Abstract
Both metazoan parasites and simple protein allergens induce Th2 regulated immune responses, but the innate immune sensing mechanisms involved are currently unknown. Specifically, the cellular source of cytokines that control Th2 differentiation in vivo has not been defined. Here we show that basophils are activated and recruited to the draining lymph nodes specifically in response to Th2 inducing allergen challenge. Furthermore, we demonstrate that the basophil is the accessory cell type required for Th2 induction in response to protease allergens. Finally, we show that basophils are directly activated by protease allergens and produce Th2 inducing cytokines, including IL-4 and TSLP, which are involved in Th2 differentiation in vivo.
Introduction
Bacterial, viral, and fungal pathogens are evolutionarily distant from the host and thus produce biochemically distinct macromolecules that can be sensed by a variety of pattern recognition receptors of the host, such as Toll-like receptors (TLRs), NODs and Dectin-1. Activation of these receptors generally results in the induction of Th1 and Th17 regulated immune responses. In contrast, little is known about the innate immune recognition mechanisms and cell types involved in the initiation of Th2 responses. Th2 regulated immune responses are induced upon helminth infections, and are characterized by activation of eosinophils, mast cells, mucosal epithelial cells and production of IgE antibodies1. It is remarkable and puzzling that Th2 and IgE responses can also be induced by simple protein allergens, as they appear to have little in common with complex parasites. Many potent allergens, however, have intrinsic protease activity2-4, and secreted proteases are essential for the infectious and reproductive cycles of helminths5. It is possible, therefore, that the innate immune system has evolved a detection mechanism based on sensing the abnormal protease activity associated with helminth infections. The ability of protease allergens to trigger the Th2 mediated response could then be explained by their ability to trigger, unintentionally, the same pathway that has evolved to detect infection by helminths6.
The differentiation of naïve T cells into Th1 and Th17 effectors is controlled by cytokines produced by dendritic cells (DCs) as well as other accessory cell types. Thus, Th1 differentiation is regulated by IFN-γ produced by NK cells recruited to the lymph nodes (LN)7, and by IL-12 produced by DCs upon TLR activation8. Th17 differentiation is controlled by DC-derived IL-6 and TGF-β (from an unknown source) and by IL-23 produced by DCs upon Dectin-1 activation9,10. In contrast, the cell types and cytokines involved in Th2 differentiation in vivo are not well defined. IL-4 is essential for Th2 differentiation11, but the cellular source of IL-4 is not clear.
Several possible cellular sources of IL-4 have been postulated; mast cells and basophils for example are known to readily produce IL-4 upon cross-linking of their FcεRI receptors12. FcεRI crosslinking on basophils leads to Th2 differentiation in a model of repeated exposure to an antigen13 and mice with increased numbers of basophils exhibit increased Th2 differentiation in response to hapten immunization14. However, whether basophils play any role in Th2 induction in the absence of antigen-specific IgE, as would occur during the primary exposure to an antigen, is unknown.
In addition to the essential role of IL-4 in Th2 differentiation, recent studies have identified an important role for thymic stromal lymphopoeitin (TSLP) in Th2-mediated immunity. TSLP plays a role in lymphocyte development, but is also produced by epithelial cells at sites of chronic allergic inflammation leading to Th2 cell recruitment and allergic inflammation15. Stimulation of DCs with TSLP leads to OX-40L upregulation and can lead to Th2 differentiation in vitro15. In addition, TSLP can contribute to Th2 immunity by inhibiting Th1 differentiation16. Finally, in vitro studies have shown that TSLP can act directly on T cells to promote Th2 differentiation17. However, because TSLP is only known to be produced in peripheral tissues, such as epithelial cells, it is unclear whether it plays a role in the initiation of Th2 immune responses in vivo, which would require TSLP production in the LNs at the time of naïve T cell activation.
Here we sought to identify the mechanisms of innate immune control of Th2 responses after primary exposure to an allergen. Although the protease activity of allergens has been shown to be required for the induction of Th2 and IgE responses, the cellular target of that protease activity is unknown4,18,19. We find that basophils directly respond to a model protease allergen, papain, by producing IL-4, TSLP and other Th2-associated cytokines and chemokines. Importantly, we find that basophils are transiently recruited to the draining LNs in response to papain immunization. Furthermore, we demonstrate that basophils are essential for the Th2 induction in response to allergen immunization. Finally, we show that TSLP is produced by basophils in the LNs and plays an important role in Th2 differentiation in vivo and in vitro.
Results
The antibody response to papain
Papain is a cysteine protease and a potent allergen associated with occupational allergy in humans20 and IgE, IgG1 production, and mast cell degranulation in mice6,19. Papain immunization without any additional adjuvants stimulated production of IgE and IgG1 antibodies in Balb/c and C57Bl/6 mice, while an inert and pure protein antigen, human serum albumin (HSA), did not (Fig. 1a-b, Fig. S1a). Papain immunization did not result in IgM or IgG2 production (Fig. S1b-c), suggesting that papain possesses specifically Th2 adjuvant activity. Consistent with previous reports19, this effect was dependent on papain's protease activity. Blocking the protease activity of papain (Fig. S1d) decreased or eliminated induction of total and papain-specific IgE (Fig. 1c & Fig. S1e-f). IgE induction by papain was not dependent on TLRs (either physiologically, or due to contaminating ligands), as it was retained in TLR2X4 double knock-out mice and MyD88 deficient mice (Fig. 1d-e). Although mast cells can degranulate in response to papain19, the papain induced IgE production independent of mast cells, as papain induced a robust IgE response in mast cell deficient mice (Fig. 1f). Finally, IgE induction was not a unique property of papain, as immunization with bromelain, another protease allergen, also induced a strong IgE response (Fig. S1g).
Figure 1.
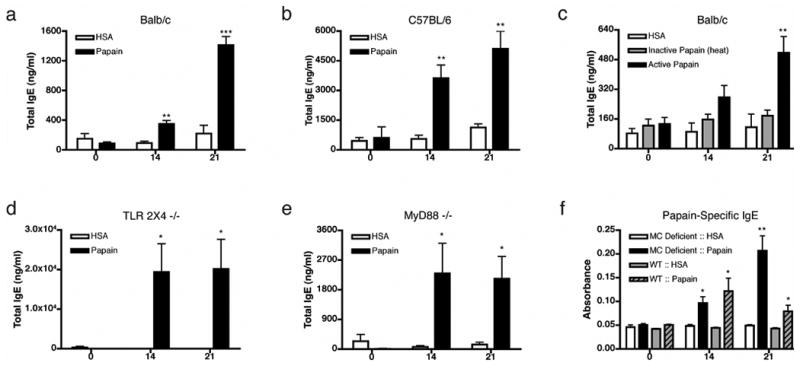
Protease active papain induces IgE secretion independent of TLR2, TLR4 or MyD88 signaling. Serum IgE levels immediately before primary immunization (day 0), before secondary immunization (day 14) and at day 21 in Balb/c (a) or C57BL/6 (b) mice are shown. (c) Total IgE response to heat inactivated papain in Balb/c mice, in TLR2/4 -/- mice (d), or MyD88 -/- mice (e). (f) Antigen specific IgE response in mast cell (MC) deficient (KitW/W-v) and littermate controls (WT). For each timepoint, n=5-10 (a-c), or n=3-5 (d-f). Data are representative of at least three separate experiments, panels d and e represent data combined from separate experiments. Error bars represent s.e.m.; p values are calculated via the Student's t test, and represent comparisons to HSA immunizations at the indicated time-points; ***, p≤0.0001; **, p≤0.001, *, p≤0.01. If not indicated p>0.05.
Th2 differentiation in response to papain
We next examined the role of protease activity in T cell responses to papain. In order to monitor Th2 responses in vivo, we used 4get mice, where cells that activate the IL-4 gene can be visualized through bicistronic expression of eGFP21. Papain immunization of 4get mice resulted in the induction of a Th2 response, evidenced by accumulation of CD69high and CD44high IL-4-eGFP+ Th2 cells in the draining LNs (Fig. 2a). As previously described21,22, T cell IL-4-eGFP expression indicated Th2 differentiation based on production of IL-4, IL-5 and IL-13 after in vitro restimulation (Fig. S2). This response peaked on day 4 post immunization (Fig. 2b), and was dependent on the protease activity of papain (Fig. 2a-b). Th2 differentiation was not seen in response to trypsin, collagenase or clostripain, indicating that certain classes of proteases are selectively able to induce Th2 differentiation. To test if protease activity had a bona fide adjuvant effect, mice were immunized with ovalbumin (OVA) together with papain, following transfer of OVA-specific 4get X DO11.10 TCR transgenic T cells. Active papain stimulated the production of OVA-specific Th2 cells in an antigen specific manner, indicating that protease activity has a Th2 adjuvant effect that is independent of papain's antigenicity (Fig. 2c). Th2 differentiation in response to papain-OVA was twice as strong as that seen in response to OVA in alum (Fig. 2c), illustrating papain's robust adjuvant effect.
Figure 2.

The protease activity of papain has adjuvant activity and leads to Th2 cell development on day 4 post immunization. (a) 4get mice were immunized with the indicated stimuli and CD4+DX5- cells were examined for IL-4-eGFP expression. (b) 4get mice were immunized with papain or heat inactivated papain and Th2 differentiation was examined on indicated days post immunization. (c) CD4+ cells from DO11.10x4get mice were transferred into Balb/c mice, immunized as indicated and KJ1.26+CD4+ T cells were examined for IL-4-eGFP expression on day 4 post immunization, except for the OVA in Alum condition, which was examined at day 11 post immunization. Graphs and percentages are representative of multiple experiments (n>3) with 2-3 mice per group in each experiment. Error bars represent s.e.m.; p values are calculated via the Student's t test, and represent comparisons to inactive papain immunization (b) at the indicated time-point or OVA immunization (c); ***, p≤0.0001; **, p≤0.001, *, p≤0.01. If not indicated p>0.05.
Papain induced dendritic cell migration
Activation of naïve T cells requires DC maturation and migration into draining LNs23. Although DCs have not been shown to be sources of Th2 inducing cytokines, OX-40L as well as the Notch ligands Jagged1 and Jagged2 on DCs have been shown to induce Th2 differentiation24,25. Subcutaneous immunization with papain induced accumulation of mature, CD86high DCs in the draining LNs (Fig. 3a, Fig. S3a), apparent 18-24 hours after immunization (Fig. S3b). DC migration and maturation was dependent on papain protease activity (Fig. 3a) and was not due to LPS contamination, as these experiments were performed in TLR4 deficient mice. Although implicated in Th2 differentiation24, OX-40L was not detectable on the resident or migrated DCs (data not shown). The DC subset that selectively accumulated in the draining LN was of dermal origin, characterized by a CD11c+CD8α-DEC205intCD11bhigh phenotype26 (Fig. 3b and Fig. S3a). Despite the in vivo effects of papain on DC migration and maturation, in vitro treatment of TLR4 deficient bone marrow derived, splenic, or LN resident DCs with papain did not induce DC maturation as measured by CD86, CD40, MHC Class II or OX-40L upregulation, nor did it induce transcription of Jagged1 or Jagged2 (Fig. S3c and data not shown). This disparity between in vivo and in vitro effects of papain as well as the lack of expression of known Th2 inducing molecules suggested that an additional cell type was involved in the in vivo Th2 response to papain.
Figure 3.

Proteolytically active papain induces migration of total CD86highCD11c+ cells as well as dermal dendritic cells into the draining LN. (a) Total live CD11c+ cells and total CD86highCD11c+ cells in the popliteal LN 22 hours post subcutaneous footpad immunization with HSA, CpG, heat inactivated papain (H.I.P.) or papain. Cell numbers were calculated by multiplying percentage of indicated cell type by total live cells. Error bars indicate s.e.m., n=2-6 per group, p>0.05. (b) Dermal DCs migrate preferentially in response to CpG and papain. Panels are gated on live, CD11c+, CD8α- cells, percentages indicate gated percentage of total live CD11c+ cells. Graphs and percentages are representative of multiple experiments (n>3).
Basophil recruitment to LNs
Since IL-4 can contribute to Th2 differentiation and is likely to be co-expressed with other Th2-inducing signals, we examined the draining LNs of 4get mice for the appearance of IL-4-eGFP+ cells in response to papain immunization. We identified a subset of DX5+CD3-CD4-CD19-CD11clow IL-4-eGFP+ cells in the draining LNs 3 days post papain immunization (Fig. 4a). These cells appeared in response to papain and further examination revealed these cells to be FcεRI+IgE+ckit-CCR3-, identifying them as basophils (Fig. 4a & Fig. S4a). This was consistent with their morphology determined by cytospin analysis as well as by electron microscopy (Fig. 4a-b). Basophil recruitment was transient and peaked at day 3, one day prior to the peak of Th2 differentiation observed under the same immunization conditions (Fig. 2b, Fig. 4c). Importantly, recruitment of basophils was induced only in response to proteolytically active papain, but not in response to HSA, LPS or inactive papain (Fig. 4c-d). Immunization with bromelain also induced basophil recruitment 3 days post immunization (Fig. 4e). LN basophils had elevated surface levels of CD62L (Fig. 4a) and they entered the LN via a CD62L dependent mechanism that was inhibited by MEL-14 blockade (Fig. 4f). Additionally, basophils could be detected in the popliteal LN by immunofluorescence only in response to proteolytically active papain (Fig. S5a). Basophils were localized in the T cell zone of the LN, amongst T cells, but not B cells (Fig. S5b). Thus we identified a well-known IL-4 producing cell type, the basophil, specifically recruited to the draining LNs during the initiation of Th2 response.
Figure 4.

Basophils enter the draining LN after papain immunization and are necessary for Th2 development. (a) Cytospin morphology, cell size and cell surface staining (IgE, ckit, CCR3, CD62L) of LN DX5+IL-4-eGFP+ cells after papain immunization. Black histogram: DX5+IL-4-eGFP+ cells; Gray histogram: total LN cells (scatter and IgE), isotype control (CD62L), peritoneal mast cells (ckit), peripheral blood eosinophils (CCR3); Dotted line: live LN cells. (b) Electron microscopy of sorted LN DX5+IL-4- eGFP+ cells 3 days post papain immunization, bar is 2μm. (c) Percentage of DX5+IL-4-eGFP+ or DX5+IgE+ cells in the LN at the indicated days post immunization. (d) Percentage and absolute numbers of LN basophils 3 days after immunization. (e) LN basophils after bromelain immunization. (f) Papain induced basophil entry after in vivo Rat Ig or MEL-14 treatment. (g) Basophil depletion from peripheral blood leukocytes 3 days after hamster IgG or MAR-1 antibody treatment of 4get mice. (h) Th2 differentiation in basophil depleted 4get mice. Data and percentages are representative of multiple experiments (n>3). Percentages indicate gated cells as percentage of total live cells, except in h where percentages indicate gated cells as percentage of total live CD4+DX5- cells. Error bars represent s.e.m.; p values are calculated via the Student's t test, and represent comparisons to inactive enzyme immunizations at the indicated time-points (c, e), HSA immunization (d) or isotype control treatment (f); ***, p≤0.001; **, p≤0.01; *, p≤0.05. If not indicated p>0.05.
Role of basophils in Th2 differentiation
Basophils comprise 1-2% of the peripheral blood leukocytes and can enter peripheral tissues in response to nematode infection12,27, but their role in innate activation of the adaptive immune response is unknown. Basophils are not normal constituents of the LN; because of their unexpected recruitment there, we hypothesized that basophils may be involved in papain induced Th2 response in vivo. Genetic models of basophil deficiency do not currently exist and so to test the role of basophils we developed a protocol for basophil depletion using the MAR-1 antibody specific for FcεRIα. During steady state conditions in mice, FcεRI is present exclusively on basophils and mast cells. Because MAR-1 specifically targets the non-signaling α chain of the FcεRI complex, MAR-1 did not activate IL-4 production from basophils in vitro, but did block subsequent staining with MAR-1 (Fig. S4a-b). Injection of MAR-1 antibody led to >90% depletion of peripheral blood basophils by day 3 post final MAR-1 treatment, and the depletion lasted for at least 8 days (Fig. 4g). Basophils, but not skin or intraperitoneal mast cells, were depleted by MAR-1 treatment in all organs examined (Fig. S4c-e). Basophil-depleted animals were subcutaneously immunized with papain and Th2 differentiation in vivo was analyzed 4 and 8 days later. The induction of a Th2 response was abolished in mice depleted of basophils, but not in mice treated with control antibody (Fig. 4h & Fig. S4f). This is in contrast to mast cell deficient mice, which exhibited no defect in IgE production or basophil migration in response to papain immunization (Fig. 1f and Fig. S4g). Therefore, based on these results, we conclude that basophils play an essential and non-redundant role in the development of the Th2 response to a protease allergen in vivo.
Papain directly induces basophil activation
In order to determine whether papain was acting directly or indirectly on basophils, we analyzed basophil responses to papain in vitro. Available methods to produce basophils from bone marrow progenitors give rise to both basophils and mast cells28. To specifically examine the effects of papain stimulation on basophils, we FACS purified basophils from the bone marrow cultures to >99% purity. As seen with human basophils29, purified basophils produced IL-4 in response to IgE crosslinking or papain stimulation, but not in response to the inactive protease (Fig. 5a). Basophil activation is associated with both cytokine production as well as secretion of inflammatory mediators such as histamine and leukotriene C412. As expected, ionomycin and cell surface IgE cross-linking induced histamine secretion (Fig. 5b). However, while papain induced IL-4, IL-6 and TNF production from basophils (Fig. 5c), papain did not induce histamine release, indicating that papain activated basophils via a pathway distinct from ionomycin and FcεRI (Fig. 5b). Furthermore, basophil activation by papain was not affected by the absence of serum, indicating that serum derived factor(s) were not involved (Fig. 5d). Therefore, papain can directly activate basophils via a distinct pathway, yet the identity of the papain activated sensor expressed by basophils remains to be identified.
Figure 5.
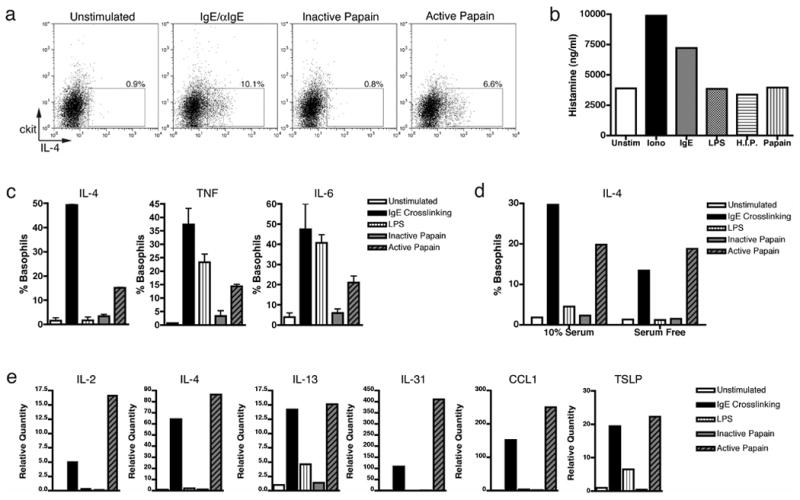
The protease activity of papain induces IL-4, TNF, and IL-6 production in bone marrow derived basophils. (a) IL-4 production from ≥99% purified basophils from bone marrow derived co-cultures were stimulated as indicated and intracellular cytokine staining was performed 6 hours post stimulation in vitro. (b) Histamine release by co-cultures in response to indicated stimuli 24 hours post stimulation. (c) Basophil and mast cell co-cultures were enriched for basophils by depletion of ckit+ cells and stimulated as indicated. Intracellular cytokine staining was performed as indicated 6 hours post stimulation in vitro. (d) IL-4 production by intracellular cytokine staining in basophil enriched co-cultures with or without serum. (e) Gene expression by Q-PCR analysis 4 hours following various stimuli. Percentages indicate that of gated population out of total basophil population. Data in (c) from triplicate samples in the experiment; error bars represent s.e.m. Data are representative of multiple (n>3) experiments.
Papain activated basophils secrete cytokines involved in Th2 initiation
Papain stimulation of purified basophils strongly induced expression of IL-2, IL-4, IL-13, IL-31, TSLP and CCL1 transcripts, which have all been associated with Th2 differentiation and recruitment (Fig. 5e)11,15,24,25,30,31. Papain did not induce transcription of IL-25, OX-40L or the Notch ligands Jagged1 or Jagged2 (data not shown), but it did induce robust levels of IL-4 transcript and protein (Fig. 5e). IL-4 is well-known to play an important role in Th2 differentiation in vivo and in vitro, but the cellular source of IL-4 remains unclear11. Blockade of IL-4Rα in vivo led to a significant defect in Th2 differentiation in response to papain immunization (Fig. S6a). Consistent with other reports 32, T cell derived IL-4 was sufficient for Th2 differentiation in an experimental system that relies on transfer of TCR transgenic T cells (Fig. S6b). However this could be explained by the abnormally high frequency of antigen-specific T cells that could compensate for the lack of exogenous IL-433. Thus, these results do not exclude a possibility that basophil derived IL-4 contributes to Th2 differentiation in vivo in wild type mice. Given the potent effect of IL-4 on Th2 differentiation, this is a likely possibility.
TSLP is an IL-7-like cytokine with reported roles in lymphocyte development, T cell homeostasis and allergic responses15. Since papain treatment induced TSLP transcription in basophils in vitro (Fig. 5e), we first asked whether TSLP was indeed produced in vivo by LN basophils after papain immunization. In accordance with Q-PCR data, we found that 31.6% + 8.5% of LN basophils expresses TSLP protein (Fig. 6a). This is likely to be an underestimate since basophils were examined directly ex vivo and without stimulation in the presence of brefeldin A. As expected, TSLP expression was only detected in the allergen-activated basophils within the LN, and not in unactivated peripheral blood basophils of papain injected mice or unimmunized mice (Fig. 6a). Importantly, LN TSLP production was specific to basophils, as TSLP could not be significantly detected in other cell types in the LN, including DCs (Fig. 6a). In the setting of chronic allergic inflammation, TSLP production in peripheral tissues has been associated with the effector Th2 response15,16,34. However, our identification of basophil-derived TSLP in the draining LN led us to hypothesize that TSLP may play an important role in Th2 initiation in vivo.
Figure 6.
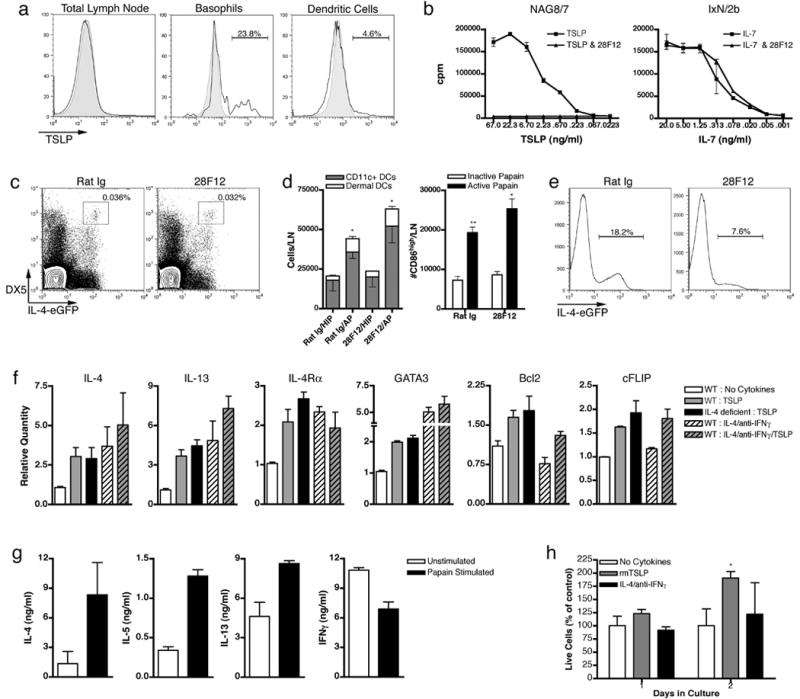
TSLP is required for basophil-mediated Th2 differentiation. Intracellular TSLP staining 3 days post papain immunization (a). Black histogram represents total LN cells, basophils, or DCs. Shaded histograms represent TSLP staining in peripheral blood basophils (basophil) or isotype control (total LN and DC). Gates indicate staining over isotype control. (b) 28F12 neutralizes TSLP dependent growth of NAG8/7 cells, but not IL-7 dependent growth of IxN/2b cells. TSLP neutralization in vivo does not effect basophil migration (c) or DC migration and maturation (d) after papain immunization. (e) Neutralization of TSLP in vivo inhibits Th2 differentiation. Percentages indicate gated population out of CD4+DX5- cells. (f) Q-PCR analysis and cell survival (g) of CD4+ T cells stimulated in vitro with the indicated cytokines/neutralizing antibodies. WT indicates Balb/c and IL-4 deficient indicates IL-4Rα -/- mice in all plots except IL-4Rα in which IL-4 -/- mice were used. Survival percentages indicate live cells from indicated cultures versus live cells from cultures without added cytokines. Data are representative of multiple (n>3) experiments, error bars illustrate s.e.m. p values are calculated via the Student's t test, and represent comparisons to inactive papain immunizations for corresponding antibody treatments; **, p≤0.01; *, p≤0.05.
Because TSLP may have effects on T cell development35, we developed a TSLP neutralizing antibody (28F12) to evaluate the role of TSLP specifically during Th2 initiation in vivo. The 28F12 monoclonal antibody effectively neutralized TSLP dependent proliferation of the NAG8/7 cell line, but not IL-7 dependent proliferation in IxN/2b cells (Fig. 6b)36,37. To address the role of TSLP in Th2 differentiation in vivo, mice were injected i.v. with 28F12 and their response to papain immunization was analyzed. Despite reported roles in DC maturation, TSLP neutralization did not inhibit papain induced maturation or migration of basophils or DCs (Fig. 6c-d). Interestingly, in vivo neutralization of TSLP led to a strong inhibition of Th2 differentiation (Fig. 6e). Since basophils are the only cell type that produced TSLP in the draining LN and since TSLP neutralization inhibited Th2 differentiation without affecting DC maturation or migration (Fig. 6a,c-d), these data indicate that basophil derived TSLP is necessary for Th2 differentiation.
CD4+ T cells activated in vitro by T cell receptor (TCR) stimulation in the presence of exogenous IL-2, TSLP and neutralizing IFN-g antibodies have been shown to produce IL-417. However, since IL-2 has been shown to promote Th2 differentiation38, we sought to determine TSLP's direct role in Th2 differentiation. Using a similar activation protocol, but without addition of exogenous IL-2 or neutralizing IFN-γ antibodies, we found that TSLP directly induces multiple genes associated with Th2 differentiation. Activation of T cells in the presence of TSLP leads to induction of Th2 specific cytokines and IL-4Rα in an IL-4 independent manner (Fig. 6f). Independent of IL-4, we found that TSLP directly upregulates transcription of a master regulator of Th2 development, GATA339 (Fig. 6f). All of the above genes can also be activated via IL-4 signaling, but we found TSLP to have non-redundant effects. TSLP, but not IL-4, led to transcription of the pro-survival factors Bcl2 and cFLIP40 (Fig. 6f). This correlated with an increased number of live cells at early time points in TSLP supplemented cultures (Fig. 6g). This increase in live cells appeared to be due to an increase in cell survival, as TSLP did not induce a significant increase in proliferation over that seen under Th2 differentiation conditions or with T cell receptor stimulation alone (Fig. S6c). Thus TSLP promotes naïve T cell competence for Th2 differentiation (by upregulating expression of IL-4Rα and GATA3) and increases their survival (by upregulating Bcl2 and cFLIP).
Discussion
Activation of the adaptive immune response is generally dependent on innate immune recognition of the infectious agents. How the innate immune system recognizes both complex metazoan parasites and simple protein allergens to induce Th2-mediated immunity has been a long-standing question. One possible solution to this conundrum is that the protease activity of allergens or proteases secreted by parasites may be sensed by the host innate immune system6 (Fig. S7). Indeed, protease activity of several allergens has been previously demonstrated to be essential for the induction of Th2 and IgE responses4,18,41. However the mechanism and the physiologically relevant cellular target linking this protease activity with the induction of Th2 differentiation has been unknown. The protease allergen Der p 1, has been shown to have in vitro effects on DCs, T cells and B cells41,42. We did not observe such effects in vivo and as none have been described to induce Th2 differentiation or IgE production their in vivo relevance remains unclear41. In our study we have identified basophils as a cell type that can be directly targeted by protease allergens with immunologically relevant consequences, such as production of Th2 promoting cytokines and induction of Th2 differentiation in vivo.
Basophils have been reported to play roles in the effector response to helminths43, memory responses44, chronic allergic inflammation13, and IL-4 production in an IgE dependent manner28,45. Due to their ability to produce IL-4, it has been hypothesized that basophils may play a role in initiating Th2-mediated immunity by providing an early source of IL-4 for Th2 differentiation12. However, this role has only been implied by observations based on basophil activation via FcεRI bound antigen specific IgE in the setting of chronic allergic inflammation, or helminth infection in mice with IL-3 mediated basophilia13,14. Here we provide evidence that basophils are activated and recruited to the draining LNs in response to protease allergens and are required for the induction of Th2 responses to these allergens. Importantly, basophil activation and recruitment occurs prior to the induction of the adaptive immune response, underscoring the basophil's role as an innate initiator of Th2 responses to protease allergens.
In addition to their essential role in Th2 initiation, we demonstrate that these allergen-activated basophils produce IL-4 and TSLP. Although the critical role of IL-4 in Th2 differentiation is well established, the cellular source of IL-4 during Th2 differentiation in vivo remains unclear. This is in part because the accessory cell type capable of supplying IL-4 for naïve T cells in the LNs has not been identified prior to this study. T cell derived IL-4 signaling has been shown to be sufficient for Th2 differentiation in vivo upon transfer of TCR transgenic T cells32. Whether IL-4 produced by T cells is sufficient under conditions of normal precursor frequency is not yet clear. By analogy with Th1 and Th17 responses, it seems likely that a critical lineage specifying cytokine would be produced by cells of the innate immune system in response to the appropriate innate immune sensing pathway. Given the potent effect of IL-4 on Th2 differentiation, it is reasonable to suggest that basophil derived IL-4 plays an important role in Th2 induction.
TSLP is another Th2-associated cytokine that we show to be produced by basophils and to be involved in allergen induced Th2 differentiation in vivo. TSLP has previously been shown to be produced in peripheral tissues, where it plays an important role in the effector stages of Th2 immunity and in allergic inflammation15,16,34. In addition, TSLP has been shown to act directly on naïve T cells, suggesting a role for this cytokine in the induction of Th2 responses17. However, it is unclear how TSLP produced in peripheral tissues may affect activation of naïve T cells. One possibility is that epithelial derived TSLP acts on DCs to induce their migration to the LNs and perhaps their “programming” for Th2 induction, as previously demonstrated for human DCs15. However, we were unable to detect TSLP protein production in keratinocytes after papain immunization (data not shown) and DC migration and maturation was unaffected by TSLP neutralization. Therefore, at least in the context of the response to protease allergens, TSLP production by basophils in the LNs is an important source of this cytokine for the initiation of Th2 responses. The peripheral sources of TSLP may be more relevant for control of effector stages of Th2-mediated immunity in both physiological46 and pathological15 conditions.
We find that TSLP can promote naïve T cell differentiation into Th2 cells in vitro via pleiotropic effects. TSLP stimulation resulted in induction of IL-4, IL-13, IL-4Ra and GATA3 transcripts, and this induction was not dependent on IL-4 production or signaling by T cells. These results suggest that TSLP may promote naïve T cell competence for Th2 differentiation. Thus the role of TSLP in Th2 induction may be analogous to the role of NK-derived IFN-γ in Th1 differentiation7. Both cytokines are derived from accessory cell types and act on naïve T cells to promote their competence for a particular differentiation fate through the induction of the appropriate cytokine receptor subunit. Finally, TSLP also upregulates expression of antiapoptotic genes in T cells and promotes survival of Th2 cells. These and additional effects of TSLP on Th2 responses in vivo will need to be investigated further in future studies.
Collectively, our findings support a model for the innate initiation of the Th2 response based on sensing the protease activity of allergens and helminths (Fig. S7)6. In this model, a host-derived sensor of proteolytic activity is cleaved by parasite or allergen proteases. This sensor, once cleaved, activates cells of the innate immune system to induce a Th2 response. This includes, but presumably is not limited to, basophil activation and recruitment to the draining LN where they provide multiple signals, including IL-4 and TSLP, that lead to Th2 differentiation. Our findings indicate that in addition to their effector functions, basophils also function as the sensors of protease allergens and possibly helminths, suggesting novel therapeutic pathways for treatment of atopic disease.
Importantly, this strategy of innate immune recognition is distinct from pattern recognition. Rather, it is based on detection of enzymatic activity associated with the presence of parasites. A similar principle is involved in innate immune recognition in plants and in fungal recognition in Drosophila47,48. It would be interesting to determine whether this strategy is also utilized in innate sensing of fungal and protozoan pathogens in mammals.
Methods
Mice
Animals were bred and maintained at the Yale Animal Resources Center at Yale University and all animal experiments were performed with approval by and in accordance with regulatory guidelines and standards set by the Institutional Animal Care and Use Committee of Yale University. Balb/c, C57Bl/6, TLR4d Balb/c (C.C3-Tlr4Lps-d), IL-4 -/- (BALB/c-Il4tm2Nnt/J), mast cell deficient (WBB6F1/J-KitW/KitW-v) and 4get mice were purchased from Jackson laboratories. TLR2X4 double knock-out mice were backcrossed 2-3 times and MyD88-/- mice were backcrossed >6 times onto the C57B/6 background. DO11.10 X 4get mice were provided by K. Bottomly and IL-4 -/- mice were provided by M. Mohrs.
Reagents and Antibodies
Papain and E64 were purchased from Calbiochem. Purified 2.4G2, RA3-6B2, MAR-1, and CD11c-allophycocyanin (APC), CD86-phycoerythrin (PE), DX5-APC, DX5-biotin, ckit-APC, I-A/I-E-fluorescein isothiocyanate (FITC) and IL-4-FITC were purchased from E bioscience. CD4-PE, CD8α-PE, CD11b-biotin, IL-4-PE, TNF-PE, and IL-6-PE were purchased from BD Pharmingen. DEC205-FITC was purchased from Serotec. Rat anti-mouse TSLP was purchased from R&D systems. KJ1.26-biotin was provided by K. Bottomly. SEA was provided by E. Pearce. Histamine ELISA was performed using Histamine ELISA kit from IBL. E64 inactivated papain was reduced with 5mM cysteine-HCl (Sigma), followed by addition of E64 in 100M excess to papain. After incubation, inactivated papain was dialyzed to remove excess E64, protein concentration was determined using the Micro BCA Protein Assay Kit (Pierce) and then activity was tested by Ig cleavage. Alternatively, papain was heat inactivated by heating to 100°C for 10 minutes.
Generation of 28F12 Antibody
Sprague-Dawley rats were repeatedly immunized with purified murine TSLP and alum and their spleens were used to generate hybridomas by standard methodologies. Hybridomas were screened for the production of anti-TSLP antibodies by ELISA assay. NAG8/7 cells were stimulated with indicated amounts of TSLP and 1μg/ml 28F12 as indicated. IxN/2b cells were stimulated with indicated amounts of murine IL-7 and 1μg/ml 28F12 as indicated.
Immunizations, Depletions and Cell Transfer
Mice were immunized subcutaneously in rear footpads with 50μl of PBS into which 50μg HSA (Sigma), Papain (active, heat inactivated or E64 inactivated), Bromelain (Calbiochem), OVA, SEA (Fig. 4) or 20μg SEA (Fig. S4), was dissolved, with or without 10μg LPS (Sigma) or CpG. For OVA in Alum immunizations, OVA dissolved in PBS was mixed 1:1 with Imject Alum (Pierce) and mice were immunized with 50μg OVA in 20μg alum. Immediate hypersensitivity was assessed by ear swelling 1 hour after injection of the pinna with 10μl of PBS or 1mg/ml papain or HSA in PBS. For basophil depletions, mice were intravenously immunized with 100μg MAR-1 antibody 90 and 72 hours before subcutaneous immunization. CD4+ DO11.10 T cells were positively selected using CD4 microbeads followed by MACS purification (Miltenyi). 5E5-1E6 purified CD4+ cells were transferred into recipient mice. For TSLP neutralization, mice were injected i.v. with 250μg of 28F12, concurrent with papain s.c. immunization.
Serum Antibodies
Serum samples were taken immediately before antigen immunization at days 0 and 14. Final serum samples were taken at 21 days. Total IgE and IgG1 were detected using anti-mouse IgE and IgG1 antibodies (BD Pharmingen) and detected using biotinylated anti-mouse IgE and IgG1 antibodies (BD Pharmingen). Anti-DNP-IgE and purified IgG1-κ were used as controls (BD Pharmingen). Papain (50μg/ml) and E64 (100x molar excess) were co-plated to coat plates overnight. After 1%BSA blocking, serum was plated at multiple dilutions, and papain-specific IgE was detected with a biotinylated anti-mouse IgE. Absorbance values at 1:25 serum dilution were used to indicate papain-specific IgE. Bone Marrow DC Cultures. Bone marrow was cultured for 5 days in GM-CSF supplemented media. Cultures were then stimulated with heat inactivated or active protease (100μg/ml) or CpG (5μM) for 18 hours.
Bone Marrow Mast Cell and Basophil Cultures
Erythrocyte depleted mouse bone marrow was initially seeded at 5E6 cells/ml and then replated every 3–4 days at 1E6 cells/ml for a total of 12 days culture in IL-3 (Peptrotech) supplemented (30ng/ml) RPMI/10%FCS. Basophils were enriched by MACS (Miltenyi) positive selection of DX5+ cells, or mast cells were depleted by negative selection of ckit+ cells. Cultures were stimulated by ionomycin (500ng/ml, Calbiochem), LPS (100ng/ml, Sigma), heat inactivated or active protease (100μg/ml). Activation by IgE cross-linking was performed by first incubating with mouse IgE (10μg/ml) followed by incubation with anti-mouse IgE (10μg/ml).
Flow Cytometry and Sorting
Lymphocytes and erythrocyte depleted hepatic leukocytes, splenocytes and peripheral blood leukocytes were first blocked with 2.4G2 to eliminate Fc mediated antibody binding. Cells were then incubated with indicated antibodies on ice for 20 min. Cells were analyzed on a FACSCalibur Flow Cytometer (BD Biosciences) and data was analyzed using FloJo software (Tree Star). For sorting, samples were run on a MoFlo cell sorter (BD Biosciences) at 30psi and basophils were selected by being ckit-Gr1-DX5+. Intracellular cytokine staining was performed using the BD Cytofix/Cytoperm Plus (with GolgiPlug) kit (BD Pharmingen) according to manufacturer's instructions. Sorted basophils were adhered to slides using Cytospin and stained with Diff-Quick (VWR). Transmission electron microscopy of sorted basophils was performed by the Yale Center for Cellular and Molecular Imaging.
Immunohistochemistry
LNs were fixed in Periodate-Lysine-Paraformaldehayde fixative overnight at 4°C. Organs were then incubated in increasing percentages of Sucrose solutions for cryoprotection. Organs were then frozen in O.C.T. (Tissue-Tek), 6-8μm sections were cut on a Kryostat (Leica), and kept at -20°C. Sections were rehydrated in PBS, blocked in PBS/0.1%Tween-20/5%BSA then stained with anti-GFP-FITC (Rockland), MAR-1, and anti-CD4 (GK1.5) or anti-B220 (RA3-6B2) (eBioscience). Streptavidin-AlexaFluor594 and anti-rat-AlexaFluor647 (Molecular Probes) were used as secondary antibodies. Vectashield (Vector) mounting medium was used to prevent fading while examining sections.
Supplementary Material
Figure S1 Protease allergens induce IgE and IgG1 production. Serum IgG1 levels in response to papain immediately before primary immunization (day 0), before secondary immunization (day 14) and at day 21 in Balb/c (a) mice are shown. Serum IgM (b) and IgG2 (c) levels in Balb/c mice. Activity of E64-inactivated papain was measured by IgG cleavage assay (d), and total IgE response in Balb/c mice to E64 inactivated papain was assayed (e). (f) Antigen specific IgE to E64 inactivated papain immunization. (g) Total IgE response to Bromelain in Balb/c mice. For each timepoint, n=5-10. Data are representative of at least three separate experiments. Error bars represent s.e.m.; p values are calculated via the Student's t test, and represent comparisons to HSA immunizations at the indicated time-points; ***, p≤0.0001; **, p≤0.001, *, p≤0.01. If not indicated p>0.05.
Figure S2 Cytokine production from sorted T cell populations after 3 days of in vitro anti-CD3/anti-CD28 restimulation. The indicated cell populations were FACS sorted from unimmunized (none) or papain immunized (papain) 4get mice and cytokine concentrations were determined via ELISA. ND: not detected; error bars represent s.e.m. Data are representative of three separate experiments.
Figure S3 Dermal DCs migrate to the popliteal LN 22 hours post subcutaneous footpad immunization with papain. (a) Gating strategy for determination of dermal DCs. The CD8α- cell population is next gated as seen in figure 3b. (b) Dermal dendritic cells as a portion of total dendritic cells in the popliteal LN after papain immunization (dermal DCs – white bar, remaining CD11c+ cells – gray bar). (c) Bone marrow derived DCs from Balb/c T4d mice do not upregulate CD86 in response to papain in vitro. Filled histograms represent unstimulated DCs, black histograms from indicated stimulus. p values determined using Student's T test; *, p≤0.05; **, p≤0.01. Unless indicated, p>0.05. Plots and percentages are representative of multiple experiments (n>3).
Figure S4 The role of mast cells and basophils in papain induced Th2 initiation. (a)in vitro incubation of bone marrow derived basophils with MAR-1 blocks subsequent staining with MAR-1. (b) Bone marrow derived basophils are not activated by the MAR-1 antibody. (c) MAR-1 treatment leads to basophil depletion from spleen and liver, even after papain immunization. (d) i.v. treatment with MAR-1 leads to basophil depletion in peripheral blood, spleen, bone marrow and liver. (e) i.v immunization with MAR-1 depletes peripheral blood basophils and basophils do not migrate into the draining LN 3 days after subcutaneous papain immunization in depleted mice. (e) Mast cells in the peritoneal lavage and skin are not depleted by MAR-1 treatment. (f) Basophil depletion with MAR-1 leads to lasting inhibition of Th2 development 8 days after papain immunization; plots are gated on CD4+DX5- cells. (g) Basophils migrate to draining LNs in response to immunization with papain in mast cell deficient mice (ckit=KitW/W-v, WT= littermate control). Plots and percentages are representative of multiple experiments (n>3). p values determined using Student's T test; *, p≤0.05.
Figure S5 Immunofluorescence of basophils in the popliteal LN following subcutaneous footpad immunization. (a) Basophils can only be localized in the draining LN after papain immunization (40×, insets 63×). B220 (purple), FcεRI (red), IL-4-eGFP (green); basophils can be identified based on FcεRI+IL-4-eGFP+. (b) Basophils can be found in direct contact with T cells, but not B cells (63×); B220 (purple, left panel), CD4 (purple, right panel). Data is representative of multiple experiments (n>3).
Figure S6 IL-4 dependence of papain-induced Th2 differentiation. (a) Th2 differentiation in response to papain in the presence or absence of IL-4Rα blockade. (b) Th2 development of OVA specific DO11 x IL-4-eGFP cells transferred into Wild type (Balb/c) or IL-4 deficient recipients and immunized as indicated. (c) CFSE dilution in CD4+ cells stimulated for 2 days in vitro with plate-bound anti-CD3/anti-CD28 and in the presence of the indicated cytokines/neutralizing antibodies. Filled histogram represents the no cytokine control, black histogram corresponds to indicated stimulation. Percentages given are of total CD4+DX5- cells (a) or of OVA specific CD4+ cells (b), percentages and graphs are representative of two experiments, with n=2-4 animals per immunization, per experiment. p values determined using Student's T test with comparison to Isotype control; *, p≤0.05; **, p≤0.01.
Supplementary Figure 1 Protease allergens induce IgE and IgG1 production. (a-c) Serum IgG1 (a), IgM (b) and IgG2 (c) concentrations in response to papain immediately before primary immunization (day 0), before secondary immunization (day 14) and at day 21 in Balb/c mice. (d) Activity of E64-inactivated papain as measured by IgG cleavage assay. (e,f) Total (e) and antigen-specific (f) IgE responses in Balb/c mice immunized with E64-inactivated papain. (g) Total IgE response in Balb/c mice immunized with bromelain. For each timepoint, n=5-10. Data are representative of at least three separate experiments. Error bars represent s.e.m.; P-values are calculated via the Student's t-test, and represent comparisons to HSA immunizations at the indicated timepoints; ***, P≤0.0001; **, P≤0.001, *, P≤0.01. If not indicated P>0.05.
Supplementary Figure 2 IL-4-eGFP expression marks TH2 cells. T cell populations were sorted from 4get mice immunized as indicated, and were restimulated with anti-CD3 and anti-CD28 for 3 days in vitro. Cytokine concentrations were determined via ELISA. ND: not detected; error bars represent s.e.m. Data are representative of three separate experiments.
Supplementary Figure 3 Dermal DCs migrate to the popliteal LN 22 hours post-subcutaneous footpad immunization with papain. (a) Gating strategy for identification of dermal DCs. The CD8α– cell population is gated as indicated. (b) Dermal DCs as a portion of total DCs in the popliteal LN after papain immunization. White bar, dermal DCs; gray bar, remaining CD11c+ cells. (c) Upregulation of CD86 on bone marrow-derived DCs from Balb/c Tlr4–/– mice stimulated with papain in vitro. Filled histograms represent unstimulated DCs, black histograms represent indicated stimulus. P-values determined using Student's t-test; *, P≤0.05; **, P≤0.01. Unless indicated, P>0.05. Plots and percentages are representative of at least three experiments.
Supplementary Figure 4 The role of mast cells and basophils in papain-induced TH2 response initiation. (a) Incubation of bone marrow-derived basophils with FcεR1α-specific MAR-1 in vitro blocked subsequent staining with MAR-1. (b) Bone marrow-derived basophils were treated with the indicated stimuli and IL-4 production was measured by intracellular cytokine staining. (c) MAR-1–mediated basophil depletion from spleen and liver, even after papain immunization. (d) Basophil depletion from peripheral blood, spleen, bone marrow and liver after intravenous treatment with MAR– 1. (e) Left, reduced basophil migration into the draining LN 3 days after subcutaneous papain immunization in MAR-1-treated mice. Right, preservation of mast cell populations in the peritoneal lavage and skin after MAR-1 treatment. (f) Impaired TH2 differentiation in MAR-1-treated mice 8 days after papain immunization; plots are gated on CD4+DX5– cells. (g) Basophil migration to draining LNs in response to immunization with papain in mast cell (MC) deficient mice (KitW/W-v). Plots and percentages are representative of at least three experiments. P-values determined using Student's t-test; *, P≤0.05.
Supplementary Figure 5 Immunofluorescence of basophils in the popliteal LN following subcutaneous footpad immunization with indicated stimuli. (a) B220 (purple), FcεRI (red), IL-4-eGFP (green); basophils can be identified as FcεRI+IL-4-eGFP+. Original magnification ×40; insets original magnification ×63. (b) Left panel, B220 (purple), right panel, CD4 (purple). FcεRI (red), IL-4-eGFP (green). Original magnification ×63. Data represent at least three experiments.
Supplementary Figure 6 IL-4 and TSLP dependence of papain-induced TH2 differentiation. (a) TH2 differentiation in vivo in response to papain immunization in the presence or absence of antibodies blocking IL-4Rα or neutralizing TSLP. (b) TH2 development of OVA-specific DO11 × 4get T cells transferred into Balb/c or IL-4-deficient recipients, which were immunized as indicated. (c) Naïve T cells were stimulated for 48 hours in vitro in the presence of the indicated cytokines and/or neutralizing antibodies, and indicated cytokines were measured by ELISA. ND, none detected; N/A, not tested. (d) CFSE dilution in CD4+ cells stimulated for 2 days in vitro with plate-bound anti-CD3 and anti-CD28 and in the presence of the indicated cytokines and/or neutralizing antibodies. Filled histogram represents the no cytokine control, open histogram corresponds to indicated stimulation. Percentages are of total CD4+DX5– cells (a) or of OVA-specific CD4+ cells (b); percentages and graphs are representative of two experiments, with 2-4 animals per immunization, per experiment. P-values determined using Student's t-test with comparison to isotype control; *, P≤0.05; **, P≤0.01.
Supplementary Figure 7 Model for protease activation of TH2 responses. Proteases derived from allergens or parasites cleave a proteolytically sensitive sensor expressed by basophils. Cleavage activates basophils to produce IL-4 and TSLP, which leads to TH2 differentiation.
Acknowledgments
We thank R. Locksley for sharing 4get mice, E. Pearce for providing SEA, K. Bottomly for DO11.10 X 4get transgenic mice and M. Mohrs for providing IL4-/- mice. We would also like to thank S. Holley and C. Annicelli for technical assistance.
Footnotes
Competing Interests Statement. The authors declare they have no competing financial interests.
References and Notes
- 1.Else KJ, Finkelman FD. Intestinal Nematode Parasites, Cytokines and Effector Mechanisms. Int J Parasitol. 1998;28:1145–1158. doi: 10.1016/s0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 2.Chua KY, et al. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J Exp Med. 1988;167:175–82. doi: 10.1084/jem.167.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grobe K, Becker WM, Schlaak M, Petersen A. Grass group I allergens (beta-expansins) are novel, papain-related proteinases. Eur J Biochem. 1999;263:33–40. doi: 10.1046/j.1432-1327.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- 4.Kheradmand F, et al. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–11. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 5.McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. Proteases in Parasitic Diseases. The Annual Review of Pathology: Mechanisms of Disease. 2006;1:497–536. doi: 10.1146/annurev.pathol.1.110304.100151. [DOI] [PubMed] [Google Scholar]
- 6.Finkelman FD, Urban JF., Jr Cytokines: making the right choice. Parasitol Today. 1992;8:311–4. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 9.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 10.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 11.Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunol Rev. 2004;202:203–22. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 12.Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624–31. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 13.Mukai K, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109:2921–7. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- 15.Liu YJ, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–39. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 18.Gough L, Schulz O, Sewell HF, Shakib F. The cysteine protease activity of the major dust mite allergen Der p 1 selectively enhances the immunoglobulin E antibody response. J Exp Med. 1999;190:1897–902. doi: 10.1084/jem.190.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers L, et al. Enzymatically active papain preferentially induces an allergic response in mice. Biochem Biophys Res Commun. 1998;253:837–40. doi: 10.1006/bbrc.1998.9862. [DOI] [PubMed] [Google Scholar]
- 20.Novey HS, Marchioli LE, Sokol WN, Wells ID. Papain-induced asthma--physiological and immunological features. J Allergy Clin Immunol. 1979;63:98–103. doi: 10.1016/0091-6749(79)90198-2. [DOI] [PubMed] [Google Scholar]
- 21.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 22.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–29. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 24.Ohshima Y, et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 25.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 26.Henri S, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–8. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 27.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–17. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–72. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 29.Phillips C, Coward WR, Pritchard DI, Hewitt CR. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol. 2003;73:165–71. doi: 10.1189/jlb.0702356. [DOI] [PubMed] [Google Scholar]
- 30.Dillon SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 31.Zingoni A, et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–51. [PubMed] [Google Scholar]
- 32.Liu Z, et al. IL-2 and autocrine IL-4 drive the in vivo development of antigen-specific Th2 T cells elicited by nematode parasites. J Immunol. 2005;174:2242–9. doi: 10.4049/jimmunol.174.4.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noben-Trauth N, Hu-Li J, Paul WE. Conventional, naive CD4+ T cells provide an initial source of IL-4 during Th2 differentiation. J Immunol. 2000;165:3620–5. doi: 10.4049/jimmunol.165.7.3620. [DOI] [PubMed] [Google Scholar]
- 34.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 35.Al-Shami A, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–68. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friend SL, et al. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–8. [PubMed] [Google Scholar]
- 37.Park LS, Friend DJ, Schmierer AE, Dower SK, Namen AE. Murine interleukin 7 (IL-7) receptor. Characterization on an IL-7-dependent cell line. J Exp Med. 1990;171:1073–89. doi: 10.1084/jem.171.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cote-Sierra J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–5. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 40.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–42. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 41.Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19:313–6. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 42.Ghaemmaghami AM, Gough L, Sewell HF, Shakib F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin Exp Allergy. 2002;32:1468–75. doi: 10.1046/j.1365-2745.2002.01504.x. [DOI] [PubMed] [Google Scholar]
- 43.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–77. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 44.Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med. 2004;200:857–70. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luccioli S, et al. IgE(+), Kit(-), I-A/I-E(-) myeloid cells are the initial source of Il-4 after antigen challenge in a mouse model of allergic pulmonary inflammation. J Allergy Clin Immunol. 2002;110:117–24. doi: 10.1067/mai.2002.125828. [DOI] [PubMed] [Google Scholar]
- 46.Zaph C, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–6. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 47.Gottar M, et al. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–37. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–9. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Protease allergens induce IgE and IgG1 production. Serum IgG1 levels in response to papain immediately before primary immunization (day 0), before secondary immunization (day 14) and at day 21 in Balb/c (a) mice are shown. Serum IgM (b) and IgG2 (c) levels in Balb/c mice. Activity of E64-inactivated papain was measured by IgG cleavage assay (d), and total IgE response in Balb/c mice to E64 inactivated papain was assayed (e). (f) Antigen specific IgE to E64 inactivated papain immunization. (g) Total IgE response to Bromelain in Balb/c mice. For each timepoint, n=5-10. Data are representative of at least three separate experiments. Error bars represent s.e.m.; p values are calculated via the Student's t test, and represent comparisons to HSA immunizations at the indicated time-points; ***, p≤0.0001; **, p≤0.001, *, p≤0.01. If not indicated p>0.05.
Figure S2 Cytokine production from sorted T cell populations after 3 days of in vitro anti-CD3/anti-CD28 restimulation. The indicated cell populations were FACS sorted from unimmunized (none) or papain immunized (papain) 4get mice and cytokine concentrations were determined via ELISA. ND: not detected; error bars represent s.e.m. Data are representative of three separate experiments.
Figure S3 Dermal DCs migrate to the popliteal LN 22 hours post subcutaneous footpad immunization with papain. (a) Gating strategy for determination of dermal DCs. The CD8α- cell population is next gated as seen in figure 3b. (b) Dermal dendritic cells as a portion of total dendritic cells in the popliteal LN after papain immunization (dermal DCs – white bar, remaining CD11c+ cells – gray bar). (c) Bone marrow derived DCs from Balb/c T4d mice do not upregulate CD86 in response to papain in vitro. Filled histograms represent unstimulated DCs, black histograms from indicated stimulus. p values determined using Student's T test; *, p≤0.05; **, p≤0.01. Unless indicated, p>0.05. Plots and percentages are representative of multiple experiments (n>3).
Figure S4 The role of mast cells and basophils in papain induced Th2 initiation. (a)in vitro incubation of bone marrow derived basophils with MAR-1 blocks subsequent staining with MAR-1. (b) Bone marrow derived basophils are not activated by the MAR-1 antibody. (c) MAR-1 treatment leads to basophil depletion from spleen and liver, even after papain immunization. (d) i.v. treatment with MAR-1 leads to basophil depletion in peripheral blood, spleen, bone marrow and liver. (e) i.v immunization with MAR-1 depletes peripheral blood basophils and basophils do not migrate into the draining LN 3 days after subcutaneous papain immunization in depleted mice. (e) Mast cells in the peritoneal lavage and skin are not depleted by MAR-1 treatment. (f) Basophil depletion with MAR-1 leads to lasting inhibition of Th2 development 8 days after papain immunization; plots are gated on CD4+DX5- cells. (g) Basophils migrate to draining LNs in response to immunization with papain in mast cell deficient mice (ckit=KitW/W-v, WT= littermate control). Plots and percentages are representative of multiple experiments (n>3). p values determined using Student's T test; *, p≤0.05.
Figure S5 Immunofluorescence of basophils in the popliteal LN following subcutaneous footpad immunization. (a) Basophils can only be localized in the draining LN after papain immunization (40×, insets 63×). B220 (purple), FcεRI (red), IL-4-eGFP (green); basophils can be identified based on FcεRI+IL-4-eGFP+. (b) Basophils can be found in direct contact with T cells, but not B cells (63×); B220 (purple, left panel), CD4 (purple, right panel). Data is representative of multiple experiments (n>3).
Figure S6 IL-4 dependence of papain-induced Th2 differentiation. (a) Th2 differentiation in response to papain in the presence or absence of IL-4Rα blockade. (b) Th2 development of OVA specific DO11 x IL-4-eGFP cells transferred into Wild type (Balb/c) or IL-4 deficient recipients and immunized as indicated. (c) CFSE dilution in CD4+ cells stimulated for 2 days in vitro with plate-bound anti-CD3/anti-CD28 and in the presence of the indicated cytokines/neutralizing antibodies. Filled histogram represents the no cytokine control, black histogram corresponds to indicated stimulation. Percentages given are of total CD4+DX5- cells (a) or of OVA specific CD4+ cells (b), percentages and graphs are representative of two experiments, with n=2-4 animals per immunization, per experiment. p values determined using Student's T test with comparison to Isotype control; *, p≤0.05; **, p≤0.01.
Supplementary Figure 1 Protease allergens induce IgE and IgG1 production. (a-c) Serum IgG1 (a), IgM (b) and IgG2 (c) concentrations in response to papain immediately before primary immunization (day 0), before secondary immunization (day 14) and at day 21 in Balb/c mice. (d) Activity of E64-inactivated papain as measured by IgG cleavage assay. (e,f) Total (e) and antigen-specific (f) IgE responses in Balb/c mice immunized with E64-inactivated papain. (g) Total IgE response in Balb/c mice immunized with bromelain. For each timepoint, n=5-10. Data are representative of at least three separate experiments. Error bars represent s.e.m.; P-values are calculated via the Student's t-test, and represent comparisons to HSA immunizations at the indicated timepoints; ***, P≤0.0001; **, P≤0.001, *, P≤0.01. If not indicated P>0.05.
Supplementary Figure 2 IL-4-eGFP expression marks TH2 cells. T cell populations were sorted from 4get mice immunized as indicated, and were restimulated with anti-CD3 and anti-CD28 for 3 days in vitro. Cytokine concentrations were determined via ELISA. ND: not detected; error bars represent s.e.m. Data are representative of three separate experiments.
Supplementary Figure 3 Dermal DCs migrate to the popliteal LN 22 hours post-subcutaneous footpad immunization with papain. (a) Gating strategy for identification of dermal DCs. The CD8α– cell population is gated as indicated. (b) Dermal DCs as a portion of total DCs in the popliteal LN after papain immunization. White bar, dermal DCs; gray bar, remaining CD11c+ cells. (c) Upregulation of CD86 on bone marrow-derived DCs from Balb/c Tlr4–/– mice stimulated with papain in vitro. Filled histograms represent unstimulated DCs, black histograms represent indicated stimulus. P-values determined using Student's t-test; *, P≤0.05; **, P≤0.01. Unless indicated, P>0.05. Plots and percentages are representative of at least three experiments.
Supplementary Figure 4 The role of mast cells and basophils in papain-induced TH2 response initiation. (a) Incubation of bone marrow-derived basophils with FcεR1α-specific MAR-1 in vitro blocked subsequent staining with MAR-1. (b) Bone marrow-derived basophils were treated with the indicated stimuli and IL-4 production was measured by intracellular cytokine staining. (c) MAR-1–mediated basophil depletion from spleen and liver, even after papain immunization. (d) Basophil depletion from peripheral blood, spleen, bone marrow and liver after intravenous treatment with MAR– 1. (e) Left, reduced basophil migration into the draining LN 3 days after subcutaneous papain immunization in MAR-1-treated mice. Right, preservation of mast cell populations in the peritoneal lavage and skin after MAR-1 treatment. (f) Impaired TH2 differentiation in MAR-1-treated mice 8 days after papain immunization; plots are gated on CD4+DX5– cells. (g) Basophil migration to draining LNs in response to immunization with papain in mast cell (MC) deficient mice (KitW/W-v). Plots and percentages are representative of at least three experiments. P-values determined using Student's t-test; *, P≤0.05.
Supplementary Figure 5 Immunofluorescence of basophils in the popliteal LN following subcutaneous footpad immunization with indicated stimuli. (a) B220 (purple), FcεRI (red), IL-4-eGFP (green); basophils can be identified as FcεRI+IL-4-eGFP+. Original magnification ×40; insets original magnification ×63. (b) Left panel, B220 (purple), right panel, CD4 (purple). FcεRI (red), IL-4-eGFP (green). Original magnification ×63. Data represent at least three experiments.
Supplementary Figure 6 IL-4 and TSLP dependence of papain-induced TH2 differentiation. (a) TH2 differentiation in vivo in response to papain immunization in the presence or absence of antibodies blocking IL-4Rα or neutralizing TSLP. (b) TH2 development of OVA-specific DO11 × 4get T cells transferred into Balb/c or IL-4-deficient recipients, which were immunized as indicated. (c) Naïve T cells were stimulated for 48 hours in vitro in the presence of the indicated cytokines and/or neutralizing antibodies, and indicated cytokines were measured by ELISA. ND, none detected; N/A, not tested. (d) CFSE dilution in CD4+ cells stimulated for 2 days in vitro with plate-bound anti-CD3 and anti-CD28 and in the presence of the indicated cytokines and/or neutralizing antibodies. Filled histogram represents the no cytokine control, open histogram corresponds to indicated stimulation. Percentages are of total CD4+DX5– cells (a) or of OVA-specific CD4+ cells (b); percentages and graphs are representative of two experiments, with 2-4 animals per immunization, per experiment. P-values determined using Student's t-test with comparison to isotype control; *, P≤0.05; **, P≤0.01.
Supplementary Figure 7 Model for protease activation of TH2 responses. Proteases derived from allergens or parasites cleave a proteolytically sensitive sensor expressed by basophils. Cleavage activates basophils to produce IL-4 and TSLP, which leads to TH2 differentiation.


