Abstract
Aims
A prolonged QT interval is associated with increased risk of Torsades de pointes (TdP) and may be fatal. We sought to investigate the extent to which clinical covariates affect the change in QT interval among ‘real-world’ patients treated with sotalol and followed in an electronic medical record (EMR) system.
Methods and results
We used clinical alerts in our EMR system to identify all patients in whom a new prescription for sotalol was written (2001–11). Rate-corrected QT (QTc) was calculated by Bazett's formula. Correlates of sotalol-induced change in the QTc interval and sotalol discontinuation were examined using linear and logistic regression, respectively. Overall, 541 sotalol-exposed patients were identified (n = 200 women, 37%). The mean first sotalol dose was 86 ± 39 mg, age 64 ± 13 years, and BMI 30 ± 7 kg/m2. Atrial fibrillation/flutter was the predominant indication (92.2%). After initial exposure, the change in the QTc interval from baseline was highly variable: ΔQTc after 2 h = 3 ± 42 ms (P = 0.17) and 11 ± 37 ms after ≥48 h (P < 0.001). Multivariable linear regression analysis identified female gender and age, reduced left ventricular ejection fraction, high sotalol dose, hypertrophic cardiomyopathy, and loop diuretic co-administration as correlates of increased ΔQTc at ≥48 h (P < 0.05 for all). Within 3 days of initiation, 12% discontinued sotalol of which 31% were because of exaggerated QTc prolongation. One percent developed TdP.
Conclusion
In this EMR-based cohort, the increase in QTc with sotalol initiation was highly variable, and multiple clinical factors contributed. These data represent an important step in ongoing work to identify real-world patients likely to tolerate long-term therapy and reinforces the utility of EMR-based cohorts as research tools.
Keywords: Arrhythmia, Long QT syndrome, Torsades de pointes, Beta-blocker, Atrial fibrillation, Electronic medical records
What's new?
Clinical alerts incorporated into an electronic medical record (EMR) are useful for identifying ‘real-world’ clinical patients who can be studied and highlights the utility of an EMR as a research tool.
Correlates of increased ΔQTc interval during sotalol administration were also associated with actual sotalol discontinuation during follow-up.
Traditional correlates of QT prolongation are applied in the specific setting of sotalol administration.
Introduction
Exaggerated prolongation of the QT interval following drug exposure has been termed ‘acquired long QT syndrome’ (aLQTS) and is associated with increased risk of developing the arrhythmia, Torsades de pointes (TdP) which can be fatal.1 The QT interval is a marker for myocardial repolarization and is currently the best available tool for risk stratification for individuals susceptible to aLQTS, although the positive predictive value of the QT interval is imperfectly associated with TdP.2,3 Antiarrhythmic drugs that prolong QT interval by blocking the repolarizing potassium current IKr are among the commonest causes of aLQTS, and among these, sotalol is a common culprit.4 As with other drugs of this type, the effects of sotalol on QT are variable and unpredictable in individual patients, leading to clinical recommendations that the QT interval be closely monitored after initiation of therapy.5
We report here a 10-year experience examining the effect of sotalol on the QT interval during initiation of therapy. The study was enabled by the implementation of alerts in an electronic medical record (EMR) environment, allowing us to capture all patients in whom the drug was started. The goal was to identify predictors of QT prolongation in a large ‘real-world’ environment and highlights the utility of an EMR as a research tool.6 These data represent an important step in ongoing work to identify patient subsets in which therapy with this class of drugs should be targeted or avoided.
Methods
We developed and implemented a clinical alerts system in the Vanderbilt University Medical Center (VUMC) EMR system to identify patients for whom a new prescription was written for the following antiarrhythmic drugs: quinidine, sotalol, dofetilide, ibutilide, disopyramide, or procainamide. Computer algorithms deployed into the VUMC EMR prompts (i.e. clinical alerts) research staff when administration of a drug of interest is scheduled. Once prompted, the clinical alert is confirmed by a medical administration database (MAR), which holds information on actual time of drug administration, the type of drug, route, and strength. Patients are approached for informed consent to allow follow-up of outcomes in the EMR when administration of the drug of interest has been confirmed in the MAR. After initial patient identification, we then performed a manual chart review to re-confirm that patients did start the drug of interest and were ≥18 years old. This cohort made up the QT-prolonging antiarrhythmics QT Star Panel Study.
No testing was ordered for the purposes of this study and all data reported here were collected as part of routine clinical care and retrieved from the VUMC EMR. All patients had baseline electrocardiograms (ECGs) performed before antiarrhythmic drug administration and periodically thereafter. For sotalol, this was typically done at baseline and at 2 h after drug administration and on Days 2 and 3 following initial drug exposure. We also assessed whether the drug was still being prescribed at Day 3 and at 3 months and recorded the reason for discontinuation if available.
Study population
Overall, we identified a total of 845 patients although alerts in the EMR system, and informed consent was obtained in ≥95%. For the present study, we excluded patients who started an antiarrhythmic drug treatment outside of VUMC, had a pacemaker or an implantable cardioverter-defibrillator (ICD) with ventricular capture, had a complete atrioventricular block, or initiated treatment with disopyramide, ibutilide, or procainamide as a limited number of patients received these drugs (n = 18, n = 21, and n = 17, respectively). We required that all included patients had an ECG performed at baseline and at least one ECG at 2 hours, Day 2, or Day 3 after sotalol exposure. The study population selection process is shown in Figure 1.
Figure 1.
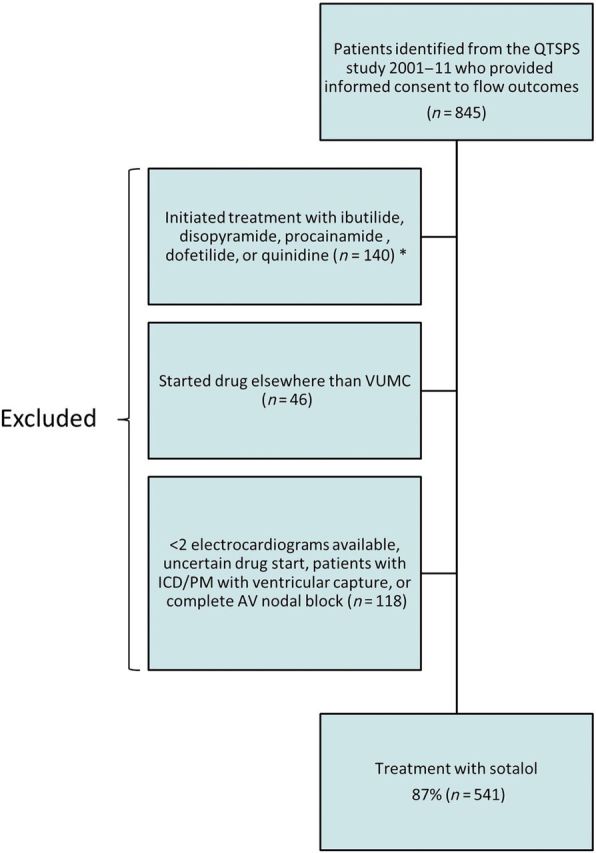
Flow chart of study population. Legend: Patients refusing consent comprise an estimated <5% of all patients approached for informed consent. QTSPS, QT Star Panel Study; VUMC, Vanderbilt University Medical Center; ICD, implantable cardioverter-defibrillator; PM, pacemaker; AV, atrioventricular.
Baseline co-variables
Serum potassium and calcium levels were obtained at baseline or up to 3 days prior to drug initiation and were determined from the EMR. Data on height and weight closest to baseline were identified and body mass index (BMI, kg/m2) for each patient was calculated. We gathered information on left ventricular ejection fraction (LVEF, %, estimated from echocardiogram or ventriculography) closest to baseline for each patient, if available. Information on concomitant pharmacotherapy was gathered from each patient at baseline.
Electrocardiographic analysis
Electrocardiograms were performed with a standard 12-lead tracing at 25 mm/s paper speed and 10 mm/mV gain. We defined the start of the QT interval as the first deflection of the QRS complex away from baseline (isoelectric line) and the end of the QT interval was defined as the point, where a component of the descending T-wave intersected or crossed baseline. Rate-corrected QT (QTc) was calculated by Bazett's (QTc = QT/RR1/2) and Fridericia's formulae (QTc = QT/RR1/3). We calculated the drug-induced change in the QTc interval (ΔQTc) as the difference between the baseline ECG and ECGs performed at 2 hours, Day 2, and Day 3 after start of therapy. If patients had ECGs performed at both Day 2 and Day 3 (i.e. ≥48 h), we used the mean of the ΔQTc. Electronically measured QT intervals were in all cases manually validated or re-measured by a physician. If discrepancies between the electronically and manually evaluated QT intervals were encountered, the latter was used. Among patients in atrial fibrillation, the QT interval was determined by the average measured QT interval over 10 beats.7
Statistical analyses
Continuous data are presented as means ± standard deviation. To test the ΔQTc we used Student's paired t-test. Multivariate linear regression analyses were used to examine the impact of covariates on ΔQTc (Bazett's correction) and multivariate logistic regression analyses were used to determine predictors of sotalol discontinuation within 3 days. Results from the latter are presented as odds ratios (ORs) and 95% confidence intervals (CIs). Owing to the small number of patients who initiated dofetilide or quinidine, we were not able to perform any type of multivariate regression analyses (58 and 26, respectively).
For all analyses, a two-sided P < 0.05 was considered statistically significant. All analyses were performed using Stata statistical package version 11.1 (Stata-Corp. LP).
Ethics
All participating patients gave written informed consent before study inclusion. The study protocol was approved by the Vanderbilt University Institutional Review Board.
Results
Overall, we identified 845 patients initiating treatment with quinidine, sotalol, dofetilide, ibutilide, disopyramide, or procainamide. Of these, 672 were patients initiating sotalol and 7 of these (1%) developed TdP after sotalol administration. Among the patients who developed TdP, the number of sotalol doses administered before TdP onset were one dose for one TdP patient, two doses for three TdP patients, three and four doses for two TdP patients. In one patient, the TdP event was identified on an ICD because it was uncertain in relation to which sotalol dose TdP occurred. Sotalol was discontinued in all instances. After applying exclusion criteria, our final study population comprised of 641 consented patients identified through clinical alerts in the VUMC EMR system of which 86.5% initiated treatment with sotalol (Figure 1). Clinical characteristics are listed in Table 1 for patients on sotalol where the overall age was 63.6 (±12.9) years, 37.0% were women, and atrial fibrillation or flutter was the predominant indication for therapy (92.2%). Patients who initiated treatment with sotalol at baseline had a mean BMI of 30.5 ± 7.0 and a mean ejection fraction of 52 ± 12%. In 7.1%, the serum potassium was <3.5 mEg/L at the time of drug initiation. Calcium levels were within the normal range (Table 1). The clinical characteristics for patients who initiated dofetilide or quinidine are listed in Supplementary material online, Table S1.
Table 1.
Clinical characteristics of patients who initiated sotalol therapy
| Baseline characteristics | Sotalol |
|---|---|
| N, (%) | 541 (86.6) |
| Age, years (±SD) | 63.6 (±12.9) |
| Women, n (%) | 200 (37.0) |
| Ethnicity white, n (%) | 524 (96.9) |
| Ejection fraction, n (±SD) | 52% (±12)a |
| BMI, kg/m2 (±SD) | 30.5 (±7.0)b |
| Drug dose | |
| Start dose (±SD) | 86 mg (±39)c |
| Accumulated 24 h dose (±SD) | 175 mg (±102)c |
| Index rhythm, n (%) | |
| Atrial fibrillation or flutter | 499 (92.2) |
| Ventricular tachycardia | 40 (7.4) |
| History of, n (%) | |
| Diabetes (type 1 and 2) | 103 (19.0) |
| Hypertension | 360 (66.5) |
| Myocardial infarction | 79 (14.6) |
| Hypertrophic cardiomyopathy | 12 (2.2) |
| ICD or PM | 82 (15.2) |
| Coronary artery bypass graft | 110 (20.3) |
| Concomitant medication, n (%) | |
| Digoxin | 68 (12.7) |
| Calcium channel blocker | 187 (35.0) |
| Warfarin | 329 (61.5) |
| Amiodarone | 13 (2.6) |
| Loop diuretic | 192 (35.9) |
| Thiazide | 48 (9.0) |
| Lab values, (±SD) | |
| Baseline potassium, mEg/L (±SD) | 4.1 (±0.44)d |
| Baseline calcium, mg/dL (±SD) | 8.7 (±0.65)e |
SD, standard deviation; ICD, implantable cardioverter-defibrillator; PM, pacemaker.
Number of patients:
an=526.
bn=410.
cn=525.
dn = 489.
en = 406.
Electrocardiogram data in sotalol-exposed patients (Table 2) showed a statistically significant increase in the QTc interval from baseline to ≥48 h (11 ± 37 ms, P < 0.001). The increase in the QTc interval from baseline to 2 h was not statistically significant (3 ± 42 ms, P = 0.17). Variability in ΔQTc among patients treated with sotalol after 2 h and ≥48 h is depicted in Figure 2. The QT interval, heart rate, QTc interval, and ΔQTc data for patients treated with quinidine and dofetilide are available in Supplementary material online, Tables S2 and S3. Patients in concurrent treatment with amiodarone (n = 13) also experienced an increase in ΔQTc on average (12.2 ± 42.1 ms).
Table 2.
QT variability and change compared with baseline after sotalol administration
| QT variability | Baselinea | 2 hb | ≥48 hc |
|---|---|---|---|
| QT, ms (±SD) | 384 (±50) | 403 (±53) | 431 (±45) |
| Heart rate, b.p.m. (±SD) | 83 (±24) | 78 (±22) | 69 (±16) |
| QTc Bazett, ms (±SD) | 443 (±37) | 445 (±39) | 453 (±37) |
| ΔQTc Bazett, ms (±SD) | – | 3 (±42) | 11 (±37) |
| QTc, Fridericia, ms (±SD) | 421 (±34) | 429 (±34) | 444 (±34) |
| ΔQTc Fridericia, ms (±SD) | – | 10 (±38) | 25 (±37) |
QT corrections: QTc Bazett QT/RR1/2, QTc Fridericia QT/RR1/3.
SD, standard deviation; b.p.m., beats per minute.
Information available for:
an = 541.
bn = 377.
cn = 503.
Figure 2.
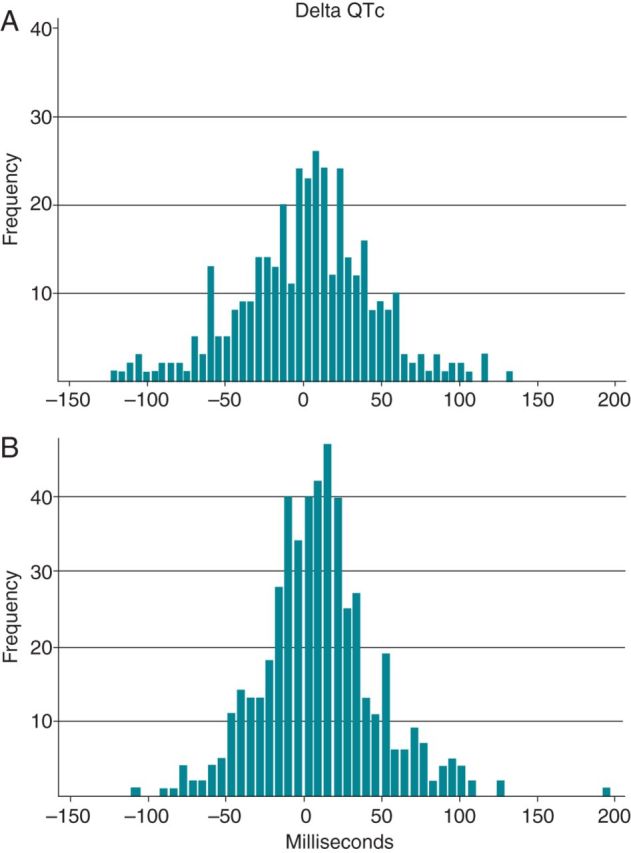
Changes in QTc from baseline after sotalol administration (ΔQTc) at 2 and ≥48 h. Legend: (A) 2 h, n= 382; (B) ≥48 h, n = 511.
Follow-up information at 3 days/3 months was available for 98.9% of patients on sotalol. Overall, 152 (29%) patients discontinued sotalol therapy ≤3 months and 10.5% of these were caused by the development of a ‘long QT interval’. The reasons for discontinuation during follow-up for sotalol-treated patients are listed in Table 3. Overall, we identified 61 patients (11.6%) who discontinued sotalol ≤3 days and 91 patients (22.9%) who were in treatment for 3 days but then discontinued therapy by ≤3 months. Common reasons for sotalol discontinuation ≤3 days were ‘long QT interval’ and ‘Other’ (31.1%, n = 19/61 for both). The ‘Other’ category includes development of hypotension, palpitations, complete atrioventricular heart block, and renal insufficiency. Among patients who were on sotalol therapy for >3 days but then discontinued therapy within ≤3 months, the most common causes for sotalol discontinuation were ‘Other’ (36.3%, n = 33 of 91) and ‘recurrence of arrhythmia’ (30.8%, n = 28 of 91) (Table 3).
Table 3.
Clinical outcomes after initiating sotalol therapy
| ≤3 days | ≤3 monthsa | |
|---|---|---|
| N | 525 | 397 |
| Continued sotalol (%) | 464 (88.4) | 306 (77.1) |
| Discontinued sotalol (%) | 61 (11.6) | 91 (22.9) |
| Reason for discontinuation: | ||
| Bradycardia (%) | 8 (1.5) | 8 (2.0) |
| Adverse event (%) | 10 (1.9) | 18 (4.5) |
| Long QT (%) | 19 (3.6) | 4 (1.0) |
| Recurrence of arrhythmia (%) | 5 (1.0) | 28 (7.1) |
| Other (%) | 19 (3.6) | 33 (8.3) |
aPatients who did not discontinue sotalol treatment ≤3 days.
Patients who discontinued sotalol for reasons not related to sotalol were not included (n = 10).
Clinical predictors of ΔQTc ≥ 48 h among sotalol-exposed patients are depicted in Figure 3 and listed in Supplementary material online, Table S4. Female gender was associated with an increase in ΔQTc as was age (7.1 ms for female gender, CI: 0.8–14.1, and 2.3 ms per 5 year increase in age, CI: 0.2–14.4). Similarly, a high sotalol dose (160 mg/day) was associated with an increase in ΔQTc (9.2 ms, CI: 0.9–17.4). The biggest overall effects on ΔQTc were seen among patients with a history of hypertrophic cardiomyopathy (22.8 ms, CI: 2.1–43.5) or concomitant treatment with loop diuretics (19.4 ms, CI: 11.2–27.6). No statistically significant associations were found between ΔQTc and reduced ejection fraction (<55%) in the main analyses (7.4 ms, CI: −0.9–15.7).
Figure 3.
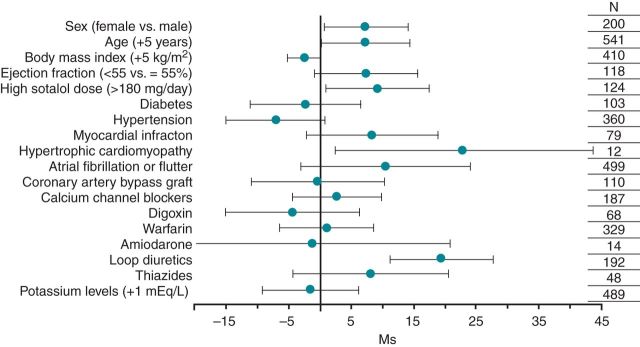
Estimated effect of clinical variables on QTc changes following sotalol administration according to multivariable linear regression analyses. Legend: ΔQTc = (≥48 h sotalol QTc−baseline QTc); patients who contributed to the analysis: *n = 337; model controlled for baseline QTc interval; For dichotomous variables N denotes the number of patients with variables of interest and for continuous variables N denotes the number of patients with non-missing values.
The clinical covariates associated with sotalol discontinuation within 3 days are shown in Figure 4. Statistically significant predictors of sotalol discontinuation were female gender (OR = 2.2, CI: 1.2–4.1), reduced LVEF (≤35%) (OR = 2.7, CI: 1.1–6.5), and concomitant amiodarone treatment (OR = 4.2, CI: 1.1–15.3). No other predictors were identified.
Figure 4.
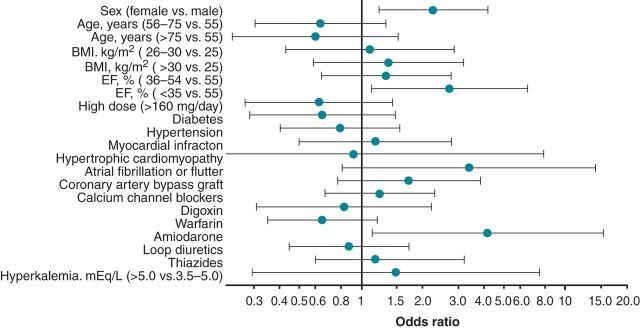
Likelihood of sotalol discontinuation within 3 days according to multivariable logistic regression.
Other analyses
Post hoc multiple linear regression without BMI as an independent variable due to missing information in 22% of patients confirmed all associations from the main analyses, but also identified a statistically significant association between a reduced LVEF (<55%) and ΔQTc (9.3 ms, CI: 1.9–16.6).
We identified all discontinuations ≤3 days caused by a ‘long QT interval’ at follow-up and repeated the multivariable logistic regression analysis to identify clinical covariates of sotalol-induced ‘long QT interval’ discontinuation. Statistically significant associations were identified for female gender (OR = 5.2, CI: 1.5–18.1), reduced LVEF (OR = 9.3, CI: 2.3–37.3), and hypokalaemia (OR = 4.9, CI: 1.0–23.5).
Discussion
In the present study, we identified patients through an EMR system who started sotalol therapy. Electrocardiogram monitoring of all patients before and after sotalol exposure showed a highly variable effect of sotalol on the QTc interval. Identified predictors of sotalol-related changes on the QTc interval included female gender, high sotalol dose, age, hypertrophic cardiomyopathy, reduced LVEF, and concomitant use of loop diuretics. Correlates of sotalol discontinuation ≤3 days were female gender, reduced LVEF, and concomitant amiodarone pharmacotherapy. Overall, ∼33% of all patients discontinued sotalol therapy ≤3 months.
This study offers novel insights into specific clinical predictors of ΔQTc among real-world patients treated with sotalol. We used an EMR to identify patients and gather data on a wide range of parameters that would otherwise not have been obtainable unless a large prospective study had been conducted. Using this approach enabled us to describe patient characteristics of treated patient and provide information on current sotalol use in clinical practice. We have previously used the same approach to interrogate genetic determinants of response to warfarin.6 Furthermore, in the present study, we were also able to evaluate whether identified associations between clinical covariates and ΔQTc also translated into sotalol discontinuation within 3 days.
Long QT syndrome has two well-characterized aetiologies; those due to congenital Mendelian syndromes and acquired forms, often seen with administration of QT-prolonging drugs. Additional risk factors for acquired TdP include left ventricular hypertrophy, heart failure, myocardial infarction, female gender, bradyarrhythmias, hypokalaemia, and conversion of atrial fibrillation to sinus rhythm.8–11
Torsades de pointes-inducing drugs include not only antiarrhythmics, but also non-cardiovascular drugs such as methadone, terfenadine, or haloperidol, which has led to restrictions and high-profile withdrawals of marketed drugs.12–15 Concomitant use of loop diuretics was common among sotalol-treated patients (35.9%) and associated with QTc interval prolongation. There are multiple potential mechanisms for this association. First, these drugs are commonly prescribed for patients with heart failure. Secondly, hypokalaemia is common with loop diuretic treatment and this leads to reduced IKr and greater sensitivity of IKr to drug block.16 Although post hoc analyses did show a robust association between loop diuretics and ΔQTc after controlling for baseline potassium and LVEF, it is important to note that serum potassium is an imperfect marker for intracellular potassium levels. While there are data that thiazides block a separate potassium current, IKs, which may potentiate the QT prolongation seen with IKr block, such findings cannot readily be extrapolated to loop diuretics.17–20 In addition, no effect of thiazides on the QTc interval was found in any of our regression models.
Selective IKr blocking antiarrhythmic drugs such as sotalol are known to prolong the QT interval and induce TdP in a dose-dependent manner.20,21 In agreement with this observation, patients with a high sotalol dose (>160 mg/day) were more likely to develop a longer QTc interval compared with patients on a lower dose (9.2 ms, CI: 0.9–17.4) (Figure 3, see Supplementary material online, Table S4).
It is standard-of-care to correct serum potassium to normal levels before initiating patients on QT-prolonging drugs, such as sotalol, and we assume that the fact that most (>90%) of the patients we studied had baseline potassium values in the normal range (3.5–5 mEq/L) reflects this practice. This could explain why we did not see an association between ΔQTc and plasma potassium levels (Figure 3, see Supplementary material online, Table S4). However, in the sub-analysis we identified a positive correlation between hypokalaemia and sotalol discontinuation caused by ‘long QT interval’ (OR = 4.9, CI: 1.0–23.5). Although this association is in accordance with previous findings, we only identified 19 patients who discontinued because of ‘long QT interval’ which is reflected in the wide CIs. Nevertheless, these data further reinforce the desirability of correcting hypokalaemia prior to initiating drugs such as sotalol.
The most common reasons for sotalol discontinuation within 3 days and after 3 days were recurrence of an arrhythmia, ineffectiveness, and long QT interval. These findings are in agreement with previous findings by Soyka et al.13 who studied the safety profile in 1288 patients treated with sotalol primarily for ventricular arrhythmias, and found that the most common cause of sotalol discontinuation was ineffectiveness. In that study, the incidence of TdP was 1.9% (24 of 1288) compared with 1.0% (7 of 672) in the present study.
Female gender and reduced LVEF have been shown to be strong predictors of a prolonged QTc (and TdP)22 in prior studies as well as the present study.9,11 Notably, both these also translated into overall sotalol discontinuation before 3 days as well as sotalol discontinuation because of a ‘long QT interval’. Similarly, co-administration of amiodarone was also associated with overall sotalol discontinuation, which probably reflects the fact that these two drugs are generally combined only in patients with serious and difficult to control arrhythmias; this co-prescription was only seen in 14 patients.
Limitations
One limitation of this study is inherent in its observational design. Hence, being unable to gather all baseline information at the time of initial drug administration as well as not having all covariates available in all patients may have masked potential associations. Although we tried to eliminate potential confounders in the present analyses, it is possible that residual confounding may have influenced our results. Moreover, a measured variable (e.g. serum potassium) does not always reflect the intracellular state which may have affected our results. In all instances, QT intervals were manually confirmed or re-measured if deemed necessary. Although comparing the QT intervals pre- and post-drug exposure is a measure of sotalol-induced effects on repolarization, we recognize that beat-to-beat variation does exist which may have influenced our results. In addition, we were unable to determine how much of reported QT variability was due to sotalol, as repeated QTc measurements in the absence of a change in drug therapy (e.g. sotalol) were unavailable. We also acknowledge that the calculated QTc interval, using Bazett's formula, may not reflect real QTc changes if the heart rate was substantially affected at the time of ECG obtainment.
Conclusion
Using clinical alerts in an EMR, we were able to identify ‘real-world’ clinical patients who initiated antiarrhythmic drug treatment. Sotalol was the most prescribed drug, and the increase in the QTc interval associated with sotalol initiation was highly variable among patients. Risk factors for significant increases in QT interval included female gender, high sotalol dose, age, hypertrophic cardiomyopathy, reduced LVEF, and concomitant pharmacotherapy with loop diuretics. Of these risk factors, female gender and reduced LVEF were also associated with sotalol discontinuation due to prolonged QT interval. These data represent a key step in ongoing work to identify real-world patients likely to tolerate long-term therapy and further reinforce the utility of EMR-based cohorts as research tools.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: none declared.
Funding
P.W. was funded by an unrestricted research grant from the Tryg Foundation (J.nr. 7343–09, TrygFonden, Denmark), supported in part by U19 HL065962.
Supplementary Material
References
- 1.Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med. 2006;259:59–69. doi: 10.1111/j.1365-2796.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 2.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 3.Hondeghem LM. Thorough QT/QTc not so thorough: removes torsadogenic predictors from the T-wave, incriminates safe drugs, and misses profibrillatory drugs. J Cardiovasc Electrophysiol. 2006;17:337–40. doi: 10.1111/j.1540-8167.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:651–745. doi: 10.1093/europace/eul097. [DOI] [PubMed] [Google Scholar]
- 5.Chung MK, Schweikert RA, Wilkoff BL, Niebauer MJ, Pinski SL, Trohman RG, et al. Is hospital admission for initiation of antiarrhythmic therapy with sotalol for atrial arrhythmias required? Yield of in-hospital monitoring and prediction of risk for significant arrhythmia complications. J Am Coll Cardiol. 1998;32:169–76. doi: 10.1016/s0735-1097(98)00189-2. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A, et al. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358:999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA. 2003;289:2120–7. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 8.Gaudron P, Kugler I, Hu K, Bauer W, Eilles C, Ertl G. Time course of cardiac structural, functional and electrical changes in asymptomatic patients after myocardial infarction: their inter-relation and prognostic impact. J Am Coll Cardiol. 2001;38:33–40. doi: 10.1016/s0735-1097(01)01319-5. [DOI] [PubMed] [Google Scholar]
- 9.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–7. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 10.Choy AM, Darbar D, Dell'Orto S, Roden DM. Exaggerated QT prolongation after cardioversion of atrial fibrillation. J Am Coll Cardiol. 1999;34:396–401. doi: 10.1016/s0735-1097(99)00226-0. [DOI] [PubMed] [Google Scholar]
- 11.Davey PP, Barlow C, Hart G. Prolongation of the QT interval in heart failure occurs at low but not at high heart rates. Clin Sci (Lond) 2000;98:603–10. [PubMed] [Google Scholar]
- 12.Torp-Pedersen C, Moller M, Bloch-Thomsen PE, Kober L, Sandoe E, Egstrup K, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish investigations of arrhythmia and mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–65. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 13.Soyka LF, Wirtz C, Spangenberg RB. Clinical safety profile of sotalol in patients with arrhythmias. Am J Cardiol. 1990;65:74A–81A. doi: 10.1016/0002-9149(90)90207-h. discussion 2A-3A. [DOI] [PubMed] [Google Scholar]
- 14.De Ponti F, Poluzzi E, Cavalli A, Recanatini M, Montanaro N. Safety of non-antiarrhythmic drugs that prolong the QT interval or induce torsade de pointes: an overview. Drug Saf. 2002;25:263–86. doi: 10.2165/00002018-200225040-00004. [DOI] [PubMed] [Google Scholar]
- 15.Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:2215–20. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- 16.Yang T, Roden DM. Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation. 1996;93:407–11. doi: 10.1161/01.cir.93.3.407. [DOI] [PubMed] [Google Scholar]
- 17.Roden DM, Yang T. Protecting the heart against arrhythmias: potassium current physiology and repolarization reserve. Circulation. 2005;112:1376–8. doi: 10.1161/CIRCULATIONAHA.105.562777. [DOI] [PubMed] [Google Scholar]
- 18.Fiset C, Drolet B, Hamelin BA, Turgeon J. Block of IKs by the diuretic agent indapamide modulates cardiac electrophysiological effects of the class III antiarrhythmic drug dl-sotalol. J Pharmacol Exp Ther. 1997;283:148–56. [PubMed] [Google Scholar]
- 19.Turgeon J, Daleau P, Bennett PB, Wiggins SS, Selby L, Roden DM. Block of IKs, the slow component of the delayed rectifier K+ current, by the diuretic agent indapamide in guinea pig myocytes. Circ Res. 1994;75:879–86. doi: 10.1161/01.res.75.5.879. [DOI] [PubMed] [Google Scholar]
- 20.Kehoe RF, Zheutlin TA, Dunnington CS, Mattioni TA, Yu G, Spangenberg RB. Safety and efficacy of sotalol in patients with drug-refractory sustained ventricular tachyarrhythmias. Am J Cardiol. 1990;65:58A–64. doi: 10.1016/0002-9149(90)90204-e. discussion 5A-6A. [DOI] [PubMed] [Google Scholar]
- 21.Stanton MS, Prystowsky EN, Fineberg NS, Miles WM, Zipes DP, Heger JJ. Arrhythmogenic effects of antiarrhythmic drugs: a study of 506 patients treated for ventricular tachycardia or fibrillation. J Am Coll Cardiol. 1989;14:209–15. doi: 10.1016/0735-1097(89)90074-0. discussion 16–7. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann MH, Hardy S, Archibald D, quart B, MacNeil DJ. Sex difference in risk of Torsades de pointes with d,l-sotalol. Circulation. 1996;94:2535–41. doi: 10.1161/01.cir.94.10.2535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


