Abstract
Over the past decades, some scientific progress has been made in understanding and treating cancer-related fatigue (CRF). However, three major problems have limited further progress: lack of agreement about measurement, inadequate understanding of the underlying biology, and problems in the conduct of clinical trials for CRF. This commentary reports the recommendations of a National Cancer Institute Clinical Trials Planning Meeting and an ongoing National Cancer Institute working group to address these problems so that high-priority research and clinical trials can be conducted to advance the science of CRF and its treatment. Recommendations to address measurement issues included revising the current case definition to reflect more rigorous criteria, adopting the Patient Reported Outcomes Measurement Information System fatigue scales as standard measures of CRF, and linking legacy measures to the scales. With regard to the biology of CRF, the group identified the need for longitudinal research to examine biobehavioral mechanisms underlying CRF and testing mechanistic hypotheses within the context of intervention research. To address clinical trial issues, recommendations included using only placebo-controlled trial designs. setting eligibility to minimize sample heterogeneity or enable subgroup analysis, establishing a CRF severity threshold for participation in clinical trials, conducting dissemination trials of efficacious interventions (such as exercise), and combining nonpharmacologic and pharmacologic interventions to exploit the potential synergy between these approaches. Accomplishing these goals has the potential to advance the science of CRF and improve the clinical management of this troubling symptom.
Introduction
Over the past decades, we have made progress in our understanding of cancer-related fatigue (CRF) including its definition, measurement in adults and children (1,2), and identification of a few effective therapies (3–11). Recent research has also explored biomarkers and causal mechanisms of CRF. Despite this progress, there has been considerable diversity in the conceptual and operational definition of CRF limiting the generalizability of research findings; incomplete understanding of its biologic basis resulting in few pharmacologic targets for treatment; and limited clinical dissemination of efficacious behavioral interventions such as exercise.
The Clinical Trials Planning Meeting
The National Cancer Institute convened a Clinical Trials Planning Meeting (CTPM) on CRF in 2010 to examine the issues and initiate a process for developing a focused agenda to advance the science of symptom management in the community setting. Participants included representatives of academia, community oncology, government, the pharmaceutical industry, and the patient community. The overall goal of the CTPM was to set priorities for clinical trial investigations of CRF and make recommendations for high-priority research over the next several years. Objectives of the meeting were to 1) examine what is known about the biologic, psychological, and social factors related to fatigue and propose new studies to uncover the biopsychosocial mechanisms of CRF; 2) synthesize “lessons learned” from randomized clinical trials of CRF as a basis for setting new directions for clinical intervention research; 3) review the definition and measurement of CRF (patient-reported, case definition, and biomarker measures); and 4) explore clinical trial design and analysis issues in CRF research. These activities were directed to the development of a research agenda that builds on current knowledge and provides directions for future research. The CTPM spawned a working group that has continued the discussion. This commentary incorporates both the issues and recommendations of the CTPM and the updated consensus of the ongoing working group.
Background
Fatigue is the most common symptom experienced by adults and children with cancer (12–18). It can be associated with the cancer itself, cancer treatment, and/or other symptoms such as depression or poor sleep. CRF is a complex multidimensional problem characterized by reduced energy and increased need for rest unrelated to recent sleep or activity that is known to affect quality of life adversely by reducing mental and physical functioning, disturbing mood, and interfering with usual activities (16,19). CRF is also emerging as a dose-limiting toxicity associated with established and newer therapies including targeted agents (such as tyrosine kinase inhibitors) that can ultimately limit the effectiveness of treatment (20). CRF is not always easily differentiated from everyday fatigue without careful diagnostic evaluation.
The true prevalence of CRF is unknown because differences in measurement yield a wide range of prevalence estimates depending on the domains of fatigue considered (21). For example, studies that simply ask about self-reported fatigue presence or severity have yielded prevalence estimates in the range of 70–99% (22). Studies requiring fatigue to exceed a threshold of severity, duration, or functional impairment have produced estimates in the 30–70% range (23). When CRF has been defined as a syndrome with specific diagnostic criteria inclusive of severity, functional impairment, and duration, prevalence estimates have been considerably lower, in the range of 15–30% (24,25).
CRF has been documented before the initiation of treatment (26), during cancer therapy (27–29), in disease-free survivors (24,30), and at the end of life (13,31,32). Patterns of fatigue during treatment have varied with context. A cyclic pattern of CRF has been documented with each cycle of chemotherapy followed by a gradual decline after completion of treatment (33,34). During radiotherapy, CRF follows a gradually increasing pattern until the end of treatment with a gradual decline after completion (27,35). Chronic fatigue has been documented in some disease-free survivors and in most individuals at the end of life (13,24,36). CRF has been associated with a variety of comorbid problems including sleep disturbance, psychiatric disturbances such as depression or anxiety, unrelieved pain, medication side effects, nutritional imbalance, physical inactivity, and certain coping styles (25,27,37–40).
Causal mechanisms of CRF are not well understood. Recent evidence suggests that inflammatory processes, dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis, disruption of circadian rhythms, and disturbance of monoamine pathways that regulate serotonin, dopamine, and norepinephrine may cause or contribute to CRF (41–47). However, CRF is known to be rooted in both biology and behavior, so a simple biological or psychosocial explanation is unlikely; rather, the presence of biopsychosocial causal mechanisms is more likely. Because of the number and complexity of factors that could contribute to CRF, a model is provided as an organizational framework (Figure 1).
Figure 1.
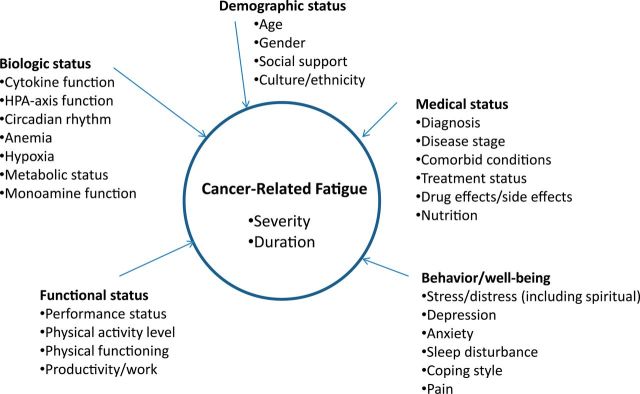
Correlates of cancer-related fatigue. HPA axis = hypothalamic-pituitary-adrenal axis.
Definition and Measurement of Cancer-Related Fatigue
Conceptual Definition
The NCCN conceptual definition of CRF is widely endorsed and cited: CRF is “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (21). Furthermore, subjective experience in these domains must interfere with functioning. The clarity of this conceptual definition and its general acceptance are congruent with the full case definition of CRF.
Case Definition
A conceptual definition alone lacks the specificity to diagnose a clinical case of CRF because threshold criteria including type, number, severity, and chronicity are not clearly articulated. To identify a case of CRF, one must determine whether a symptom of tiredness must be present across all three functional (physical, emotional, and cognitive) domains or whether one domain only (eg, physical) is sufficient (Table 1). Likewise, it is important to decide how severe the fatigue must be and for what period of time it must be present. To address these limitations, a set of diagnostic criteria to define CRF was proposed by the Fatigue Coalition (48) and recognized for inclusion in the International Classification of Diseases and Related Problems, 10th edition (49,50).
Table 1.
Proposed International Classification of Disease-10 (ICD-10) criteria for diagnosis of cancer-related fatigue*
| A | Six or more of the following symptoms have been present every day or nearly every day during the same 2-week period in the past month and at least one of the symptoms is (A1) significant fatigue. |
| A1 | Significant fatigue, diminished energy, or increased need to rest, disproportionate to any recent change in activity level |
| A2 | Complaints of generalized weakness or limb heaviness |
| A3 | Diminished concentration or attention |
| A4 | Decreased motivation or interest to engage in usual activities |
| A5 | Insomnia or hypersomnia |
| A6 | Experience of sleep as unrefreshing or nonrestorative |
| A7 | Perceived need to struggle to overcome inactivity |
| A8 | Marked emotional reactivity (e.g., sadness, frustration, or irritability) to feeling fatigued |
| A9 | Difficulty completing daily tasks attributed to feeling fatigued |
| A10 | Perceived problems with short-term memory |
| A11 | Post-exertional malaise lasting several hours |
| B | The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| C | There is evidence from the history, physical examination, or laboratory findings that the symptoms are a consequence of cancer or cancer therapy |
| D | The symptoms are not primarily a consequence of comorbid psychiatric disorders such as major depression, somatization disorder, somatoform disorder, or delirium |
This CRF case definition requires the presence of fatigue and/or related sensations and a specific number of related symptoms, evidence of impact on functioning, and inclusion/exclusion criteria (Table 1) (24,25,31,36,49,50,51–54). However, this case definition could benefit from further revision and validation including specification in the A1 criterion of “significant fatigue” in a specific time frame such as “most of the day and nearly every day.” The “B” criterion might benefit from a statement that “fatigue and associated symptoms” are causing distress, as well as specification of the duration and pervasiveness of the impairment due to fatigue. Finally, the “D” criterion needs to be clarified to indicate when a psychiatric disorder is permitted and when it is not. In the context of a depressive disorder, a concurrent diagnosis of CRF might be considered only if the fatigue is pervasive and disabling, consistent with the suggested revisions of A1 and B criteria. Although the current case definition provides a good starting point, further validation of the CRF criteria is recommended to set a rigorous standard for classifying CRF cases (24,25,36,54, 55).
Self-Report Measures of Cancer-Related Fatigue
As a subjective symptom, CRF is measured most efficiently via self-report. Numerous reviews of valid and reliable self-report measures for adults are appropriate for clinical research (1,56–58). Several reliable and valid instruments are available to evaluate fatigue in children with cancer including two validated proxy report instruments (parent and clinician) (2,59–62). Consistent with the conceptual definition, most self-report scales address both the sensation and impact domains of CRF (21), and some scales include additional domains such as reduced motivation, energy or vitality, or diurnal variation (1). Variability in the outcome domains assessed by self-report CRF measures has hindered meaningful comparison across studies and the translation of results into clinical practice.
Patient Reported Outcomes Measurement Information System as a Common Metric
Recent developments in the measurement of patient-reported outcomes have focused on the establishment of so-called item banks that consist of a large number of patient-reported outcomes questions/items that have undergone extensive qualitative and quantitative evaluation to support their validity and reliability. All items in the item bank are calibrated on a common metric using item response theory models to allow comparison of scores from different item sets within the same item bank. In other words, a fatigue item bank allows the development of multiple fatigue short forms that can be targeted to the needs of any clinical trial. Fatigue scores from the short forms can be compared or combined across multiple studies (63–66). Recognizing the importance of patient-reported outcomes item banks, the National Institutes of Health launched the Patient Reported Outcome Measurement Information System (PROMIS) initiative to develop a publicly available set of standardized self-report measures of symptoms and other health domains including both pediatric and adult measures of fatigue (64). The adult PROMIS item bank includes 95 items, 54 of which were retained in a cancer-specific application, measuring an individual’s fatigue experience and the impact of fatigue on daily living. The pediatric PROMIS item bank includes 23 items measuring key domains of energy and capacity for physical functioning, psychosocial effects, and anemia-specific concerns. The pediatric PROMIS fatigue measure also differentiates between 8- to 18-year-olds who are on treatment or in survivorship (67,68).
The adult PROMIS fatigue measure has been validated with fatigue measures commonly used in research (referred to as “legacy” scales) including the 13-item Functional Assessment of Chronic Illness Therapy-Fatigue and the 4-item SF-36 Vitality Scale (69,70). The PROMIS fatigue measure was found to be highly correlated with the legacy measures, and PROMIS scores statistically significantly differentiated cancer survivors at different points of the care continuum and different stages of disease. In addition, the PROMIS measure differentiated individuals with different levels of performance status using the rating scale developed by the Eastern Cooperative Oncology Group (71,72). Demographic correlates of PROMIS fatigue in the US general population included sex (women more than men), marital status (married more than unmarried), and age (younger more than older) (65). Race/ethnicity was also collected, and the PROMIS fatigue measure did not show differential item function by race (non-Hispanic whites, African Americans, Hispanics), but more analyses need to be conducted.
Future studies are planned to estimate minimally important differences (MIDs) for the pediatric and adult PROMIS measures. An MID is defined as the minimal change in fatigue level that is perceived by patients or clinicians as meaningful. Different from statistical significance, the MID can serve as a clinically meaningful indicator of the safety or efficacy of an intervention (73). The MID has been addressed in advanced cancer with varied results depending on the approach used to estimate the MID (73).
A potential benefit of PROMIS is that it offers an opportunity to link legacy CRF measures such as the Functional Assessment of Chronic Illness Therapy-Fatigue to the PROMIS fatigue measure and vice versa. This would enable comparisons across research results (74). Linking other legacy measures with PROMIS is encouraged. This reconciliation process could be especially beneficial in pediatrics that relies on age-specific measures. Children as young as 7 years of age can describe CRF in simple terms such as activity and lifestyle limitations; older school-age children can describe it in greater detail (75,76). Adolescents describe CRF with more abstract detail highlighting mental tiredness and physical fatigue (59,67). As a child moves from one age group to another, different forms of developmentally appropriate CRF measures that have been reconciled could be used to allow for longitudinal comparisons across different time points. Efforts are underway to validate common items between pediatric and adult CRF with a goal of developing a life span fatigue item bank.
Recommendations
Update and revise the current case definition of CRF to reflect a more rigorous and specific description of the criteria.
Adopt the PROMIS Fatigue item banks and their short forms (pediatric and adult) as standard measures of CRF severity and impact.
Identify and take opportunities to link legacy CRF measures with PROMIS fatigue measures.
Examine the validity of the PROMIS-Fatigue item bank in predicting behavioral outcomes such as work performance for adults and school performance for children.
Examine differences in CRF levels by race/ethnicity.
Evaluate minimally important differences in CRF for different clinical groups.
The Biology of CRF
To date, CRF has been understood largely as a subjective patient-reported experience, limiting our knowledge of its pathophysiology. Research has shown that biologic mechanisms are involved in a broad range of psychosocial and behavioral sequelae including CRF. However, the precise physiologic pathways involved in the development of CRF and its relationship to the cancer experience are poorly understood. Inflammatory processes, HPA-axis function, and circadian rhythms are interrelated regulatory networks that communicate with each other through multiple signaling pathways, and all have been proposed as mechanisms underlying CRF (77–79). Understanding the biologic processes underlying CRF is critical to the identification of potential targets for therapeutic intervention. There is evidence of biologic dysregulation across cancer diagnoses and treatment modalities (80,81). However, due to a lack of systematic study of these potential confounding variables, it is not known if the mechanisms of symptom development and persistence differ by tumor and/or treatment type, although recent findings do not show substantial effects of treatment type in breast cancer survivors (47).
By far the most studied mechanism is the inflammatory process. Animal studies have shown that tissue damage or infection results in the release of proinflammatory cytokines that can signal the central nervous system, leading to a constellation of behavior changes known as “sickness behavior” (82–84). In humans, cytokines are released in response to cancer and/or its treatment resulting in self-reported symptoms similar to animal sickness behaviors, of which fatigue is prominent. Findings from numerous studies over the past decade support an association between cytokine activity and fatigue, although results have been mixed (85–87). Research focused on pathways by which cytokines interact with neurocircuits in the brain have also been explored (88). Several studies have shown that inflammatory cytokines as well as inflammatory stimuli lead to changes in neural activity in the basal ganglia that in turn have been associated with symptoms of fatigue (89).
Upregulated cytokine signaling has been associated with other processes including HPA-axis dysfunction (89,90). In the normal physiologic environment, cortisol is released from the adrenal glands in a circadian pattern. One of its functions is to inhibit proinflammatory cytokine production and activity during acute stressful events (91). During chronic exposure to proinflammatory cytokines (as may occur in cancer and cancer therapies), the sensitivity of the HPA axis is thought to be blunted; this decrease in cortisol production has been associated with CRF (91,92).
Circadian rhythms are the regular daily cycles of activity and rest controlled by the suprachiasmatic nucleus in the brain. Robust synchronized circadian rhythms are important to health and well-being (78,93). HPA axis dysfunction and inflammation have been associated with disruption of circadian rhythms (94) and result in symptoms such as fatigue, sleep disturbance, and depression. Disrupted circadian rhythms have also been associated with mortality in older men and women, dementia, and cancer (95–99). Emerging evidence suggests that individuals with CRF have dysregulated circadian activity/rest and sleep/wake rhythms including less daytime activity, later sleep onset at night, and more frequent awakening (53,93,100,101). CRF that worsens during chemotherapy has also been associated with progressively worse and more enduring impairment of circadian activity rhythm (46).
Recent investigations have focused on genetic influences on symptoms including CRF. Technological advances and completion of the mapping of the human genome have enabled the development of new tools for scanning the entire genome or examining candidate genes that could control the development and persistence of CRF. Several investigations have identified gene polymorphisms or variants that characterize individual differences in the severity of CRF (44). Most of this work on the genetics of CRF has focused primarily on genes involved in inflammatory pathways (102,103). Other research has focused on gene expression as an explanation of underlying mechanisms because expression involves not only the heritable aspects (gene variants) but also alterations due to environmental changes (41,104,105). The study of gene polymorphisms and gene expression has the potential to yield important information about the mechanisms that control CRF including onset, persistence, and resolution (106). However, it would require large study samples.
Recommendations
Conduct longitudinal research to examine the interrelated biobehavioral mechanisms underlying CRF.
Test mechanistic hypotheses within the context of CRF intervention research.
Test new hypotheses about CRF mechanisms in animal models that control for the specific effects of tumor and treatment.
Interventions for Cancer-Related Fatigue
Nonpharmacologic Interventions
The research literature on nonpharmacologic intervention for CRF is substantial encompassing several broad categories of psychosocial therapies and physical activity. Three meta-analyses showed that psychosocial interventions had a small to moderate effect on CRF (5,7,8). However, psychosocial therapies comprise a diverse set of educational, supportive, and behavioral interventions, so conclusions cannot be drawn about the benefit of specific components (8,107). To date, numerous meta-analyses have shown that exercise intervention had a statistically significant effect on CRF with magnitudes in the small to moderate range (3–5,8,9,11,108–110). Exercise with aerobic and strength training components (150 minutes per week of moderate to strenuous intensity activity and two to three weekly sessions focused on major muscle groups, respectively) was found to be more effective in reducing CRF than aerobic exercise alone (4), and supervised exercise was found to be more effective than home-based exercise (4). Recent studies have also demonstrated the benefit of physical activity for individuals with advanced cancer (111,112). Most research on the mechanisms underlying the benefit of exercise for cancer patients and survivors is derived from research in healthy populations (113,114). A few studies have examined inflammatory changes or insulin resistance related to exercise in cancer survivors but not in connection with CRF (115–119).
The wealth of positive meta-analysis results for psychosocial and exercise interventions suggest the need for further research aimed to increase the effect size of beneficial interventions as well as dissemination studies to evaluate beneficial interventions such as exercise in community settings. Future studies should address motivational factors and barriers to implementation of exercise and psychosocial interventions as well as adherence (120,121). The patient advocates who participated in the CTPM recommended the conduct of exercise intervention trials for survivors that are practical and exportable, addressing safety issues, appropriate regimens for different climates, and motivation as a key factor related to uptake of the intervention. Based on the evidence, the American College of Sports Medicine has published extensive exercise guidelines for different types of cancer that have implications for CRF (122).
There are many remaining questions about nonpharmacologic interventions that would be worthy of pursuit including yoga (123–125), mindfulness-based stress reduction (126,127), cognitive behavioral therapy for insomnia (128–133), and light treatment for prevention of CRF (134). In the current climate of personalized medicine, there may be a place for trials of tailored interventions powered for subgroup analysis with regard to efficacy for different groups based on demographic, medical, nutritional, and functional status. The ability to conduct large nonpharmacologic intervention trials would require additional resources. Nonpharmacologic interventions are generally labor intensive, and the intervention often requires delivery by staff with specialized training. Investigators may also consider more efficient use of resources by conducting telephone- and/or Internet-based intervention studies.
Pharmacologic Interventions for Cancer-Related Fatigue
In contrast to the sizable amount of research on nonpharmacologic interventions for CRF, the pool of studies of pharmacologic interventions is much smaller and based largely on benefit in other diseases (such as multiple sclerosis) or conditions (such as advanced cancer). There has been only one systematic review and meta- analysis of 27 randomized controlled trials of pharmacologic treatment for CRF (135). The analysis was done by drug type, and the overall effect size for all drug classes was small. Based on two studies, methylphenidate, a sympathomimetic psychostimulant, was shown to be more effective than placebo. The antidepressant paroxetine was evaluated in two trials, but no CRF benefit was observed. Three studies examined progestational steroids, but no CRF benefit was found. Although 10 studies of erythropoietin in anemic cancer patients undergoing chemotherapy found that it was superior to placebo and four trials of darbepoetin demonstrated superiority, current concerns about cardiovascular safety and reduced disease control suggest that these drugs should be used with caution for the management of CRF associated with anemia (136,137).
Another wakefulness-promoting agent, modafinil, was evaluated in one randomized controlled trial (138). The trial was negative overall; in secondary analysis, modafinil was found to be effective for individuals presenting with severe fatigue, suggesting the need for further evaluation of this drug. Another drug with potential for CRF management is bupropion, a norepinephrine dopamine reuptake inhibitor, with favorable results in two open-label trials (139). Nonsteroidal antiinflammatory drugs and other drugs with direct and indirect cytokine antagonistic effects should also be considered for future intervention trials.
Overall, advancement of the science of CRF management requires new approaches to the study of both nonpharmacologic and pharmacologic interventions. First, most previous research has lacked a theoretical framework and hypothesis testing about the potential biopsychosocial mechanisms underlying CRF. Moving forward, it is imperative that intervention efficacy research be coupled with examination of hypotheses about the mechanisms by which interventions achieve their effects. Second, the strength of evidence in favor of physical activity intervention (including aerobic and strength- building components) suggests that dissemination research is in order. Future studies need to focus on overcoming barriers to implementation in the community setting and strategies to increase uptake and effect size of physical activity interventions. Third, given the strong likelihood of the placebo effect related to interventions for CRF, placebo-controlled studies are critical to the demonstration of efficacy and remain the gold standard (140). Fourth, there is great potential for synergy between nonpharmacologic and pharmacologic interventions that should be exploited to enhance the effect size of interventions. Fifth, CRF is a heterogeneous condition that is likely to be affected by demographic, medical, nutritional, and functional status. Careful consideration needs to be given to eligibility for participation in clinical trials of CRF management to minimize heterogeneity. Moreover, consideration needs to be given to the severity of CRF as an eligibility criterion because at least one study found that efficacy of a drug intervention was limited to individuals with severe fatigue (138), and most previous intervention trials did not have a specific inclusion criterion limiting eligibility to fatigued individuals (8).
Recommendations
Conduct research with randomized placebo-controlled trial designs, and consider the use of recently developed strategies such as the doubly randomized preference trials.
Carefully consider eligibility for CRF intervention trials to minimize sample heterogeneity or enable subgroup analysis.
Establish a threshold of symptom severity for participation in CRF clinical trials.
Conduct dissemination trials of efficacious interventions (such as exercise) focused on overcoming barriers to implementation and strategies to increase uptake and effect size of interventions.
Combine nonpharmacologic and pharmacologic interventions to exploit the potential synergy between these two approaches.
Summary
Although much has been accomplished over the past decades, there is a need to focus future research in critical directions to advance the science of CRF management. This commentary reports the recommendations of participants in a National Cancer Institute CTPM and an ongoing National Cancer Institute working group for high-priority clinical trial investigations of CRF over the next several years. Three areas of science have been addressed in this commentary: measurement, biology, and intervention for CRF. Implementation of these recommendations has the potential to 1) advance our understanding of the biobehavioral mechanisms of CRF; 2) identify new targets for intervention to prevent or treat CRF; 3) accomplish the dissemination of efficacious interventions into the community; and 4) leverage the yield beyond individual studies by pooling and comparing data across studies. Accomplishing these goals will advance the science of CRF and improve the clinical management of this troubling symptom.
Acknowledgments
Acknowledgment of Participants in Clinical Trials Planning Meeting
| Name | E-mail Address | Institution |
|---|---|---|
| Cynthia Chauhan | Canceradvocacy@aol.com | North Central Cancer Treatment Group |
| Marilyn Hockenberry, PhD, PNP, RN-CS | mjhocken@txccc.org | Baylor College of Medicine |
| Julie Bower, PhD | jbower@ucla.edu | University of California, Los Angeles (UCLA) |
| Barbara Piper, DNSc, RN, AOCN, FAAN | bpiper@shc.org | Virginia G. Piper Cancer Center |
| Charles Cleeland, PhD | ccleeland@mdanderson.org | University of Texas M.D. Anderson Cancer Center |
| Christine Miaskowski, RN, PhD, FAAN | chris.miaskowski@nursing.ucsf.edu | University of California, San Francisco |
| Debra Barton, PhD, RN, AOCN, FAAN | Barton.debra@mayo.edu | Mayo Clinic, Rochester, MN |
| Jeff A. Sloan, PhD | jsloan@mayo.edu | Mayo Clinic, Rochester, MN |
| Paddy Stone, MA, MD, MRCP | pstone@sgul.ac.uk | St. George’s University of London |
| Thomas R. Belin, PhD | tbelin@ucla.edu | University of California, Los Angeles (UCLA) |
| Deborah Bruner, PhD, RN, FAAN | Deborah.w.bruner@emory.edu | Emory University |
| Patricia Ganz, MD | Pganz@mednet.ucla.edu | UCLA Jonsson Comprehensive Cancer Center |
| Gary Morrow, PhD, MS | gary_morrow@urmc.rochester.edu | University of Rochester |
| Mary Lou Smith, JD | mlsadvocate@sbcglobal.net | Research Advocacy Network |
| Lori Minasian, MD | minasilo@mail.nih.gov | National Cancer Institute |
References
- 1. Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J. Pain Symptom Manage. 2010;39(6):1086–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hockenberry MJ, Hinds PS, Barrera P, et al. Three instruments to assess fatigue in children with cancer: the child, parent and staff perspectives. J. Pain Symptom Manage. 2003;25(4):319–328 [DOI] [PubMed] [Google Scholar]
- 3. Arnold M, Taylor NF. Does exercise reduce cancer-related fatigue in hospitalised oncology patients? A systematic review. Onkologie. 2010;33(11):625–630 [DOI] [PubMed] [Google Scholar]
- 4. Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–133 [DOI] [PubMed] [Google Scholar]
- 5. Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors—a meta-analysis. Psychooncology. 2011;20(2):115–126 [DOI] [PubMed] [Google Scholar]
- 6. Goedendorp MM, Gielissen MF, Verhagen CA, et al. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev. 2009;(1):CD006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26(6):660–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kangas M, Bovbjerg DH, Montgomery GH, et al. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134(5):700–741 [DOI] [PubMed] [Google Scholar]
- 9. McMillan EM, Newhouse IJ. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: a meta-analysis. Appl Physiol Nutr Metab. 2011;36(6):892–903 [DOI] [PubMed] [Google Scholar]
- 10. Velthuis MJ, May AM, Koppejan-Rensenbrink RA, et al. Physical Activity during Cancer Treatment (PACT) study: design of a randomised clinical trial. BMC Cancer. 2010;10:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008;(2):CD006145. [DOI] [PubMed] [Google Scholar]
- 12. Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat. 2008;112(1):5–13 [DOI] [PubMed] [Google Scholar]
- 13. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34(1):94–104 [DOI] [PubMed] [Google Scholar]
- 14. Theunissen JM, Hoogerbrugge PM, van Achterberg T, et al. Symptoms in the palliative phase of children with cancer. Pediatr Blood Cancer. 2007;49(2):160–165 [DOI] [PubMed] [Google Scholar]
- 15. Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2396–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butt Z, Rosenbloom SK, Abernethy AP, et al. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw. 2008;6(5):448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perdikaris P, Merkouris A, Patiraki E. Changes in children’s fatigue during the course of treatment for pediatric cancer. Int Nurs Rev. 2008;55:412–419 [DOI] [PubMed] [Google Scholar]
- 18. Whitsett SF, Gudmundsdottir M, Davies B, et al. Chemotherapy-related fatigue in childhood cancer: correlates, consequences, and coping strategies. J Pediatr Oncol Nurs. 2008;25(2):86–96 [DOI] [PubMed] [Google Scholar]
- 19. Scott JA, Lasch KE, Barsevick AM, Piault-Louis E. Patients’ experiences with cancer-related fatigue: a review and synthesis of qualitative research. Oncol Nurs Forum. 2011;38(3):E191–E203 [DOI] [PubMed] [Google Scholar]
- 20. Cornelison M, Jabbour EJ, Welch MA. Managing side effects of tyrosine kinase inhibitor therapy to optimize adherence in patients with chronic myeloid leukemia: the role of the midlevel practitioner. J Supportive Oncol, 2012;10(1):14–24 [DOI] [PubMed] [Google Scholar]
- 21. Berger AM, Abernethy AP, Atkinson A, et al. Cancer-related fatigue. J Natl Compr Canc Netw 2010;8(8):904–931 [DOI] [PubMed] [Google Scholar]
- 22. Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360 [DOI] [PubMed] [Google Scholar]
- 23. Servaes P, Prins J, Verhagen S, Bleijenberg G. Fatigue after breast cancer and in chronic fatigue syndrome: similarities and differences. J Psychosom Res. 2002;52(6):453–459 [DOI] [PubMed] [Google Scholar]
- 24. Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116(24):5740–5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, Jacobsen PB. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Oncol. 2005;23(27):6613–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goedendorp MM, Gielissen MF, Verhagen CA, et al. Severe fatigue and related factors in cancer patients before the initiation of treatment. Br J Cancer. 2008;99(9):1408–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Purcell A, Fleming J, Bennett S, et al. A multidimensional examination of correlates of fatigue during radiotherapy. Cancer. 2010;116(2): 529–537 [DOI] [PubMed] [Google Scholar]
- 28. Hinds PS, Hockenberry MJ, Gattuso JS, et al. Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer. 2007;110(10):2321–2330 [DOI] [PubMed] [Google Scholar]
- 29. Perdikaris P, Vasilatou-Kosmidis E, Merkouris A, et al. Evaluating cancer related fatigue during treatment according to children’s, adolescents’ and parents’ perspectives in a sample of Greek young patients. Eur J Oncol Nurs. 2009;13(5):399–408 [DOI] [PubMed] [Google Scholar]
- 30. Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–758 [DOI] [PubMed] [Google Scholar]
- 31. Murphy H, Alexander S, Stone P, et al. Investigation of diagnostic criteria for cancer-related fatigue syndrome in patients with advanced cancer: a feasibility study. Palliat Med. 2006;20(4):413–418 [DOI] [PubMed] [Google Scholar]
- 32. Ullrich CK, Dussel V, Hilden JM, et al. Fatigue in children with cancer at the end of life. J. Pain Symptom Manage. 2010;40(4):483–494 [DOI] [PubMed] [Google Scholar]
- 33. Berger AM, Grem JL, Visovsky C, Marunda HA, Yurkovich JM. Fatigue and other variables during adjuvant chemotherapy for colon and rectal cancer. Oncol Nurs Forum. 2010;37(6):E359–E369 [DOI] [PubMed] [Google Scholar]
- 34. Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: a critical appraisal. Eur J Cancer. 2006; 42(7):846–863 [DOI] [PubMed] [Google Scholar]
- 35. Dhruva A, Dodd M, Paul SM, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 2010;33(3):201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45(3):384–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barsevick AM. The elusive concept of the symptom cluster. Oncol Nurs Forum. 2007;34(5):971–980 [DOI] [PubMed] [Google Scholar]
- 38. Gerber LH, Stout N, McGarvey C, et al. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Support Care Cancer 2011;19(10):1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu L, Rissling M, Natajaran L. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep. 2012;35(2):237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oh HS, Seo WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8(4):191–201 [DOI] [PubMed] [Google Scholar]
- 41. Lyon DE, McCain NL, Pickler RH, Munro C, Elswick RK., Jr Advancing the biobehavioral research of fatigue with genetics and genomics. J Nurs Scholarsh. 2011;43(3):274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Von Ah DM, Kang DH, Carpenter JS, et al. Predictors of cancer-related fatigue in women with breast cancer before, during, and after adjuvant therapy. Cancer Nurs. 2008;31(2):134–144 [DOI] [PubMed] [Google Scholar]
- 44. Aouizerat BE, Dodd M, Lee K, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11(1):27–41 [DOI] [PubMed] [Google Scholar]
- 45. Miaskowski C, Dodd M, Lee K, et al. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. J Pain Symptom Manage. 2010;40(4):531–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Savard J, Liu L, Natarajan L, et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep. 2009;32(9):1155–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cella D, Davis K, Breitbart W, Curt G; Fatigue Coalition Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19(14):3385–3391 [DOI] [PubMed] [Google Scholar]
- 49. ICD-10-CM list of codes and descriptions R53.0, Fatigue, neoplasm-related (p. 534) International Classification of Diseases. 10th revision. Centers for Disease Control and Prevention Web site. http://cdc.gov/nchs/icd/icd10cm.htm [Google Scholar]
- 50. Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology (Williston Park). 1998;12(11A):369–377 [PubMed] [Google Scholar]
- 51. Sadler IJ, Jacobsen PB, Booth-Jones M, et al. Preliminary evaluation of a clinical syndrome approach to assessing cancer-related fatigue. J Pain Symptom Manage. 2002;23(5):406–416 [DOI] [PubMed] [Google Scholar]
- 52. Young KE, White CA, Young KE, White CA. The prevalence and moderators of fatigue in people who have been successfully treated for cancer. J Psychosom Res. 2006;60(1):29–38 [DOI] [PubMed] [Google Scholar]
- 53. Fernandes R, Stone P, Andrews P, et al. Comparison between fatigue, sleep disturbance, and circadian rhythm in cancer inpatients and healthy volunteers: evaluation of diagnostic criteria for cancer-related fatigue. J Pain Symptom Manage. 2006;32(3):245–254 [DOI] [PubMed] [Google Scholar]
- 54. Van Belle S, Paridaens R, Evers G, et al. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer 2005;13(4):246–254 [DOI] [PubMed] [Google Scholar]
- 55. Piper BF, Cella D. Cancer-related fatigue: definitions and clinical subtypes. J Natl Compr Canc Netw. 2010;8(8):958–966 [DOI] [PubMed] [Google Scholar]
- 56. Hjollund NH, Andersen JH, Bech P. Assessment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health Qual Life Outcomes. 2007;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist. 2007;12(suppl 1):11–21 [DOI] [PubMed] [Google Scholar]
- 58. Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol. 2009;20(1):17–25 [DOI] [PubMed] [Google Scholar]
- 59. Hinds PS, Hockenberry M, Tong X, et al. Validity and reliability of a new instrument to measure cancer-related fatigue in adolescents. J Pain Symptom Manage. 2007;34(6):607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hinds PS, Yang J, Gattuso JS, et al. Psychometric and clinical assessment of the 10-item reduced version of the Fatigue Scale-Child instrument. J Pain Symptom Manage. 2010;39(3):572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lai JS, Cella D, Kupst MJ, et al. Measuring fatigue for children with cancer: development and validation of the pediatric Functional Assessment of Chronic Illness Therapy-Fatigue (pedsFACIT-F). J Pediatr Hematol Oncol. 2007;29(7):471–479 [DOI] [PubMed] [Google Scholar]
- 62. Mandrell BN, Yang J, Hooke MC, et al. Psychometric and clinical assessment of the 13-item reduced version of the fatigue scale-adolescent instrument. J Pediatr Oncol Nurs. 2011;28(5):287–294 [DOI] [PubMed] [Google Scholar]
- 63. Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007;25(32):5106–5112 [DOI] [PubMed] [Google Scholar]
- 65. Junghaenel DU, Christodoulou C, Lai JS, Stone AA. Demographic correlates of fatigue in the US general population: results from the patient-reported outcomes measurement information system (PROMIS) initiative. J Psychosom Res. 2011;71(3):117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lai JS, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil. 2011;92(10 suppl):S20–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lai JS, Kupst MJ, Cella D, et al. Using Q-methodology to understand perceived fatigue reported by adolescents with cancer. Psychooncology. 2007;16(5):437–447 [DOI] [PubMed] [Google Scholar]
- 68. Hinds PS, Nuss SL, Ruccione KS, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer. 2013;60(3):402–408 [DOI] [PubMed] [Google Scholar]
- 69. Ware JJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483 [PubMed] [Google Scholar]
- 70. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;4(3 suppl 2):13–19 [PubMed] [Google Scholar]
- 71. Zubrod CG, Ipsen J, Frei E, et al. Newer techniques and some problems in cooperative group studies. Natl Cancer Inst Monogr. 1960;3:277–292 [PubMed] [Google Scholar]
- 72. Hinds PS, Nuss SL, Ruccione KS, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer. 2012;60(3):402–408 [DOI] [PubMed] [Google Scholar]
- 73. Lai JS, Stucky BD, Thissen D, et al. Development and psychometric properties of the PROMIS((R)) pediatric fatigue item banks. Qual Life Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Smith E, Lai JS, Cella D. Building a measure of fatigue: the functional assessment of Chronic Illness Therapy Fatigue Scale. PM R. 2010;2(5):359–363 [DOI] [PubMed] [Google Scholar]
- 75. Hicks J, Bartholomew J, Ward-Smith P, et al. Quality of life among childhood leukemia patients. J Pediatr Oncol Nurs. 2003;20(4):192–200 [DOI] [PubMed] [Google Scholar]
- 76. Hockenberry-Eaton M, Hinds PS, Alcoser P, et al. Fatigue in children and adolescents with cancer. J Pediatr Oncol Nurs. 1998;15(3):172–182 [DOI] [PubMed] [Google Scholar]
- 77. Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(suppl 1):22–34 [DOI] [PubMed] [Google Scholar]
- 78. Berger AM, Wielgus K, Hertzog M, Fischer P, Farr l. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18(1):105–114 [DOI] [PubMed] [Google Scholar]
- 79. Hrushesky WJ, Grutsch J, Wood P, et al. Circadian clock manipulation for cancer prevention and control and the relief of cancer symptoms. Integr Cancer Ther. 2009;8(4):387–397 [DOI] [PubMed] [Google Scholar]
- 80. Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21(4):413–427 [DOI] [PubMed] [Google Scholar]
- 81. Miaskowski C, Aouizerat BE. Biomarkers: symptoms, survivorship, and quality of life. Semin Oncol Nurs. 2012;28(2):129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dantzer R, Kelley KW, Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs. 2007;23(2):99–105 [DOI] [PubMed] [Google Scholar]
- 84. Myers JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum. 2008;35(5):802–807 [DOI] [PubMed] [Google Scholar]
- 85. Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21(4):413–427 [DOI] [PubMed] [Google Scholar]
- 86. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–899 [DOI] [PubMed] [Google Scholar]
- 87. Vallance K, Liu W, Mandrell BN, et al. Mechanisms of dexamethasone-induced disturbed sleep and fatigue in paediatric patients receiving treatment for ALL. Eur J Cancer. 2010;46(10):1848–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Miller AH, Timmie WP. Mechanisms of cytokine-induced behavioral change: psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23(3):149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jager A, Sleijfer S, van der Rijt CC. The pathogenesis of cancer related fatigue: could increased activity of pro-inflammatory cytokines be the common denominator? Eur J Cancer. 2008;44(2):175–181 [DOI] [PubMed] [Google Scholar]
- 91. Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. 2007;21(7):863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bower JE, Ganz PA, Dickerson SS, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30(1):92–100 [DOI] [PubMed] [Google Scholar]
- 93. Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14(3):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rich T, Innominato PF, Boerner J, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11(5):1757–1764 [DOI] [PubMed] [Google Scholar]
- 95. Tranah GJ, Blackwell T, Ancoli-Israel S, et al. Circadian activity rhythms and mortality: the study of osteoporotic fractures. J Am Geriatr Soc. 2010;58(2):282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Paudel ML, Taylor BC, Ancoli-Israel S, et al. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiol Int. 2010; 27(2):363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gehrman PR, Marler MR, Martin JL, et al. The timing of activity rhythms in patients with demential is related to survival. J Gerontol. 2004; 59A:1050–1055 [DOI] [PubMed] [Google Scholar]
- 98. Innominato PF, Giacchetti S, Bjarnason GA, et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer. 2012;131(11):2684–2692 [DOI] [PubMed] [Google Scholar]
- 99. Innominato PF, Focan C, Gorlia T, et al. Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res. 2009; 69(11):4700–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Berger AM, Wielgus KK, Young-McCaughan S, et al. Methodological challenges when using actigraphy in research. J Pain Symptom Manage. 2008;36(2):191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fiorentino L, Rissling M, Liu L, Ancoli-Israel S. The symptom cluster of sleep, fatigue and depressive symptoms in breast cancer patients: severity of the problem and treatment options. Drug Discov Today. 2011;8(4):167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Miaskowski C, Dodd M, Lee K, et al. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. J Pain Symptom Management. 2010;40(4):531–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Landmark-Hoyvik H, Reinertsen KV, Loge JH, et al. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J. 2009;9(5):333–340 [DOI] [PubMed] [Google Scholar]
- 105. Reinertsen KV, Grenaker Alnæs GI, Landmark-Høyvik H, et al. Fatigued breast cancer survivors and gene polymorphisms in the inflammatory pathway. Brain Behav Immun. 2011;25(7):1376–1383 [DOI] [PubMed] [Google Scholar]
- 106. Bower JE, Ganz PA, Irwin MR, et al. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol. 2013;31(13):1656–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer 2012;118(8 suppl):2261–2269 [DOI] [PubMed] [Google Scholar]
- 108. Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol. 2010;22(3):208–221 [DOI] [PubMed] [Google Scholar]
- 109. Keats MR, Culos-Reed SN. A community-based physical activity program for adolescents with cancer (Project TREK): program feasibility and preliminary findings. J Pediatr Hematol Oncol 2008;30(4):272–280 [DOI] [PubMed] [Google Scholar]
- 110. Yeh CH, Man Wai JP, Lin U-S, Chiang Y-C. A pilot study to examine the feasibility and effects of a home-based aerobic program on reducing fatigue in children with acute lymphoblastic leukemia. Cancer Nurs 2011;34(1):3–12 [DOI] [PubMed] [Google Scholar]
- 111. Yeo TP, Burrell SA, Sauter PK, et al. A progressive postresection walking program significantly improves fatigue and health-related quality of life in pancreas and periampullary cancer patients. J Am Coll Surg. 2012;214(4):463–475; discussion 475–477. [DOI] [PubMed] [Google Scholar]
- 112. Albrecht TA, Taylor AG. Physical activity in patients with advanced-stage cancer: a systematic review of the literature. Clin J Oncol Nurs. 2012;16(3):293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nieman DC. Exercise immunology: practical applications. Int J Sports Med. 1997;18 (suppl 1):S91–S100 [DOI] [PubMed] [Google Scholar]
- 114. Moldoveanu AI, Shephard RJ, Shek PN. The cytokine response to physical activity and training. Sports Med. 2001;31(2):115–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Allgayer H, Nicolaus S, Schreiber S. Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detect Prev. 2004;28:208–213 [DOI] [PubMed] [Google Scholar]
- 116. Jones SB, Thomas GA, Hesselsweet SD, et al. Effect of exercise on markers of inflammation in breast cancer survivors: the Yale exercise and survivorship study. Cancer Prev Res (Phila). 2013;6(2):109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ligibel JA, Campbell N, Partridge A, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26(6):907–912 [DOI] [PubMed] [Google Scholar]
- 118. Ligibel JA, Giobbie-Hurder A, Olenczuk D, et al. Impact of a mixed strength and endurance exercise intervention on levels of adiponectin, high molecular weight adiponectin and leptin in breast cancer survivors. Cancer Causes Control. 2009;20(8):1523–1528 [DOI] [PubMed] [Google Scholar]
- 119. Linkov F, Maxwell GL, Felix AS, et al. Longitudinal evaluation of cancer-associated biomarkers before and after weight loss in RENEW study participants: implications for cancer risk reduction. Gynecol Oncol. 2012;125(1):114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shang J, Wenzel J, Krumm S, Griffith K, Stewart K. Who will drop out and who will drop in: exercise adherence in a randomized clinical trial among patients receiving active cancer treatment. Cancer Nurs. 2012;35(4):312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Matthews EE, Schmiege SJ, Cook PF, Berger AM, Aloia MS. Adherence to cognitive behavioral therapy for insomnia (CBTI) among women following primary breast cancer treatment: a pilot study. Behav Sleep Med. 2012;10(3):217–229 [DOI] [PubMed] [Google Scholar]
- 122. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426 [DOI] [PubMed] [Google Scholar]
- 123. Bower JE, Garet D, Sternlieb B. Yoga for persistent fatigue in breast cancer survivors: results of a pilot study. Evid Based Complement Alternat Med. 2011;2011:623168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17(10):1301–1309 [DOI] [PubMed] [Google Scholar]
- 126. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261–1272 [DOI] [PubMed] [Google Scholar]
- 127. Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med. 2012;35(1):86–94 [DOI] [PubMed] [Google Scholar]
- 128. Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum Online. 2007;34(5):E51–E59 [DOI] [PubMed] [Google Scholar]
- 129. Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. J Clin Oncol. 2005; 3(25):6097–6106 [DOI] [PubMed] [Google Scholar]
- 130. Fiorentino L, McQuaid JR, Liu L, et al. Individual cognitive behavioral therapy for insomnia in breast cancer survivors: a randomized controlled crossover pilot study. Nat Sci Sleep. 2010;2:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology. 2009;18(6):634–646 [DOI] [PubMed] [Google Scholar]
- 132. Berger AM, Kuhn BR, Farr LA, et al. One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. J Clin Oncol. 2009;27(35):6033–6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658 [DOI] [PubMed] [Google Scholar]
- 134. Ancoli-Israel S, Rissling M, Neikrug A, et al. Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support Care Cancer. 2012;20(6):1211–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst. 2008;100(16):1155–1166 [DOI] [PubMed] [Google Scholar]
- 136. Hershman DL, Buono DL, Malin J, et al. Patterns of use and risks associated with erythropoiesis-stimulating agents among Medicare patients with cancer. J Natl Cancer Inst. 2009;101(23):1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. National Comprehensive Cancer Network (NCCN) The Complete Library of NCCN Clinical Practice Guidelines in Oncology. Fort Washington, PA: NCCN; 2012 [Google Scholar]
- 138. Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116(14):3513–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Breitbart W, Alici Y. Pharmacologic treatment options for cancer-related fatigue: current state of clinical research. Clin J Oncol Nurs. 2008;12(5 suppl):27–36 [DOI] [PubMed] [Google Scholar]
- 140. Bruera E, Valero V, Driver L, et al. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2006;24(13):2073–2078 [DOI] [PubMed] [Google Scholar]


