Abstract
In rodents, many aspects of sociosexual behavior are mediated by chemosignals released by opposite-sex conspecifics. These chemosignals are relayed via the main (MOS) and accessory olfactory systems (AOS) to the medial amygdala (Me). The Me is subdivided into anterior (MeA) and posterior (MeP) subnuclei, and lesions targeting these regions have different effects on proceptive courtship behaviors in female mice. Differential behavioral effects of MeA vs. MeP lesions could reflect a difference in the projections of neurons located in these Me subnuclei. To examine this question, we injected female mice with the anterograde tracer, Fluoro-Ruby into either the MeA or MeP and quantified labeled puncta in 11 forebrain target sites implicated in courtship behaviors using confocal fluorescence microscopy. We found that the MeP more densely innervates the medial and intermediate regions of the posterior bed nucleus of the stria terminalis (pBNST) and the posteromedial cortical amygdala (PMCo), while the MeA more densely innervates the horizontal diagonal band of Broca (HDB) and the medial olfactory tubercle (mOT), a region that may be a component of the circuitry responsible for olfactory-mediated motivated behaviors.
Keywords: Medial Amygdala, axon terminal, sociosexual behavior, olfactory, anterograde tracing
1. Introduction
Female mice prefer to seek out and investigate pheromonal odors released by male conspecifics, even in the absence of prior experience with those odors. These chemosignals are detected and processed by two separate olfactory systems, the main (MOS) and accessory (AOS) olfactory systems, which detect primarily volatile and non-volatile body odor cues, respectively. Both the MOS and AOS contribute to the normal display of sociosexual behaviors in female mice (Keller et al., 2006a,b; Martel & Baum, 2009b). Female urinary stimuli that activate either the MOS or the AOS are sufficient to establish a conditioned place preference in male mice, suggesting that either olfactory system suffices to mediate the rewarding effects of opposite-sex odors (Korzan et al., 2013).
The medial amygdala (Me) is a critical node of convergent inputs from the MOS and AOS. Both the main and accessory olfactory bulbs send direct projections to the Me; Me-projecting mitral cells in the ventromedial portion of the main olfactory bulb of female mice are preferentially activated by opposite-sex urinary odors, suggesting that this circuit may relay sexually relevant olfactory information to the Me (Kang et al., 2009). The Me includes anterior, posterodorsal and posteroventral subdivisions, and it has been proposed that these subnuclei play distinct roles in the processing of conspecific body odors. In male Syrian hamsters, the anterior Me (MeA) acts as a “chemosensory filter” that distinguishes between opposite-sex and same-sex odorants (Maras & Petrulis, 2006), while the posterodorsal Me (MePD), which includes a dense population of steroid receptor-expressing neurons (Wood et al., 1993), enhances male hamsters’ attraction toward female hamster body odorants. More recently, we found that in estrous female mice, lesions targeting only the MeP eliminated females’ motivation to preferentially approach urinary odors from testes-intact, as opposed to castrated males, whereas lesions of either the MeA or MeP reduced females’ lordotic responses to male mounts (DiBenedictis et al., 2012).
We hypothesized that the ability of MeP, but not MeA, lesions to disrupt the normal preference of female mice for male chemosignals would be reflected in differences in the downstream projection targets of these two amygdalar subnuclei. Two recent studies examined differences in the efferent projections of Me subdivisions in mice (Pardo-Bellver et al., 2012; Usunoff et al., 2009). One of these studies (Pardo-Bellver et al., 2012) used a small number of subjects (n=2 in one group) with no quantitative index of the strength of efferent Me projections; the other study (Usunoff et al., 2009) examined the MePD but provided no assessment of MeA projections. Here, we largely confirmed and extended the results of Pardo-Bellver and others (2012) in outbred (Swiss Webster) female mice using a method of analysis that allowed for a quantitative assessment of MeA vs. MeP projections to downstream forebrain projection targets. We found that the MeA and MeP differentially target several forebrain sites implicated in motivated behavior, including the posterior BNST (pBNST) and medial olfactory tubercle (mOT).
2. Results
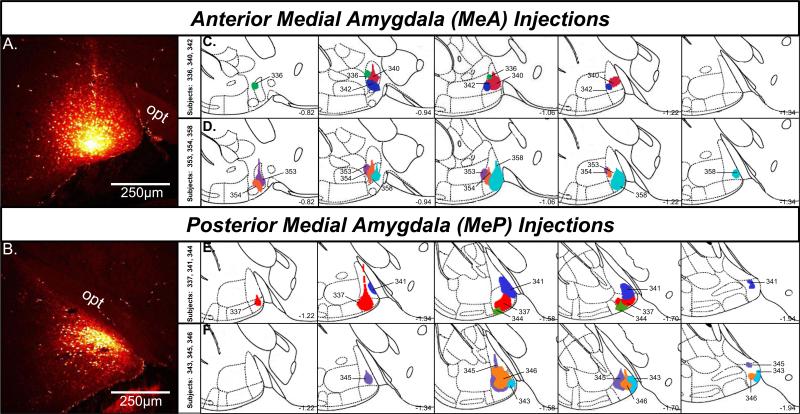
Injections of the anterograde tracer, Fluoro-Ruby, into the MeA or MeP were largely confined to the target nucleus (Fig. 1A,B), although in the case of larger injections, there was some leakage of tracer (i.e. very few labeled cell bodies) into the rostral MeP in animals given injections intended for the MeA (Fig. 1D). Likewise, there was minimal leakage into the caudal MeA in animals given injections targeting the MeP (Fig. 1E). In some cases, a few labeled cell bodies were observed lateral to the target Me nucleus, minimally affecting adjacent amygdaloid nuclei including the anterior amygdaloid area (subjects 336 and 353), and the anterior cortical amygdaloid nucleus (subjects 337 and 344; see Figure 1). However, labeling due to tracer spread in these adjacent regions was very sparse and in most cases did not occur at all. In no cases were central amygdaloid nuclei labeled.
Figure 1.
A,B, Confocal image showing Fluoro-Ruby injection sites in the anterior (A) and posterior (B) medial amygdala. C,D, Schematic reconstructions of coronal sections showing the extent of individual Fluoro-Ruby injections in female mice targeting the anterior (n=6) medial amygdala. Top row: subjects 336, 340, 342; Bottom row: subjects 353, 354, 358. E,F, Schematic reconstructions of coronal sections showing the extent of individual Fluoro-Ruby injections in female mice targeting the posterior (n=6) medial amygdala. Top row: subjects 337, 341, 344; Bottom row: subjects 343, 345, 346. Sections are ordered sequentially from anterior (left) to posterior (right), with the numbers shown on the bottom right of each plate representing the distance in mm posterior to bregma. Colored regions within each plate represent individual injections, identified by the animal code. Adapted from Franklin and Paxinos (Franklin & Paxinos, 2008). MeA, anterior medial amygdala; MeP, posterior medial amygdala; opt, optic tract.
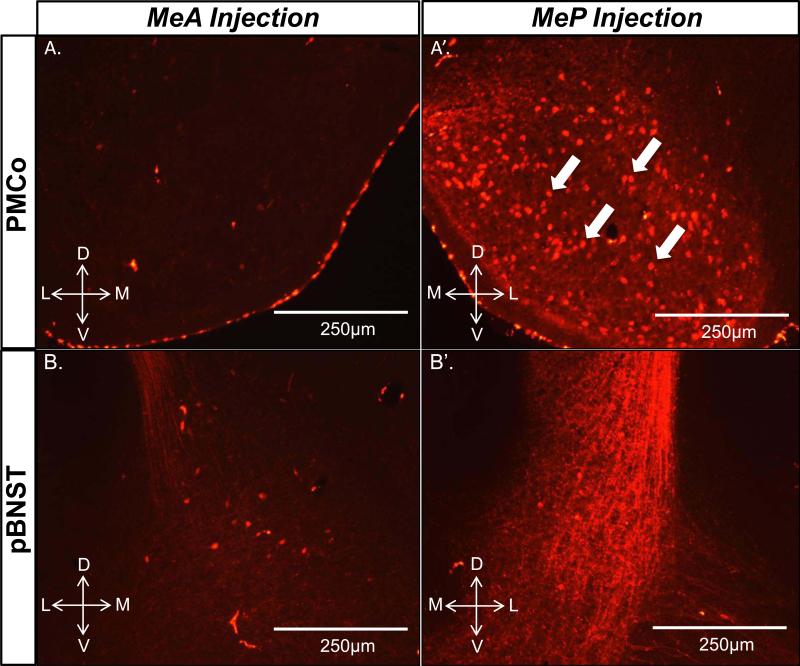
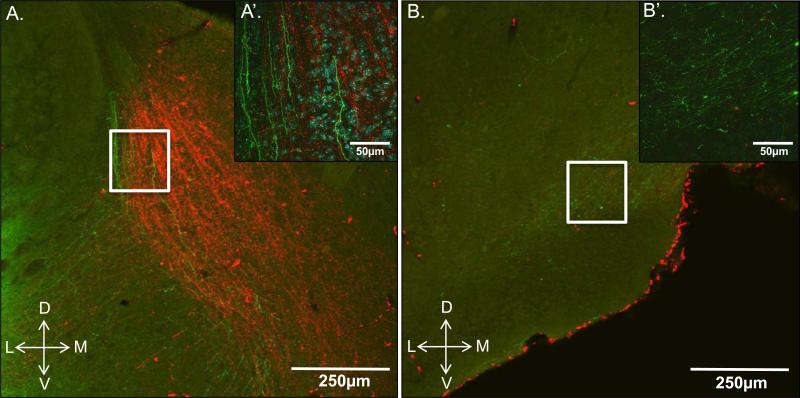
Both the posteromedial cortical amygdala (PMCo) and posterior bed nucleus of the stria terminals (pBNST) were most densely innervated by the MeP injections (Fig. 2). Many anterogradely labeled fibers originating from neurons in the MeP were observed in the PMCo. Retrogradely labeled cell bodies were also found, suggesting a dense bilateral connectivity between the MeP and PMCo (Fig. 2A’) that was not observed between the MeA and PMCo (Fig. 2A). Fluoro-Ruby is predominantly an anterograde tracer, but retrograde labeling can also occur. The pBNST also showed dense labeling in lateral, intermediate, and medial subregions (STMPL, STMPI, STMPM, respectively) in MeP-injected subjects (Fig. 2B’), while labeling was mostly observed in the STMPL in MeA-injected subjects (Fig. 2B). Several animals (not included in the quantitative analysis) were given an injection of the anterograde tracer, Phaseolus vulgaris leucoagglutinin (PHA-L) into the MeA plus an injection of Fluoro-Ruby into the MeP of the same hemisphere in order to obtain a qualitative comparison of pBNST and mOT innervation by the MeA and MeP. Fibers originating from the MeA and MeP were apparent throughout the pBNST but mainly overlapped in the STMPL (Fig. 3A,A’). In contrast to the pBNST, the mOT received relatively dense innervation from the MeA, but was only sparsely innervated by the MeP (Fig. 3B,B’).
Figure 2.
The posterior medial amygdala (MeP) densely innervates the posteromedial cortical amygdala (PMCo) and the posterior bed nucleus of the stria terminalis (pBNST). Epifluorescent images show dense anterograde fiber labeling in the PMCo and pBNST (A’, B’) in MeP Fluoro-Ruby-injected animals, but not MeA Fluoro-Ruby-injected animals (A, B). Retrogradely labeled cell bodies were also observed in the PMCo of subjects given Fluoro-Ruby injections into the MeP (A’), indicated by filled white arrows.
Figure 3.
Overlapping anterograde fiber/puncta labeling in the posterior bed nucleus of the stria terminalis (A; pBNST) and medial olfactory tubercle (B; mOT) of a female mouse given injections of PHA-L (green) and Fluoro-Ruby (red) into the MeA and MeP, respectively. A, confocal image showing that the intermediate and medial portions of the pBNST receive input mostly from the MeP (red), while the lateral portion receives relatively equal input from both the anterior and posterior Me. A’, 60× (oil) Z plane-stacked confocal image of the boxed area shown in A. Labeled fibers with puncta originating from the MeA (green) and MeP (red) were seen throughout the posterolateral stria terminalis (STMPL), shown with DAPI nuclear counterstain (blue cell bodies). B, confocal image showing synaptic input to the mOT almost exclusively from the anterior Me (green) with very sparse labeling from the MeP (red). B’, 60× (oil) Z plane-stacked confocal image of the boxed area shown in B showing innervation of the mOT predominantly by the MeA (green fibers).
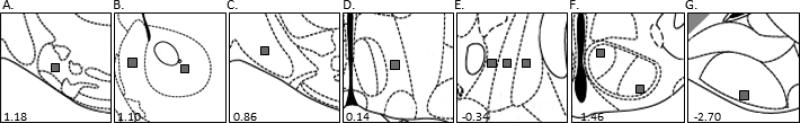
An important goal of this study was to directly compare the relative magnitude of efferent innervation of MeA vs. MeP projection targets; therefore a quantitative estimation of puncta number in projection targets of the MeA and MeP was performed. Puncta are highly variable in size (Mitchell et al., 2012), and only those that could be reliably identified were counted (Fig. 4). Puncta were quantified from confocal images stacked in the Z-plane of 11 forebrain sites that received considerable innervation by the MeA and/or MeP. These regions included the mOT, the shell and core of the nucleus accumbens (AcbSh, AcbC), the horizontal diagonal band of Broca (HDB), the medial preoptic area (MPA), pBNST subregions (STMPM, STMPI, STMPL), dorsomedial and ventrolateral subdivisions of the ventromedial hypothalamus (VMHdm, VMHvl), and PMCo (Fig. 5). These regions were chosen for two main reasons. First, preliminary qualitative results indicated that these regions received considerable innervation from the MeA and/or MeP. Second, the purpose of this study was to elucidate the degree of innervation of Me forebrain targets that have been previously implicated in odor-driven sociosexual behaviors, since deficits observed in MeA vs. MeP-lesioned animals in our previous study concerned these behaviors (DiBenedictis et al., 2012). Intra-amygdaloid connections, though important, were not included in the present study (with the exception of Me-PMCo projections) because our aim was to focus on specific regions of interest and not to document the full breadth and magnitude of MeA and MeP projections in the female mouse brain (but see Pardo-Bellver et al., 2012). The precise locations of puncta counting domains within each target nucleus analyzed were chosen based on prior studies from our laboratory in which we observed augmented Fos protein expression in response to opposite-sex odors or in response to placement in a chamber where subjects were previously exposed to an anesthetized opposite-sex conspecific (see Pankevich 2006b; Martel & Baum, 2009a; Kang et al., 2009).
Figure 4.
Z-plane stacked confocal images (60× magnification) showing anterograde fiber/puncta labeling in coronal sections of the medial portion of the posterior stria terminalis (STMPM) following Fluoro-Ruby injections into either the anterior (A, MeA) or posterior (B, MeP) medial amygdala. C, Filled white arrows point to labeled puncta, magnified from the boxed region that were included for analysis (0.8μm-1.6μm diameter); open arrows point to possible puncta that were very small (<0.8μm diameter) and therefore excluded from analysis.
Figure 5.
Modified schematic from the mouse brain atlas of Franklin and Paxinos (Franklin & Paxinos, 2008) showing the location of forebrain regions in which labeled puncta (dark gray boxes; 1002 μm) were counted. Counting regions included the medial olfactory tubercle (A), the shell (left) and core (right) of the nucleus accumbens (B), the horizontal diagonal band of Broca (C), the medial preoptic area (D), medial (left), intermediate (middle), and lateral (right) posterior bed nucleus of the stria terminalis (E), dorsomedial (left) and ventrolateral (right) subdivisions of the ventromedial hypothalamus (F), and posteromedial cortical amygdala (G). Numerical values represent the distance in mm from bregma for each section.
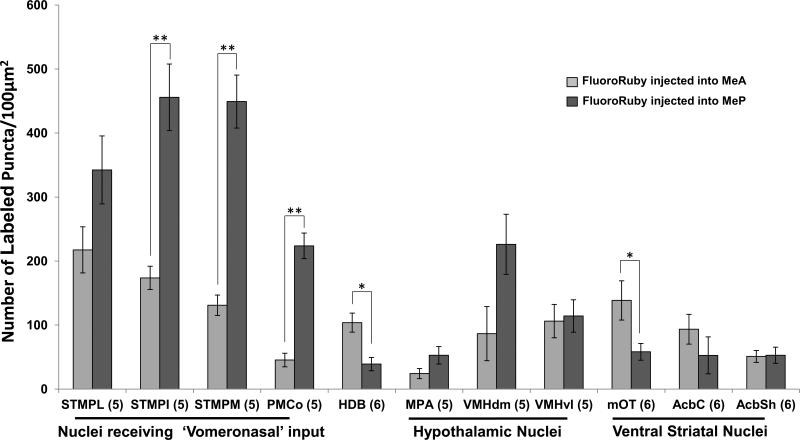
In support of qualitative observations, results of puncta analysis showed that the STMPI (t (df = 8) = 5.350, p < 0.001), STMPM (t (df = 8) = 7.445, p < 0.001), and PMCo (t (df = 9) = −8.632, p = 0.001) possessed significantly more labeled puncta on efferent fibers originating from the MeP, as compared to the MeA. In contrast, the HDB (t (df = 10) = 3.567, p = 0.005) and mOT (t (df = 10) = 2.408, p = 0.037) showed denser innervation by the MeA. Other ventral striatal and hypothalamic structures displayed relatively equal innervation from the MeA and MeP, although there was a trend toward denser innervation by the MeP in the STMPL (t (df = 8) = 2.263, p = 0.053) and VMHdm (t (df = 8) = −2.208, p = 0.058) (Fig. 6).
Figure 6.
Quantitative estimation of labeled puncta in animals given Fluoro-Ruby injections into either the MeA or MeP. Brain regions in which puncta were counted: STMPL, STMPI, STMPM, posterolateral, intermediate, medial divisions of the bed nucleus of the stria terminalis; PMCo, posteromedial cortical amygdala; HDB, horizontal diagonal band of Broca; MPA, medial preoptic area; VMHdm, VMHvl, dorsomedial, ventrolateral portions of the ventromedial hypothalamus; mOT, medial olfactory tubercle; AcbC, AcbSh, nucleus accumbens core, shell. Data are expressed as the mean (±SEM) calculated from confocal images merged in the Z-plane. Sample sizes for each group are shown in parentheses. *P<0.040, **P<0.001 (Two-tailed Student's t-test).
3. Discussion
The present results demonstrate that the MeA and MeP differentially target multiple ventral telencephalic sites that have been previously implicated in the control of sociosexual behaviors. The pBNST is the major recipient of efferent projections from the Me as a whole, but our analysis revealed that the STMPI and STMPM subdivisions receive significantly more input from the MeP, while the STMPL receives an equal, but lesser degree of input from both the MeA and MeP. Aspects of this pattern of innervation of the pBNST by the Me differs from those observed by Pardo-Bellver et al., (2012). Specifically, those workers reported that the MeA most densely innervates the STMPI, while the STMPM is equally innervated by all Me subnuclei. While it is possible that this discrepancy could reflect strain differences (C57BL/J6 & CD1 vs. Swiss Webster in the present study), an alternative explanation is that puncta analysis, averaged across tracer injections given in multiple animals, is more sensitive to discrete differences in the pattern of innervation of these subnuclei. For example, the MeA and MeP may send a similar number of axonal projections to the STMPI (as has been previously reported using qualitative measurements of fiber innervation). However, projections from the MeP may make more en passant synaptic connections within the region, as indexed by labeled puncta, while the majority of fibers from the MeA may represent fibers of passage, producing fewer labeled puncta. Puncta evaluation is therefore a fundamentally different method of analysis, which makes direct comparisons with past studies using qualitative measures of fiber density potentially confounding. A major strength of the current method is that puncta values are averaged across multiple animals, which accounts for individual variability and may provide a more complete view (i.e. rostal to caudal) of the outputs of a given brain region. Our approach contrasts with the alternative approach (Pardo-Bellver et al., 2012; Usunoff et al., 2009) in which as few as one or two discrete tracer injections, which may or may not cover a substantial volume of the targeted nucleus, are used to assess efferent projections of amygdalar subnuclei.
Puncta are clearly defined boutons sprouting from labeled fibers and have been shown to contain synaptic proteins (Bozdagi et al., 2000). They are therefore thought to denote the presence of synapses. For example, long-term potentiation in hippocampal neurons, which is associated with an increase synapse numbers (Toni et al., 1999), is accompanied by long lasting increases in puncta number (Antonova et al., 2009). In the present study, we did not probe for the presence of pre and/or post-synaptic proteins and cannot therefore define a punctum as representing a synapse, but rather as a method for quantifying the amount of labeling observed in Me projection targets.
The BNST has been directly implicated in the control of sociosexual behaviors. In female hamsters, an intact BNST is necessary for the display of precopulatory behaviors including ultrasonic vocalizations and vaginal scent marking (Kirn & Floody, 1985; Martinez & Petrulis, 2011), while in male hamsters excitotoxic lesions of the BNST eliminate opposite-sex odor preference (Been & Petrulis, 2010). The BNST as a whole, but particularly the anterior BNST, densely innervates the ventral tegmental area (VTA) predominantly via GABAergic inputs. The BNST is thus a potent regulator VTA activity, principally acting by disinhibiting dopaminergic projection neurons (Georges & Aston-Jones, 2001; Kudo et al., 2012). This polysynaptic pathway linking the MeP with the VTA (via the BNST) may underlie the preference of female mice for opposite-sex odors (DiBenedictis et al., 2012). The activation of this pathway may also contribute to the increase in Fos expression previously observed in the VTA in response to opposite-sex odors (Moncho-Bogani et al., 2005) in lieu of any major direct Me-VTA projections. Moreover, amygdaloid fibers terminating in the AcbC and AcbSh also traverse the BNST (Novejarque et al., 2011).
The VMH and MPA play important roles in controlling sexual behavior in females of many rodent species (Blaustein & Erskine, 2002; Clark et al., 1981; Malsbury et al., 1977; Meerts & Clark, 2009), and the MePD projection to the VMH conveys reproductively significant olfactory information in mice and ferrets (Choi et al., 2005; Wersinger & Baum, 1997). In the present study we found that these regions receive relatively equal input from the MeA and MeP. This result corroborates our previous work showing that both MeA and MeP-lesioned female mice exhibited deficits in their display of lordosis in response to male mounts (DiBenedictis et al., 2012).
The posteromedial cortical amygdala receives substantial input (as measured by labeled puncta) from both the MeA and MeP, with the most robust innervation originating from the MeP. These projections are reciprocal, as has been previously described in rats and hamsters (Coolen & Wood, 1998; Pitkänen et al., 2000), suggesting that the pattern of Me output may be modified by intra-amygdaloid interactions. Insofar as the PMCo directly targets regions of the ventral striatum, including the islands of Calleja and adjoining olfactory tubercle complex (Novejarque et al., 2012; Ubeda-Banon et al., 2008), this circuit may also be involved in motivating appetitive behavioral responses to salient opposite-sex chemosignals.
In contrast to the BNST and PMCo, we observed significantly more labeled puncta on fibers originating from the MeA in the horizontal limb of the diagonal band of Broca (HDB). The HDB is a major cholinergic brain center whose activity has been linked to attention. Pharmacological disruption of cholinergic signaling has been shown to impair performance in olfactory-based behavioral tasks (Roman et al., 1993), while enhancing acetylcholine levels improved olfactory discrimination (Doty et al., 1999). Furthermore, the HDB provides major centrifugal inputs to the MOB (Ma & Luo, 2012), which presumably modulates odor perception. It is possible that salient pheromonal information reaching the MeA is passed to the HDB, which in turn heightens the animal's level of attention toward the pheromonal stimulus, thereby enhancing olfactory acuity and promoting chemoinvestigation.
We also observed a pathway directly linking the MeA (and to a lesser extent, the MeP) with the ventral striatum, including the mOT, AcbSh, and AcbC. While Meaccumbal projections were relatively sparse, there was robust innervation of the mOT by the MeA, but not by the MeP. Because this projection is monosynaptic, pheromonal information transmitted by this pathway may be subject to limited signal modification and could be involved in mediating the reinforcing effects of opposite-sex odors. Indeed, the mOT has been implicated in the reinforcing effects of cocaine, MDMA, and amphetamines (Ikemoto, 2003; Shin et al., 2008; Shin et al., 2010) and in male rat sexual behavior (Hitt et al., 1973). The mOT has also been hypothesized to play an important role in odor hedonics (Wesson & Wilson, 2011). We propose that male pheromonal signals reach the Me via the main and accessory olfactory bulbs and that this information is relayed to the ventral striatum by way of both indirect polysynaptic (MeP-BNST-VTA-mOT and MeP-PMCo-mOT) and direct monosynaptic (MeA-mOT) pathways which may act synergistically to motivate female mice to seek out and investigate these odors, a critical first step leading to successful procreation. Based on our previous work (DiBenedictis et al., 2012), it is likely that indirect inputs to the mOT, originating from the MeP, are alone sufficient to drive proceptive chemoinvestagatory behaviors, while the direct pathway from the MeA is not. Future work will assess the functional role of the mOT in hardwired olfactory-driven motivated behaviors in female mice.
4. Experimental Procedure
Thirty-five ovary-intact female Swiss Webster mice (Charles River Laboratories, Wilmington, MA, USA) were purchased at 5-6 weeks of age and maintained on a reversed 12:12 h light:dark cycle with food and water available ad libitum. All procedures were approved by the Boston University Charles River Campus Institutional Animal Care and Use Committee. Female subjects used for quantitative analysis were assigned to one of two groups: MeA Fluoro-Ruby-injected or MeP Fluoro-Ruby-injected. Subjects were injected with tracer in both hemispheres to maximize the probability of obtaining an accurate injection. However, projections were quantified only within a single hemisphere for each animal, and only mice with accurate injections in either the MeA (n=6) or MeP (n=6) were included in this study. Mice with inaccurate injection placement and/or appreciable spread of the anterograde tracer into adjacent amygdaloid nuclei were not used (n=21).
Subjects were administered carprofen analgesic (5 mg/kg, s.c.) preoperatively and for 2 days after surgery. Mice were anesthetized under continuous 2% isoflurane vapor, and the head was secured in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). A midline incision was made to expose the skull, and a small craniotomy was then drilled bilaterally over each injection site. For both MeA- and MeP-injected animals, lateral coordinates were 2.0 mm and depth coordinates (from dura) were 4.7 mm. Anterior-Posterior (from interaural line) coordinates were 4.0 mm (MeA) and 2.7 mm (MeP). A pulled glass micropipette (20 m tip diameter) filled with the fluorescent anterograde tracer, Fluoro-Ruby (5% in 0.01 M PB, pH 8.0, Molecular Probes), was lowered into either the MeA or MeP. Next, a (+5μA) pulsatile cathodal current (7s on, 7s off) was passed for 2 minutes to deliver the tracer iontophoretically. The electrode was then retracted while passing a constant (−5μA) anodal current. A separate group of animals received dual anterograde tracer injections in the MeA and MeP (n=3): the anterograde tracer, PHA-L; 2.5% in 0.01 M PB, pH 8.0) was injected into the MeA, and Fluoro-Ruby was injected into the MeP within the same hemisphere. Data were not collected from these mice; they were used only to depict qualitatively the overlap in MeA vs MeP projections shown in Figure 3. Craniotomies were covered with bone wax, and the incision was closed by suture and treated with 2% topical lidocaine. Five days after surgery, subjects were perfused and their brains were subsequently removed, fixed, cryoprotected and sectioned coronally at 30 μm using a cryostat (DiBenedictis et al., 2012).
Fluorescent immunocytochemistry was used to visualize PHA-L labeling in dual anterograde tracer-injected animals. Tissues were blocked for 1 h in 5% donkey serum and subsequently incubated with primary (Vector Laboratories, goat anti-PHA-L, 1:4000, overnight at 4°C) and secondary antibodies (Life Technologies, Alexa Flur 488 donkey anti-goat, 1:600, 1 h at room temperature) followed by a 10 min incubation in 4′,6-diamidino-2-phenylindole (DAPI; Sigma, 1:200, RT) to label cell nuclei.
Fluorescently labeled (Fluoro-Ruby) puncta (terminal and en passant boutons) were counted to quantify the relative magnitude of efferent projections from the MeA vs. MeP. Eleven forebrain regions previously implicated in courtship behaviors were chosen for analysis. Confocal images (60X) of projection targets were obtained using 30 laser scans (every 1.0 μm) in 30 μm depth samples using an Olympus FluoView 10i confocal microscope. These images were then stacked in the depth (‘Z’) plane, and a 100-μm2 area was selected for each region of interest. Puncta were counted in each region using the ‘cell counter’ plugin in ImageJ by an investigator blind to location of the tracer injection. Only puncta that were clearly visible and sufficiently large (between 0.8 μm and 1.6 μm in diameter, similar in range to that used by Pro-Sistiaga et al., 2009) were included in our analysis. Limitations of this analysis, such as the amount of tracer delivered, the percentage of neurons that took up the tracer, and the counting of spurious puncta were assumed to be the same between groups, rendering this type of analysis suitable for a comparative (targets of MeA vs. MeP input) study (Pro-Sistiaga et al., 2007). The number of labeled puncta for each animal was averaged for each group and compared in each forebrain region between the MeA and MeP-injected subjects using a 2-tailed Student's t-test.
Highlights.
The anterior and posterior Me differentially innervate downstream targets
The MeP, but not the MeA, densely innervates the BNST and PMCo
The MeA, but not the MeP, densely innervates the HDB and mOT
Circuits from the Me to the mOT may underlie innate approach behaviors
Acknowledgments
Supported by NIH grant DC008962 awarded to JAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonova I, Lu F-M, Zablow L, Udo H, Hawkins RD. Rapid and long-lasting increase in sites for synapse assembly during late phase potentiation in rat hippocampal neurons. Plos One. 4(11):e7690. doi: 10.1371/journal.pone.0007690. doi:10.1371/journal.pone.0007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Lesions of the posterior bed nucleus of the stria terminalis eliminate opposite-sex odor preference and delay copulation in male Syrian hamsters: role of odor volatility and sexual experience. Eur. J. Neurosci. 2010;32:483–493. doi: 10.1111/j.1460-9568.2010.07277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent brain. In: Pfaff DW, Arnold AP, Al E, editors. Hormones and Behavior. Academic Press; New York: 2002. pp. 139–214. [Google Scholar]
- Bozdagi O, Shan W, Tanaka H, Bensen DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Clark AS, Pfeifle JK, Edwards DA. Ventromedial hypothalamic damage and sexual proceptivity in female rats. Physiol. Behav. 1981;27:597–602. doi: 10.1016/0031-9384(81)90228-6. [DOI] [PubMed] [Google Scholar]
- Coolen L, Wood R. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J. Comp. Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Ingraham KR, Baum MJ, Cherry JA. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol. Behav. 2012;105:554–559. doi: 10.1016/j.physbeh.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Bagla R, Kim N. Physostigmine enhances performance on an odor mixture discrimination test. Physiol. Behav. 1999;65:801–804. doi: 10.1016/s0031-9384(98)00238-8. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. third ed. Academic Press; California: 2008. [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J. Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt JC, Bryon DM, Modianos DT. Effects of rostral medial forebrain bundle and olfactory tubercle lesions upon sexual behavior of male rats. J. Comp. Physiol. Psych. 1973;82:30–36. doi: 10.1037/h0033797. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J. Neurosci. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur. J. Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur. J. Neurosci. 2006;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem. Sens. 2006;31:315–323. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn J, Floody OR. Differential effects of lesions in three limbic areas on ultrasound production and lordosis by female hamsters. Behav. Neurosci. 1985;6:1142–1152. doi: 10.1037//0735-7044.99.6.1142. [DOI] [PubMed] [Google Scholar]
- Korzan WJ, Freamat M, Johnson AG, Cherry JA, Baum MJ. Either main or accessory olfactory system signaling can mediate the rewarding effects of estrous female chemosignals in sexually naïve male mice. Behav. Neurosci. 2013 doi: 10.1037/a0033945. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J. Neurosci. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Luo M. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J. Neurosci. 2012;32:10105–10116. doi: 10.1523/JNEUROSCI.0058-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsbury CW, Kow LM, Pfaff DW. Effects of medial hypothalamic lesions on the lordosis response and other behaviors in female golden hamsters. Physiol. Behav. 1977;19:223–237. doi: 10.1016/0031-9384(77)90331-6. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur. J. Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway from to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur. J. Neurosci. 2009a;29:368–376. doi: 10.1111/j.1460-9568.2008.06564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Adult testosterone treatment but not surgical disruption of vomeronasal function augments male-typical sexual behavior in female mice. J. Neurosci. 2009b;29:7658–7666. doi: 10.1523/JNEUROSCI.1311-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Petrulis A. The bed nucleus of the stria terminalis is critical for sexual solicitation, but not for opposite-sex odor preference, in female Syrian hamsters. Horm. Behav. 2011;60:651–659. doi: 10.1016/j.yhbeh.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts SH, Clark AS. Lesions of the medial preoptic area interfere with the display of a conditioned place preference for vaginocervical stimulation in rats. Behav. Neurosci. 2009;123:752–757. doi: 10.1037/a0016077. [DOI] [PubMed] [Google Scholar]
- Mitchell N, Petralia RS, Currier DG, Wang Y-X, Kim A, Mattson MP, Yao PJ. Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. J. Cell Sci. 2012;125:4207–4213. doi: 10.1242/jcs.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncho-Bogani J, Martinez-Garcia F, Novejarque A, Lanuza E. Attraction to sexual pheromones and associated odorants in female mice involves activation of the reward system and basolateral amygdala. Eur. J. Neurosci. 2005;21:2186–2198. doi: 10.1111/j.1460-9568.2005.04036.x. [DOI] [PubMed] [Google Scholar]
- Novejarque A, Gutiérrez-Castellanos N, Lanuza E, Martínez-García F. Amygdaloid projections to the ventral stria- tum in mice: direct and indirect chemosensory inputs to the brain reward system. Front. Neuroanat. 2011;5 doi: 10.3389/fnana.2011.00054. doi: 10.3389/fnana.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Accessory olfactory neural Fos responses to a conditioned environment are blocked in male mice by vomeronasal organ removal. Physiol. Behav. 2006b;87:781–788. doi: 10.1016/j.physbeh.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Bellver C, Cádiz-Moretti B, Novejarque A, Martínez-García F, Lanuza E. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front. Neuroanat. 2012;6 doi: 10.3389/fnana.2012.00033. doi: 10.3389/fnana.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Ann. N. Y. Acad. Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del Mar Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J. Comp. Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Roman FS, Simonetto I, Soumireu-Mourat B. Learning and memory of odor-reward association: selective impairment following horizontal diagonal band lesions. Behav. Neurosci. 1993;107:72–81. doi: 10.1037//0735-7044.107.1.72. [DOI] [PubMed] [Google Scholar]
- Shin R, Qin M, Liu Z-H, Ikemoto S. Intracranial self-administration of MDMA into the ventral striatum of the rat: Differential roles of the nucleus accumbens shell, core and olfactory tubercle. Psychopharm. 2008;198:261–270. doi: 10.1007/s00213-008-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Cao J, Webb SM, Ikemoto S. Amphetamine administration into the ventral striatum facilitates behavioral interaction with unconditioned visual signals in rats. Plos One. 2010;5 doi: 10.1371/journal.pone.0008741. doi:10.1371/journal.pone.0008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Ubeda-Banon I, Novejarque A, Mohedano-Moriano A, Pro-Sistiaga P, Insausti R, Martinez-Garcia F, Lanuza E, Martinez-Marcos A. Vomeronasal inputs to the rodent ventral striatum. Brain Res. Bull. 2008;75:467–473. doi: 10.1016/j.brainresbull.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Usunoff K, Schmitt O, Itzev D, Haas JS, Lazarov N, Rolfs A, Wree A. Efferent projections of the anterior and posterodorsal regions of the medial nucleus of the amygdala in the mouse. Cell Tiss. Org. 2009;190:256–285. doi: 10.1159/000209233. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Wilson DA. Sniffing out the contributions of the olfactory tubercle to the sense of smell: hedonics, sensory integration, and more? J. Neurobio. Rev. 2011;35:655–668. doi: 10.1016/j.neubiorev.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Baum MJ. Sexually dimorphic processing of somatosensory and chemosensory inputs to forebrain luteinizing hormone- releasing hormone neurons in mated ferrets. Endocrinology. 1997;138:1121–1129. doi: 10.1210/endo.138.3.4969. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Mating activates androgen receptor-containing neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1993;614:65–77. doi: 10.1016/0006-8993(93)91019-o. [DOI] [PubMed] [Google Scholar]