular staining of the cells [26].
Statistical analysis
A Wilcoxon’s log-rank test was performed to determine the statistical difference between various experimental and control groups, using the SPSS package (SPSS Inc, Chicago, IL) [30]. A ‘p’ value less than 0.05 was considered significant.
Results
Tumor tissues from malignant glioma patients contain Th17 (CD4+IL-17+) cells, and express TGF-β1 and IL-6
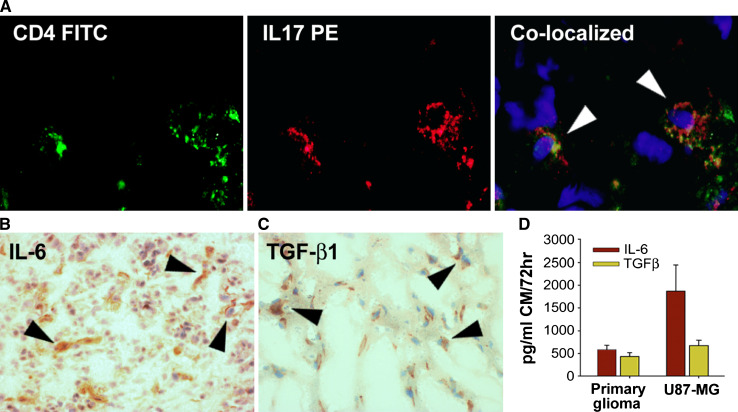
Five tumor tissues obtained from patients with malignant gliomas were analyzed by immunofluorescence microscopy (CD4 and IL-17) and IHC using DAB (IL-6 and TGF-β1). We observed co-localization of CD4 and IL-17 in glioma-infiltrating Th17 cells (Fig. 1a). Lymph node tissues obtained from a normal (non-cancer) cadaver donor was used as a positive control for Th17 staining (data not shown). Representative micrographs of a malignant glioma tissue showing about 100 total cells and a few Th17 cells as well as the negative (isotype) control staining have been shown in Supplementary Figure S1. IL-6 and TGF-β1 staining by IHC in representative glioma tissues are shown in Fig. 1b, c, respectively.
Fig. 1.
Prevalence of Th17 cells and the expression of IL-6 and TGF-β1 in gliomas. Frozen or paraffinized tumor sections, obtained from patients with malignant glioma were analyzed by IHC to determine the presence of CD4+IL-17+ Th17 cells (a) as well as expression of TGF-b1 (b) and IL-6 (c). Result shown is from one representative paraffinized glioma specimen out of five studied. The micrographs were imaged at ×400 (a) and ×200 (b, c) magnifications. Conditioned media (CM) from 72 h culture of a primary glioma and U87-MG glioma cell line were analyzed for the secretion of IL-6 and TGF-β1, by Bioplex assay and ELISA, respectively, as described in the Sect. “Materials and Methods”. The data are expressed in pg/ml of CM (d)
In order to confirm the secretion of IL-6 and TGF-β1 by gliomas, a primary glioma and U87-MG cells were cultured (1 × 106 cells/ml) for 72 h and conditioned media (CM) were harvested for cytokine analysis as described in the Materials and Methods section. Consistent with the IHC data, nearly 500 pg/ml each of IL-6 and TGF-β1 were detected in the CM of primary glioma, and 1,800 pg/ml IL-6 and 700 pg/ml TGF-β1 from U87-MG cells (Fig. 1d).
All five glioma tissues analyzed were positive for IL-17, CD11b and IL-6, while 4 out of 5 tumors were positive for TGF-β1, at varying degrees of percent positivity (Table 1). The number of samples analyzed was not sufficient to examine any possible correlation between the level of Th17 infiltration and tumor grade. Notably, one of the tumors that was negative for TGF-β1 expression was still positive for IL-17 (Table 1) and also showed presence of Th17 by IHC, prompting further investigations into possible TGFβ1-independent mechanism of Th17 induction in gliomas.
Table 1.
Percentage of cells expressing Th17 (IL-17) and Myeloid (CD11b) cell markers, and Th17-inducing cytokines (TGF-β1 and IL-6) in malignant gliomas
| Gliomas | IL-17 | CD11b | TGF-β1 | IL-6 |
|---|---|---|---|---|
| #1 Grade IV | 1 | 3 | 9 | 12 |
| #2 Grade IV | 0.8 | 1.6 | 8 | 9 |
| #3 Grade III | 0.5 | 2 | 0 | 6 |
| #4 Grade III | 0.6 | 1.8 | 4 | 4 |
| #5 Grade II | 1 | 2 | 8 | 10 |
Five tumor tissues obtained from patients with gliomas were analyzed by immunofluorescence microscopy (IL-17 and CD11b) and IHC using DAB (IL-6 and TGF-β1). Data is expressed as percentage positive cells per total cells counted
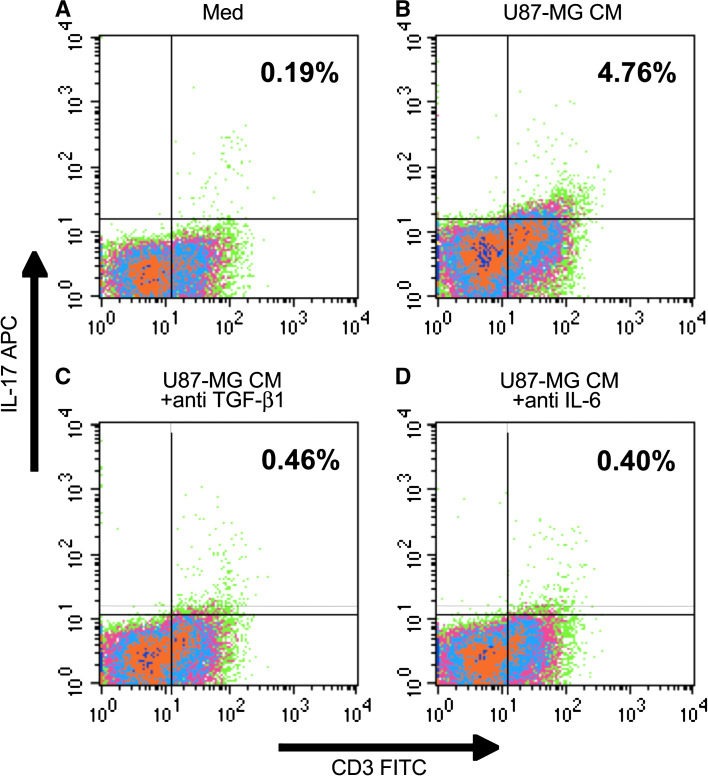
Glioma CM induces IL-17 expression in T lymphocytes, which is inhibited by antibodies to TGF-β1 and IL-6
In order to examine the role of glioma-derived factors in the induction of Th17 phenotype (IL-17), non-adherent (monocyte depleted) PBL, obtained from normal donors, were cultured in the Th17 (IL-17) induction model, in the presence or absence of U87-MG CM (50 % v/v) as described in the Materials and Methods section. The naïve T cell cultures used serum-free X-VIVO 20 medium; complete DMEM (50 % v/v) was added in the ‘control’ cultures. After 6 days of culture, the cells were analyzed by flow cytometry. PBL cultured in presence of U87-MG CM contained significantly higher frequency of Th17 cells (4.76 %) compared to the control cultures (0.19 %) (Fig. 2b vs. a). Anti-IL-6 or anti-TGF-β1 completely inhibited the U87-MG CM mediated induction of Th17 cells (Fig. 2c, d, respectively). The results show that gliomas can induce Th17 phenotype, albeit at low levels, via secretion of TGF-β1 and IL-6.
Fig. 2.
Glioma CM induces Th17 cells, which is inhibited by anti-TGF-β1 and anti-IL-6. Non-adherent (monocyte depleted) PBL, obtained from normal donors, were cultured in the Th17 generation model in the presence of Gl-CM, as described in the Sect. “Materials and Methods”. After 6 days of culture, the cells were analyzed by flow cytometry. The data are from one representative experiment out of three experiments performed with similar results
Glioma-activated monocytes secrete high levels of Th17 promoting cytokines IL-1β and IL-23
Myeloid cells are known to comprise a predominant infiltrating immune cell population in gliomas [16]. We investigated the role of glioma-associated cytokines in the induction of Th17 cells in gliomas. We observed prevalence of IL-1β co-expressing CD11b+ myeloid cells in glioma tissues (Fig. 3a). Next, we investigated if glioma cells or glioma-derived factors induced secretion of IL-1β and IL-23 in monocytes. Monocytes were cultured either in presence of U87-MG CM or were co-cultured with U87-MG cells at 1:1 ratio for 72 h. cytokines were analyzed as described in the Materials and Methods section. Low levels of IL-1β and IL-23 were detectable in the medium control group (Fig. 3b, c, respectively). U87-MG CM itself contained negligible amount of IL-1β or IL-23, but significantly upregulated the secretion of these cytokines by monocytes. Levels of IL-1β (Fig. 3b) as well as IL-23 (Fig. 3c) were significantly higher in the U87+Mo co-culture groups compared to any other group. Moreover, the quantities of cytokines detected in Mo+U87-CM groups were only marginally higher than the values obtained by adding the quantities of respective cytokines detected in individual cell cultures. On the other hand, cytokine levels in Mo+U87-MG co-culture groups were significantly higher than the Mo+U87CM or Mo+U87 (insert) group. These results suggest that secretion of Th17-promoting cytokines by glioma-associated monocytes may involve some degree of cell-to-cell interaction between gliomas and monocytes.
Fig. 3.
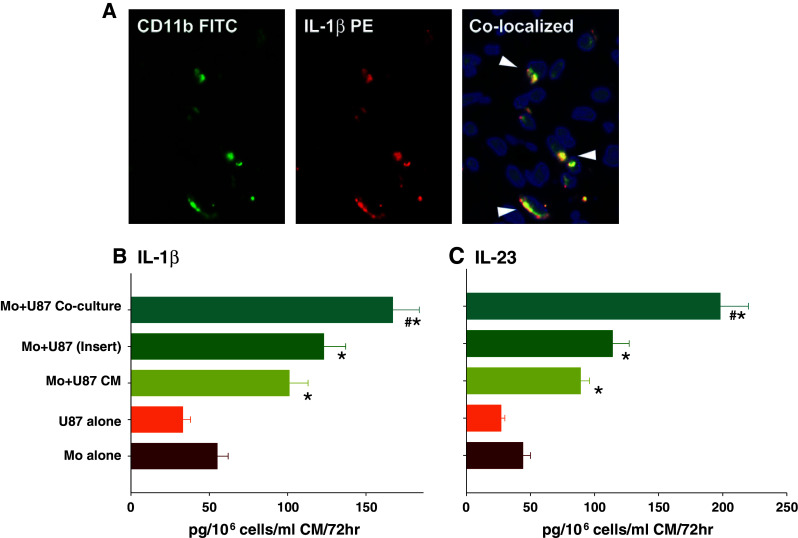
Prevalence of CD11b+IL-1β+ Myeloid cells in gliomas a tumor sections, obtained from patients with glioblastoma were analyzed by IHC to determine the presence of CD11b+IL-1β+ myeloid cells as described in the Sect. “Materials and Methods”. Result shown is from one representative frozen glioma specimen out of five studied. The micrographs were imaged at ×400 magnification. Secretion of IL-1β and IL-23 by Glioma-activated monocytes b, c peripheral blood monocytes (Mo) were either cultured in presence of U87-MG CM or with U87-MG cells (either in an insert or in a co-culture) at 1:1 ratio for 72 h. Cytokine levels in the CM were then analyzed using Bioplex cytokine array (Invitrogen) as described in the Sect. “Materials and Methods”. The data, expressed in pg/ml of CM, represents mean ± S.D. of three independent experiments. ‘*’p < 0.05 versus monocytes cultured alone; ‘#’p < 0.05 versus all other groups
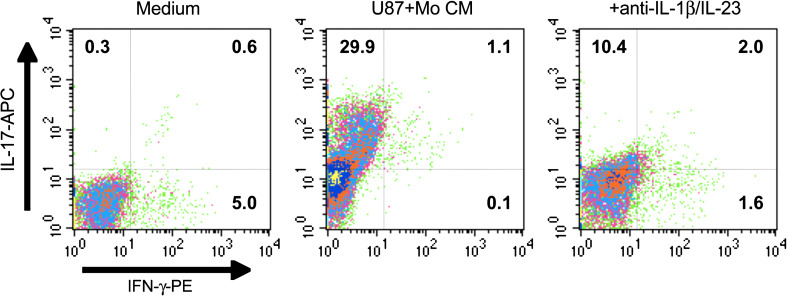
Role of glioma-activated monocyte-derived factors in the induction of Th17 cells
These sets of experiments examined whether glioma-associated monocytes and related cytokines were necessary during induction of Th17 cells in a simulated glioma microenvironment. CD4+ nT cells were cultured in the Th17 (IL-17) induction model for 6 days in the presence of CM from U87+Mo co-cultures (Mo+U87 CM) as described above. The cells were then analyzed by 3-color flow cytometry. All cells were gated on CD4-FITC. The medium control group contained less than 1 % CD4+IL-17+ (Th17) cells, but with a higher frequency of 5.6 % IL-17− IFN-γ+ cells (Fig. 4a). Addition of U87+Mo CM significantly increased the frequency of total Th17 cells (29.9 + 1.1 %, upper two quadrants), while decreasing the frequency of IFN-γ+ T cells to 1.2 % (Fig. 4b). Addition of anti-IL-1β + anti-IL-23 significantly inhibited U87+Mo CM mediated induction of Th17 cells (10.4 vs. 29.9 %) (Fig. 4c). Together with data presented in Fig. 3, these results strongly implicate glioma-associated monocytes in the induction of Th17 cells in the tumor milieu.
Fig. 4.
Role of glioma-activated monocyte-derived factors in TH17 generation. CD4+ nT cells were cultured in the Th17 (IL-17) induction model for 6 days in the presence of culture supernatants from various monocyte cultures as indicated. The cells were then analyzed by 3-color flow cytometry as described in the Sect. “Materials and Methods”. All cells were gated on CD4-FITC. The data are from one representative experiment out of three experiments performed with similar results
Glioma-activated monocyte derived factors switches differentiation of nT cells from Th1 to immune-suppressive Th17 phenotype
Data presented in Fig. 4 suggested a decrease in IFN-γ+ T cell population upon addition of Gl+Mo CM into the nT cell culture. In order to further investigate this phenomenon, and to examine the role of glioma-derived cytokines in the modulation of T cell phenotype, nT cells were cultured in Th17 (IL-17) induction model in the presence of U87-MG CM or U87+Mo CM and antibodies to IL-6 and TGF-β1 for 6 days. nT cells cultured with anti-CD2/3-loaded beads and IL-2 only (Medium control) secreted large amount of IFN-γ (721 ± 234 pg/ml; Fig. 5a) and very little IL-17 (50 pg/ml; Fig. 5b) or IL-10 (50 pg/ml; Fig. 5c). Addition of U87-MG CM significantly decreased the secretion of IFN-γ while the secretion of IL-17 as well as IL-10 was significantly enhanced. Addition of U87+Mo CM further enhanced the secretion of IL-17 and IL-10 by T cells with concomitant inhibition of IFN-γ, indicating a switch from Th1 to Th17 type T cell phenotype. Addition of anti-IL-6 along with U87+Mo CM did not significantly alter the cytokine profile, while anti-TGF-β1 inhibited IL-17 by about 40 %, restored IFN-γ secretion to almost 70 % of the control level, and inhibited IL-10 secretion by more than 50 % in U87+Mo CM-treated nT cell culture (Fig. 5). The results, once again indicated that glioma-activated monocyte derived factors induce/expand Th17 cells via a TGF-β1-independent mechanism, while presence of TGF-β appears to play a vital role in rendering Th17 cells immunosuppressive via inhibition of IFN-γ and enhancement of IL-10 secretion.
Fig. 5.
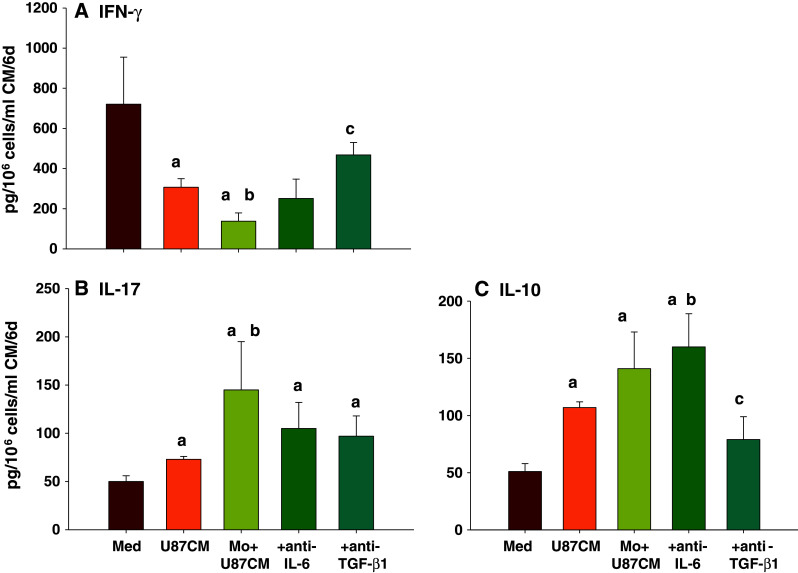
Glioma-monocyte interaction switches differentiation of nT cells from Th1 to immune-suppressive Th17 phenotype. nT cells were cultured in the Th17 (IL-17) induction model for 6 days in presence of U87-MG CM or Mo+U87 CM with anti-TGF-β1 or anti-IL-6, as indicated. Cytokine levels in the CM were then analyzed using Bioplex cytokine array (Invitrogen) or ELISA, as described in the Sect. “Materials and Methods”. The data, expressed in pg/ml of CM, represents mean ± S.D. of three independent experiments. ‘a’-p < 0.05 versus Med; ‘b’-p < 0.05 versus U87-MG CM; ‘c’-p < 0.05 versus Mo+U87 CM
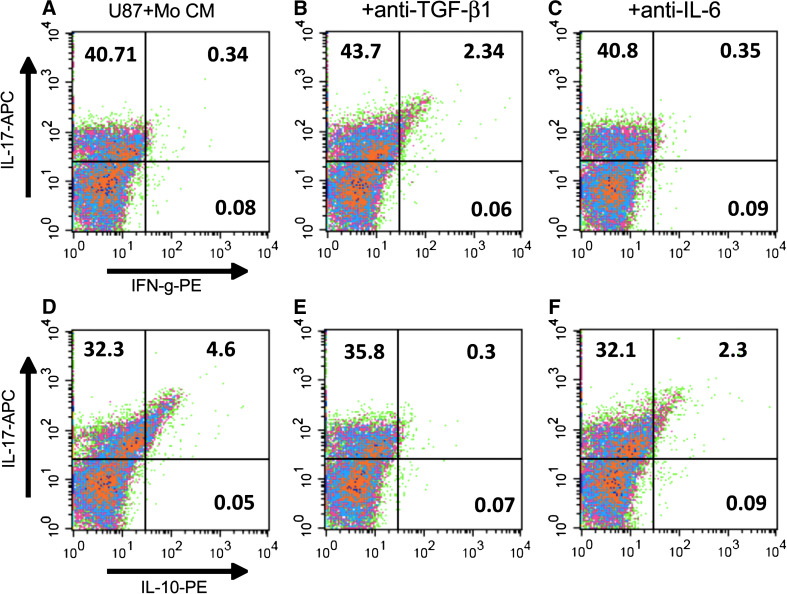
Neutralization of TGFβ1 resulted in an observed increase in IFNγ+ cells and a concomitant decrease in IL-10+ Th17 cells
In the next experiment, nT cells were cultured in Th17 (IL-17) induction model under the same conditions as described in the previous experiment, and then analyzed by flow cytometry. In agreement with the results shown in Fig. 5, we observed an increased frequency of IFN-γ+ Th17 cells (Fig. 6b vs. a) with a concomitant decrease in IL-10+ Th17 cells (Fig. 6d vs. e) following neutralization of TGF-β1 in U87+Mo CM. On the other hand, neutralization of IL-6 did not have a significant effect in the Th17 phenotype (Fig. 6c, f). Interestingly, unlike the U87-MG CM-induced Th17 culture (see Fig. 2, above), anti-TGF-β1 or anti-IL-6 alone did not alter the frequency of U87+Mo CM-induced Th17 cells, suggesting that, in the presence of myeloid-derived factors, TGF-β1 and IL-6 may regulate more than one redundant pathways for the induction of Th17 cells in glioma microenvironment.
Fig. 6.
Role of TGF-β1 in tumor-mediated generation of IFN-γ−IL10+ TH17 cells. nT cells were cultured in the Th17 (IL-17) induction model for 6 days in presence of Mo+U87 CM with anti-TGF-β1 or anti-IL-6, as indicated. The cells were then analyzed by 3-color flow cytometry. All cells were gated on CD4-FITC. The data are from one representative experiment out of three experiments performed with similar results. The data for the isotype control staining are presented in supplementary Figure S2
These results, together with results shown in Figs. 3 and 4, clearly demonstrate that gliomas induce secretion of IL-1β and IL-23 from monocytes, which can lead to induction of Th17 cells independent of TGF-β1. However, presence of TGF-β1 enhances the frequency of Th17 cells with an immune-suppressive (low IFN-γ, high IL-10) phenotype.
Discussion
The Th17 cells, a recently discovered inflammatory T cell subtype, have been reported in several extracranial tumors, where they have been implicated with either pro- or anti-tumor activity, depending on the tumor type. Th17 cells with anti-tumor activities have been reported in head and neck squamous cell carcinoma (HNSCC) [31] and ovarian cancer [32]. On the other hand, IL-17 promoted multiple myeloma (MM) cell growth and colony formation via IL-17 receptor, adhesion to bone marrow stromal cells (BMSCs) as well as increased growth in vivo in murine xenograft model of human MM [32]. Similarly, Zhang et al. have reported increased prevalence of Th17 cells in patients with gastric cancer, which was associated with poor prognosis [33]. The frequency of Th17 cells also showed negative correlation with time to disease progression in 23 prostate cancer patients receiving a dendritic cell based vaccine [34]. In another study, 23 patients with medulloblastoma had a considerable population of Th17 cells in tumor-infiltrating T cells, along with high mRNA levels for Th17-related factors (IL-17, IL-23 and ROR-γt) in tumor tissues. Moreover, the serum concentrations of IL-17 and IL-23 protein were significantly increased in patients with medulloblastoma compared to normal controls, indicating that Th17 cells may contribute to medulloblastoma pathogenesis [35]. Recently, Wainwright et al. have demonstrated mRNA expression of IL-17A in human glioma. In the same study, they also demonstrated Th17 cells in mouse brain tumors [36]. However, the prevalence of Th17 cells in human gliomas, mechanism of their induction or expansion and their immunologic functions have not been studied.
This study demonstrates, for the first time, prevalence of Th17 cell in human malignant glioma tissues. The results also showed the presence of CD11b+ myeloid cells along with Th17 promoting cytokines TGF-β, IL-6, IL-1β and IL-23 in gliomas, strongly implicating the infiltrating myeloid cells in the induction of Th17 cells in glioma microenvironment. We observed significant but small enhancement of Th17 frequency (about 5 %, Fig. 2b) by glioma-derived factors in the in vitro simulation of glioma microenvironment. However, supernatants from glioma-monocyte co-culture (U87+Mo CM) exponentially enhanced the frequency of Th17 cells to about 30 % in the in vitro simulation model, which was almost completely abrogated (10 %) by neutralization of glioma-activated monocyte derived factors IL-1β and IL-23 (Fig. 4). These data clearly suggested that glioma-monocyte interaction is required for effective induction of Th17 cells in the glioma microenvironment.
Th17 cells constitute a minor population in human peripheral blood and lymph nodes with no major frequency changes in cancer patients compared with healthy donors [24]. There is, however, a strikingly high frequency of tumor-infiltrating IL-17+ T cells in patients with diverse cancer types, including ovarian and pancreatic cancer [24, 37], suggesting that Th17 cells may be generated or expanded in the tumor microenvironment. We observed expression of Th17 inducing cytokines TGF-β1 and IL-6 in most glioma tissues as well as in malignant glioma cell lines and primary culture. However, our IHC studies also revealed prevalence of Th17 cells in one glioma tissue that did not express TGF-β1, but was positive for IL-6. This result, combined with results of our in vitro simulation studies, implicates glioma-stimulated myeloid cell-derived IL-1β and IL-23 as a possible TGF-β-independent mechanism of Th17 cell generation in gliomas. We are analyzing tumor tissues from more patients as well as conducting further in vitro studies to confirm the same.
IL-6 seems to regulate induction of Th17 cells in synergy with TGF-β (TGF-β-dependent mechanism) or in synergy with IL-1β (TGF-β-independent mechanism) [38]. A synergistic role of IL-1β and IL-6 in the induction of Th17 cells have also been demonstrated in some other models [39, 40]. IL-23 is required for maintenance and expansion of Th17 cells [41]. Our results are in agreement with some reported studies showing that IL-6 and IL-23 in combination with IL-1β effectively induced IL-17 production in naive precursors, independent of TGF-β [42]. We observed that U87+Mo CM induced significantly higher frequency of Th17 cells compared to U87 CM, which was almost completely inhibited by anti-IL-1β and anti-IL-23 antibodies, suggesting that myeloid cell derived IL-1β and IL-23 may be critical in the induction of Th17 cells in gliomas. Studies in non-cranial tumors have also demonstrated that tumor-activated monocytes secrete high levels of IL-1β and IL-23, promoting induction/expansion of IL-17 secreting T cells [23, 43]. Notably, anti-TGF-β1 or anti-IL-6 alone could not inhibit U87+Mo CM mediated Th17 generation, clearly suggesting that induction of Th17 in gliomas employ redundant mechanisms which may or may not involve TGF-β1 or IL-6. These results also corroborate with published literature showing that, unlike in mice, TGF-β+IL-6 may not be strong inducers of Th17 in humans, where myeloid-derived cytokines such as IL-1β and IL-23 are required [19–22].
Th17 cells associated with autoimmune disorders often convert to Th1-like phenotype via co-expression of IFN-γ and are implicated in cytotoxicity [44, 45]. In terms of intratumoral Th17 cells, they might not mediate direct anti-tumor activity but can promote anti-tumor immunity indirectly through the recruitment of dendritic cells and cytotoxic effector cells via production of CCL20 [46, 47]. However, anti-tumor activity of a novel population of IL17+/IFN-g+ CD8 T cells (termed as Tc17 cells) have been reported in some cancers [48, 49] and, interestingly, non-cytotoxic Tc17 cells could be rendered cytotoxic upon treatment with IL-12 [50]. These studies demonstrate that the polarity and functions of tumor-infiltrating immune cell subsets clearly depends on the cytokine milieu in the tumor microenvironment. The precise mechanism of polarization and resulting immunologic functions of Th17 cells remain to be defined in tumor types where they are associated with pro-tumor activity. Exogenous IL-17 could promote tumor growth by inducing tumor vascularization particularly in immune deficient nude mice [51]. However, effect of IL-17 on tumor development and growth might be different in immune competent hosts [52]. Moreover, as result of differences in local concentrations, bioavailability and potential targets, the biological activities of endogenous IL-17 such as IL-17 derived from Th17 cells and exogenously administered IL-17 might differ [53]. Th17 cells are very plastic and can switch to non-cytotoxic (low IFN-γ, high IL-10) functional phenotype depending on the cytokines present in the milieu [15, 54, 55]. Induction of Th17 cells in the presence of TGF-β1 may lead to generation of non-cytotoxic (low IFN-γ, high IL-10) Th17 cells [56]. These studies suggest that the pro- or anti-tumor activity of Th17 cells, as reported in several tumor types, may be dictated by the relative co-expression of IL-10 or IFN-γ in these cells, which in turn depends on the cytokine milieu of the tumor microenvironment. The exact mechanism for generation and functions of such Th17 phenotype needs to be clarified. Our results concurred with these published studies. We observed that induction of IL-17 in T cell cultures by U87+Mo CM was associated with high IL-10 co-expression and concomitant inhibition of IFN-γ secretion. The cytokine profile was reversed upon neutralization of TGF-β1 in the culture. Flow cytometric analysis also revealed about 5 % IL-10 co-expressing Th17 cells in U87+Mo CM group, which was completely obliterated by anti-TGF-β1. These results strongly suggest that glioma-associated Th17 cells are potentially non-cytotoxic (low IFN-γ) and may become immune suppressive (high IL-10 expression) upon exposure to TGF-β1. These observations are in agreement with a recent report by Zhao et al. [57], who also observed an inhibition of autologous CD8+ T cell response by CCR4+CCR6+ Th17 cells in colorectal carcinoma, and the inhibition of CD8+ T cell response by Th17 was partially dependent on TGF-β1. Further studies in this direction have shown that glioma-induced Th17 cells can in fact enhance proliferation of a small population of glioma cells via IL-17/IL-17R interaction (Parajuli, unpublished observation). We are also performing correlation studies in more patient samples in order to determine a link between the level of TGF-β1 and frequency of immune suppressive Th17 cells in gliomas.
Immunotherapy has potential to prevent recurrence of malignant gliomas by specifically targeting residual or invasive tumor cells without affecting the normal tissue. However, immunotherapeutic strategies in high-grade gliomas have met with limited success in the clinic because of glioma-mediated active immune suppression [11, 58]. Development of novel adjuvant strategies to relieve tumor-mediated immune suppression is not only desirable but necessary for combination therapy of malignant gliomas. Our data provides preliminary insight into how conditions in glioma microenvironment could regulate induction of Th17 cells with an immunosuppressive phenotype and cytokine profile. Further systematic studies of tumor-infiltrating Th17 cells, their developmental pathways and their anti/pro-tumor functions, will likely identify novel mechanisms for immune intervention in gliomas by targeting the immune suppression-inflammation axis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure S1. Prevalence of Th17 cells in gliomas. Paraffinized tumor sections, obtained from patients with malignant glioma were analyzed by IHC to determine the presence of CD4+IL-17+ Th17 cells (A); Isotype antibody control staining were also performed to rule out the background (B). The micrographs were imaged at 200x magnification (PPT 1389 kb)
Supplementary Figure S2. Isotype Controls for data presented in Figure 6. nT cells were cultured, stained with isotype control antibodies and were analyzed by flow cytometry, as described under Figure 6. The data are from one representative experiment out of three experiments performed with similar results (PPT 294 kb)
Acknowledgments
We acknowledge research support from the Fund for Medical Research and Education (FMRE) to PP. The Microscopy, Imaging and Cytometry Resources Core is supported, in part, by NIH Center grant P30CA22453 to The Karmanos Cancer Institute, Wayne State University and the Perinatology Research Branch of the National Institutes of Child Health and Development, Wayne State University. We are thankful to Dr. Indrajit Sinha for his critical evaluation of the manuscript and also for his help with the preparation of the Figures.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- CM
Conditioned medium
- nT
Naïve T cells
- GI-CM
Conditioned medium from glioma cells
- Mo-CM
Conditioned medium from monocyte culture
- TGF
Transforming growth factor
- CCL
Chemokine ligand
References
- 1.CBTRUS: CBTRUS Statistical report: primary brain and central nervous system tumors diagnosed in the United States, 2004–2006. Central Brain Tumor Registry of the United States, 2010
- 2.Cancer facts and figures. American Cancer Society, 2010
- 3.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Cole SW. Chronic inflammation and breast cancer recurrence. J Clin Oncol. 2009;27:3418–3419. doi: 10.1200/JCO.2009.21.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronte V. Th17 and cancer: friends or foes? Blood. 2008;112:214. doi: 10.1182/blood-2008-04-149260. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Paul WE. Peripheral CD4 + T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider J, Hofman FM, Apuzzo ML, Hinton DR. Cytokines and immunoregulatory molecules in malignant glial neoplasms. J Neurosurg. 1992;77:265–273. doi: 10.3171/jns.1992.77.2.0265. [DOI] [PubMed] [Google Scholar]
- 11.Parajuli P, Mathupala S, Mittal S, Sloan AE. Dendritic cell-based active specific immunotherapy for malignant glioma. Expert Opin Biol Ther. 2007;7:439–448. doi: 10.1517/14712598.7.4.439. [DOI] [PubMed] [Google Scholar]
- 12.Zou JP, Morford LA, Chougnet C, Dix AR, Brooks AG, Torres N, Shuman JD, Coligan JE, Brooks WH, Roszman TL, Shearer GM. Human glioma-induced immunosuppression involves soluble factor(s) that alters monocyte cytokine profile and surface markers. J Immunol. 1999;162:4882–4892. [PubMed] [Google Scholar]
- 13.Gomez GG, Kruse CA. Cellular and functional characterization of immunoresistant human glioma cell clones selected with alloreactive cytotoxic T lymphocytes reveals their up-regulated synthesis of biologically active TGF-beta. J Immunother. 2007;30:261–273. doi: 10.1097/01.cji.0000211339.81211.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morishima N, Mizoguchi I, Takeda K, Mizuguchi J, Yoshimoto T. TGF-beta is necessary for induction of IL-23R and Th17 differentiation by IL-6 and IL-23. Biochem Biophys Res Commun. 2009;386:105–110. doi: 10.1016/j.bbrc.2009.05.140. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST. Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol. 1997;93:518–527. doi: 10.1007/s004010050647. [DOI] [PubMed] [Google Scholar]
- 18.Takeshima H, Kuratsu J, Takeya M, Yoshimura T, Ushio Y. Expression and localization of messenger RNA and protein for monocyte chemoattractant protein-1 in human malignant glioma. J Neurosurg. 1994;80:1056–1062. doi: 10.3171/jns.1994.80.6.1056. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 20.Yu RY, Gallagher G. A naturally occurring, soluble antagonist of human IL-23 inhibits the development and in vitro function of human Th17 cells. J Immunol. 2010;185:7302–7308. doi: 10.4049/jimmunol.1002410. [DOI] [PubMed] [Google Scholar]
- 21.Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M. TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur J Immunol. 2009;39:1221–1230. doi: 10.1002/eji.200838742. [DOI] [PubMed] [Google Scholar]
- 22.Sha Y, Markovic-Plese S. A role of IL-1R1 signaling in the differentiation of Th17 cells and the development of autoimmune diseases. Self Nonself. 2011;2:35–42. doi: 10.4161/self.2.1.15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010;51:154–164. doi: 10.1002/hep.23291. [DOI] [PubMed] [Google Scholar]
- 24.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sloan AE, Parajuli P. Human autologous dendritic cell-glioma fusions: feasibility and capacity to stimulate T cells with proliferative and cytolytic activity. J Neurooncol. 2003;64:177–183. doi: 10.1007/BF02700032. [DOI] [PubMed] [Google Scholar]
- 26.Parajuli P, Mathupala S, Sloan AE. Systematic comparison of dendritic cell-based immunotherapeutic strategies for malignant gliomas: in vitro induction of cytolytic and natural killer-like T cells. Neurosurgery. 2004;55:1194–1204. doi: 10.1227/01.NEU.0000141082.20865.48. [DOI] [PubMed] [Google Scholar]
- 27.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–8873. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- 28.Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4 + CD25 + regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- 29.Parajuli P, Joshee N, Chinni SR, Rimando AM, Mittal S, Sethi S, Yadav AK. Delayed growth of glioma by Scutellaria flavonoids involve inhibition of Akt, GSK-3 and NF-kappaB signaling. J Neurooncol. 2011;101:15–24. doi: 10.1007/s11060-010-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 31.Scheck AC, Perry K, Hank NC, Clark WD. Anticancer activity of extracts derived from the mature roots of Scutellaria baicalensis on human malignant brain tumor cells. BMC Complement Altern Med. 2006;6:27. doi: 10.1186/1472-6882-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerber M, Reiss Y, Wickersheim A, Jugold M, Kiessling F, Heil M, Tchaikovski V, Waltenberger J, Shibuya M, Plate KH, Machein MR. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68:7342–7351. doi: 10.1158/0008-5472.CAN-07-6241. [DOI] [PubMed] [Google Scholar]
- 34.Derhovanessian E, Adams V, Hahnel K, Groeger A, Pandha H, Ward S, Pawelec G. Pretreatment frequency of circulating IL-17 + CD4 + T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 35.Hong X, Jiang F, Kalkanis SN, Zhang ZG, Zhang X, Zheng X, Jiang H, Mikkelsen T, Chopp M. Increased chemotactic migration and growth in heparanase-overexpressing human U251n glioma cells. J Exp Clin Cancer Res. 2008;27:23. doi: 10.1186/1756-9966-27-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wainwright DA, Sengupta S, Han Y, Ulasov IV, Lesniak MS. The presence of IL-17A and T helper 17 cells in experimental mouse brain tumors and human glioma. PLoS One. 2010;5:e15390. doi: 10.1371/journal.pone.0015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maniati E, Soper R, Hagemann T. Up for Mischief? IL-17/Th17 in the tumour microenvironment. Oncogene. 2010;29:5653–5662. doi: 10.1038/onc.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benwell RK, Lee DR. Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand-activated dendritic cells. Clin Immunol. 2010;134:178–187. doi: 10.1016/j.clim.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 42.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, Wang J, Yin XY, Li L, Zheng L. Tumor-activated monocytes promote expansion of IL-17-producing CD8 + T cells in hepatocellular carcinoma patients. J Immunol. 2010;185:1544–1549. doi: 10.4049/jimmunol.0904094. [DOI] [PubMed] [Google Scholar]
- 44.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annunziato F, Romagnani S. The transient nature of the Th17 phenotype. Eur J Immunol. 2010;40:3312–3316. doi: 10.1002/eji.201041145. [DOI] [PubMed] [Google Scholar]
- 46.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Huang ZF, Xiong G, Mo HY, Qiu F, Mai HQ, Chen QY, He J, Chen SP, Zheng LM, Qian CN, Zeng YX. Distribution, characterization, and induction of CD8 + regulatory T cells and IL-17-producing CD8 + T cells in nasopharyngeal carcinoma. J Transl Med. 2011;9:189. doi: 10.1186/1479-5876-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Hernandez Mde L, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol. 2010;184:4215–4227. doi: 10.4049/jimmunol.0902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tajima M, Wakita D, Satoh T, Kitamura H, Nishimura T. IL-17/IFN-gamma double producing CD8 + T (Tc17/IFN-gamma) cells: a novel cytotoxic T-cell subset converted from Tc17 cells by IL-12. Int Immunol. 2011;23:751–759. doi: 10.1093/intimm/dxr086. [DOI] [PubMed] [Google Scholar]
- 51.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 52.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.V99.6.2114. [DOI] [PubMed] [Google Scholar]
- 53.Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, Dong M, Yamasawa K, Tamura K. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L, Chong MM, Littman DR. Plasticity of CD4 + T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de Jager W, Evans JG, Cimaz R, Bajaj-Elliott M, Wedderburn LR. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci USA. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volpe E, Touzot M, Servant N, Marloie-Provost MA, Hupe P, Barillot E, Soumelis V. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood. 2009;114:3610–3614. doi: 10.1182/blood-2009-05-223768. [DOI] [PubMed] [Google Scholar]
- 57.Zhao F, Hoechst B, Gamrekelashvili J, Ormandy LA, Voigtlander T, Wedemeyer H, Ylaya K, Wang XW, Hewitt SM, Manns MP, Korangy F, Greten TF. Human CCR4 + CCR6 + Th17 cells suppress autologous CD8 + T cell responses. J Immunol. 2012;188:6055–6062. doi: 10.4049/jimmunol.1102918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson LA, Sampson JH. Immunotherapy approaches for malignant glioma from 2007 to 2009. Curr Neurol Neurosci Rep. 2010;10:259–266. doi: 10.1007/s11910-010-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Prevalence of Th17 cells in gliomas. Paraffinized tumor sections, obtained from patients with malignant glioma were analyzed by IHC to determine the presence of CD4+IL-17+ Th17 cells (A); Isotype antibody control staining were also performed to rule out the background (B). The micrographs were imaged at 200x magnification (PPT 1389 kb)
Supplementary Figure S2. Isotype Controls for data presented in Figure 6. nT cells were cultured, stained with isotype control antibodies and were analyzed by flow cytometry, as described under Figure 6. The data are from one representative experiment out of three experiments performed with similar results (PPT 294 kb)