Abstract
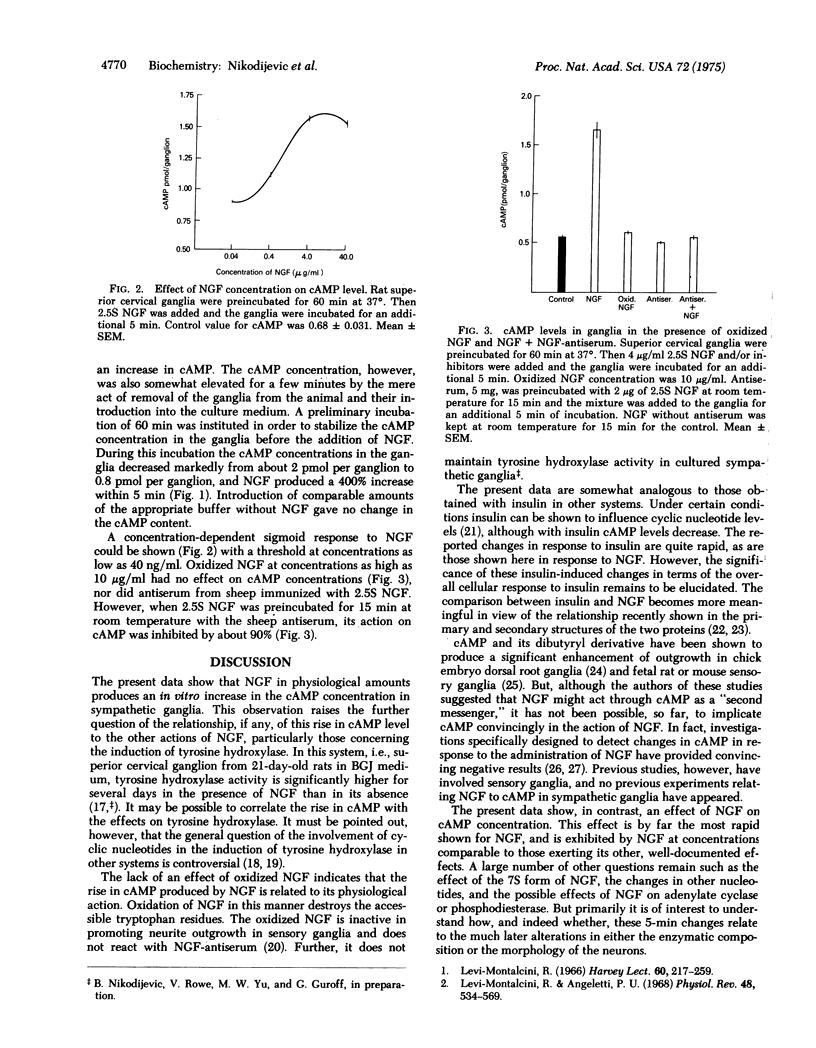
Nerve growth factor (NGF) produces a several-fold increase in the cyclic AMP concentration in rat superior cervical ganglia in organ culture within 5 min. An increase can be seen with as little as 40 ng/ml of NGF. Oxidized NGF is without effect. The increase in the cAMP concentration produced by NGF is prevented by the addition of antiserum to NGF.
Full text
PDF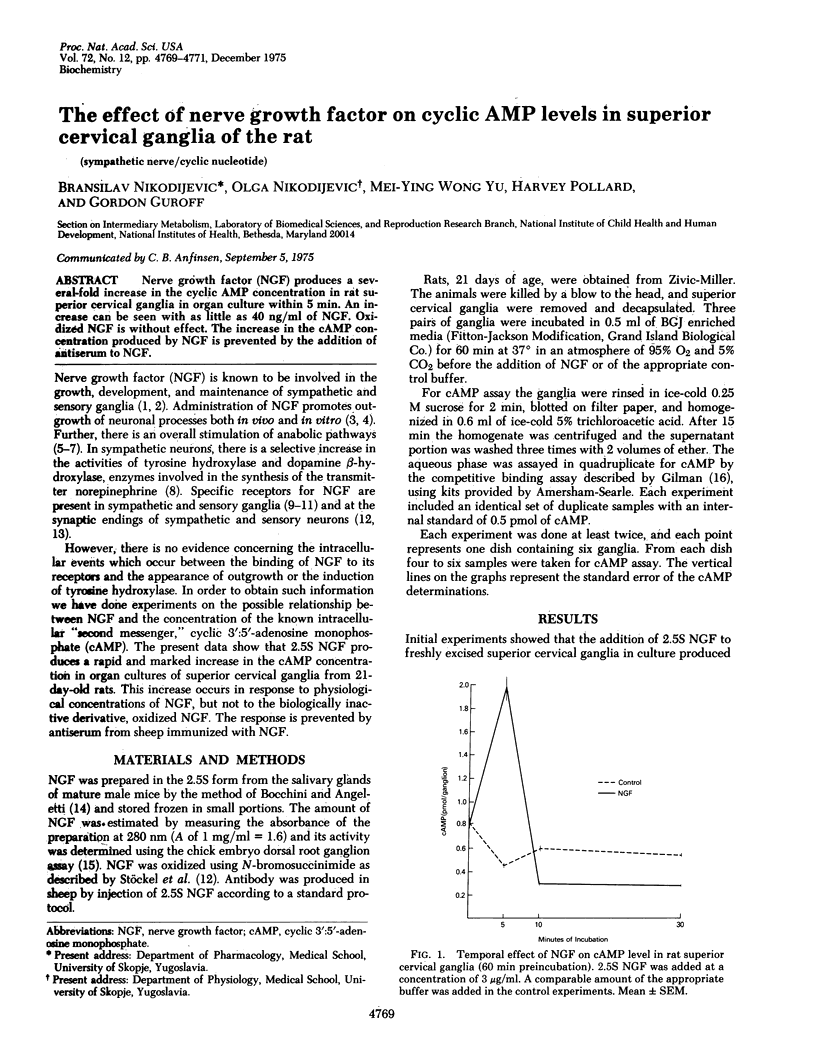

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGELETTI P. U., GANDINI-ATTARDI D., TOSCHI G., SALVI M. L., LEVI-MONTALCINI R. METABOLIC ASPECTS OF THE EFFECT OF NERVE GROWTH FACTOR ON SYMPATHETIC AND SENSORY GANGLIA: PROTEIN AND RIBONUCLEIC ACID SYNTHESIS. Biochim Biophys Acta. 1965 Jan 11;95:111–120. doi: 10.1016/0005-2787(65)90216-9. [DOI] [PubMed] [Google Scholar]
- Angeletti R. H. The role of the tryptophan residues in the activity of the nerve growth factor. Biochim Biophys Acta. 1970 Sep 29;214(3):478–482. doi: 10.1016/0005-2795(70)90307-7. [DOI] [PubMed] [Google Scholar]
- Banerjee S. P., Snyder S. H., Cuatrecasas P., Greene L. A. Binding of nerve growth factor receptor in sympathetic ganglia. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2519–2523. doi: 10.1073/pnas.70.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton E. L. Tissue culture assay of nerve growth factor and of the specific antiserum. Exp Cell Res. 1970 Mar;59(3):383–392. doi: 10.1016/0014-4827(70)90645-2. [DOI] [PubMed] [Google Scholar]
- Frazier W. A., Angeletti R. H., Bradshaw R. A. Nerve growth factor and insulin. Science. 1972 May 5;176(4034):482–488. doi: 10.1126/science.176.4034.482. [DOI] [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Bradshaw R. A. Properties of the specific binding of 125I-nerve growth factor to responsive peripheral neurons. J Biol Chem. 1974 Sep 10;249(17):5513–5519. [PubMed] [Google Scholar]
- Frazier W. A., Hogue-Angeletti R. A., Sherman R., Bradshaw R. A. Topography of mouse 2.5S nerve growth factor. Reactivity of tyrosine and tryptophan. Biochemistry. 1973 Aug 14;12(17):3281–3293. doi: 10.1021/bi00741a021. [DOI] [PubMed] [Google Scholar]
- Frazier W. A., Ohlendorf C. E., Boyd L. F., Aloe L., Johnson E. M., Ferrendelli J. A., Bradshaw R. A. Mechanism of action of nerve growth factor and cyclic AMP on neurite outgrowth in embryonic chick sensory ganglia: demonstration of independent pathways of stimulation. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2448–2452. doi: 10.1073/pnas.70.8.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Kurosawa A., Chuang D. M., Costa E. Protein kinase activation as an early event in the trans-synaptic induction of tyrosine 3-monooxygenase in adrenal medulla. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1152–1156. doi: 10.1073/pnas.72.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D. C., Hier D. B., Arnason G. W., Young M. On a possible relationship of cyclic AMP to the mechanism of action of nerve growth factor. Proc Soc Exp Biol Med. 1972 May;140(1):45–47. doi: 10.3181/00379727-140-36392. [DOI] [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta nerve growth factor receptor of avian dorsal root ganglia. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3884–3888. doi: 10.1073/pnas.70.12.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hier D. B., Arnason B. G., Young M. Nerve growth factor: relationship to the cyclic AMP system of sensory ganglia. Science. 1973 Oct 5;182(4107):79–81. doi: 10.1126/science.182.4107.79. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin: interaction with membrane receprots and relationship to cyclic purine nucleotides and cell growth. Fed Proc. 1975 Jun;34(7):1556–1563. [PubMed] [Google Scholar]
- Johnson D. G., Silberstein S. D., Hanbauer I., Kopin I. J. The role of nerve growth factor in the ramification of sympathetic nerve fibres into the rat iris in organ culture. J Neurochem. 1972 Sep;19(9):2025–2029. doi: 10.1111/j.1471-4159.1972.tb05112.x. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., ANGELETTI P. U. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol. 1963 Mar;6:653–659. doi: 10.1016/0012-1606(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: its mode of action on sensory and sympathetic nerve cells. Harvey Lect. 1966;60:217–259. [PubMed] [Google Scholar]
- Liuzzi A., Angeletti P. U., Levi-Montalcini R. Metabolic effects of a specific nerve growth factor (NGF) on sensory and sympathetic ganglia: enhancement of lipid biosynthesis. J Neurochem. 1965 Aug;12(8):705–708. doi: 10.1111/j.1471-4159.1965.tb06784.x. [DOI] [PubMed] [Google Scholar]
- Liuzzi A., Pocchiari F., Angeletti P. U. Glucose metabolism in embryonic ganglia: effect of nerve growth factor [NGF] and insulin. Brain Res. 1968 Mar;7(3):452–454. doi: 10.1016/0006-8993(68)90011-5. [DOI] [PubMed] [Google Scholar]
- Mackay A. V. The long-term regulation of tyrosine hydroxylase activity in cultured sympathetic ganglia: role of ganglionic noradrenaline content. Br J Pharmacol. 1974 Aug;51(4):509–520. doi: 10.1111/j.1476-5381.1974.tb09669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten U., Mueller R. A., Oesch F., Thoenen H. Location of an isoproterenol-responsive cyclic AMP pool in adrenergic nerve cell bodies and its relationship to tyrosine 3-monooxygenase induction. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2217–2221. doi: 10.1073/pnas.71.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisen F. J., Murphy R. A., Pichichero M. E., Braden W. G. Cyclic adenosine monophosphate stimulation of axonal elongation. Science. 1972 Jan 7;175(4017):73–74. doi: 10.1126/science.175.4017.73. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Schwab M., Thoenen H. Specificity of retrograde transport of nerve growth factor (NGF) in sensory neurons: a biochemical and morphological study. Brain Res. 1975 May 16;89(1):1–14. doi: 10.1016/0006-8993(75)90129-8. [DOI] [PubMed] [Google Scholar]
- Stöckel K., Paravicini U., Thoenen H. Specificity of the retrograde axonal transport of nerve growth factor. Brain Res. 1974 Aug 23;76(3):413–421. doi: 10.1016/0006-8993(74)90818-x. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Angeletti P. U., Levi-Montalcini R., Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1598–1602. doi: 10.1073/pnas.68.7.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]


