Abstract
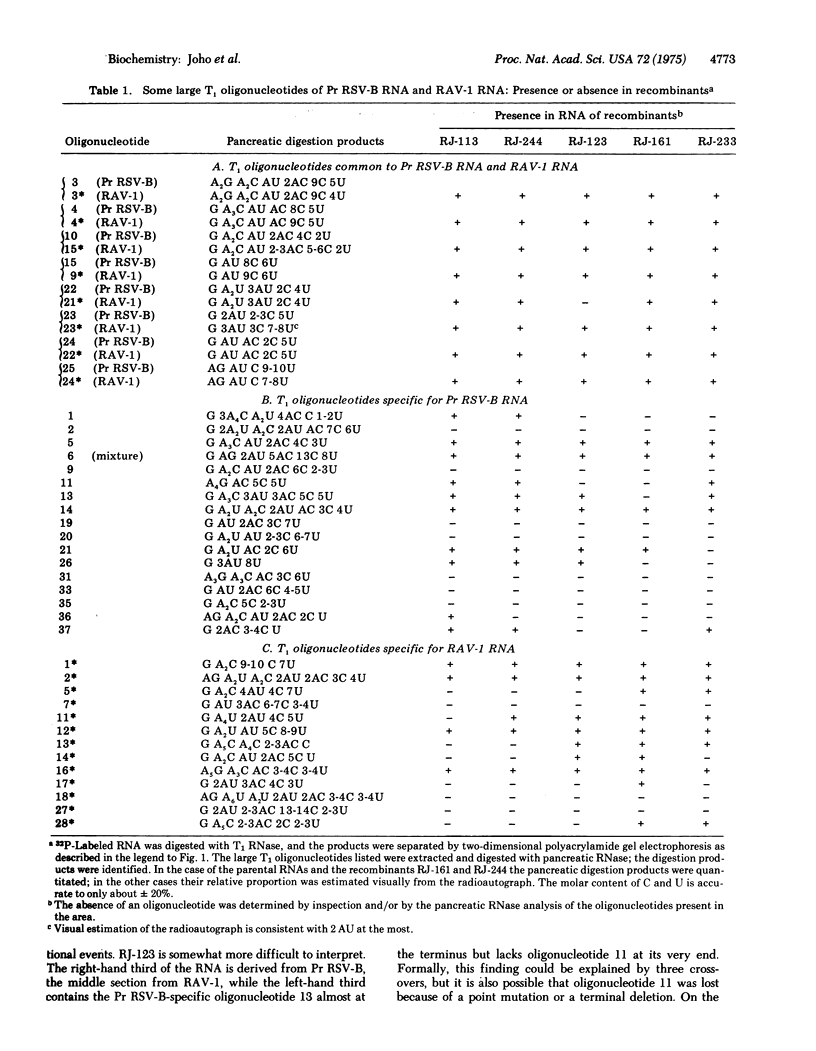
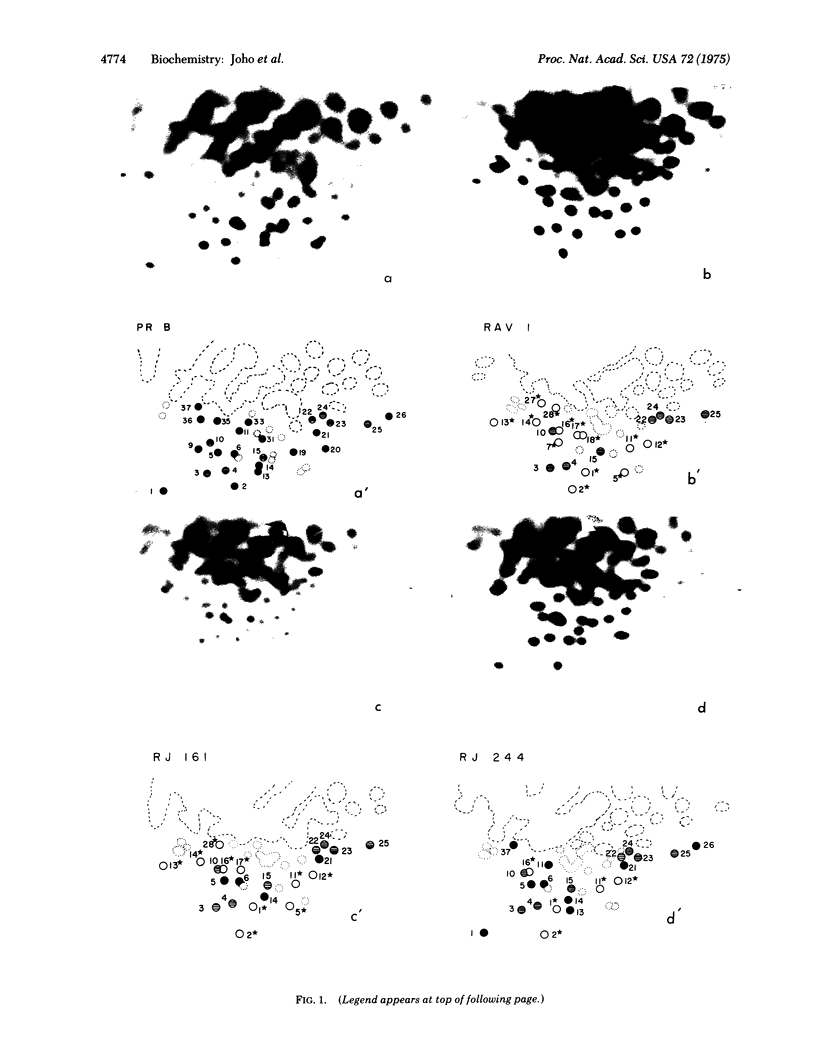
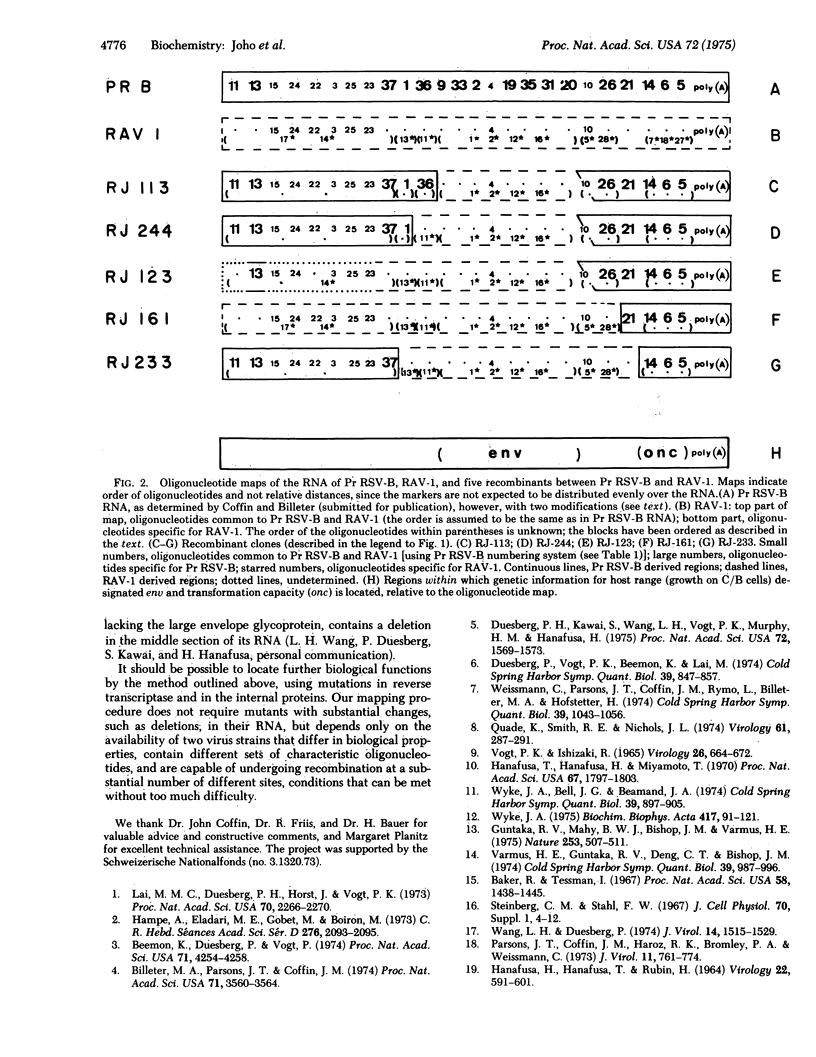
A map of the large T1 oligonucleotides of the RNA of Prague Rous sarcoma virus, strain B (Pr RSVb) has recently been established (Coffin and Billeter, submitted for publication). Since the RNA of Rous associated virus, type 1 (RAV-1) lacks many of the large 1 oligonucleotides of Pr RSV-B and contains others not present in the latter, the RNA of recombinants between RAV-1 and Pr RSV-B could be analyzed with regard to the origin of its sequences. Recombinants were selected for transforming capacity (characteristic for Pr RSV-B) and ability to grow on C/B chicken fibroblasts (characteristic for RAV-1). Four out of five recombinants examined had undergone at least two crossovers. The set of Pr RSV-B-specific oligonucleotides present in all recombinants defined an RNA region near the poly(A) segment; this must contain genetic information required for transformation required for transformation (the onc function). All recombinants lost a set of contiguous Pr RSV-B-specific oligonucleotides and concomitantly acquired a set of RAV-1-specific oligonucleotides. These define a region in the middle section of the oligonucleotide map, all or some of which must be required for determining growth capacity on C/B cells (the env function).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R., Tessman I. The circular genetic map of phage S13. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1438–1445. doi: 10.1073/pnas.58.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Kawai S., Wang L. H., Vogt P. K., Murphy H. M., Hanafusa H. RNA of replication-defective strains of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1569–1573. doi: 10.1073/pnas.72.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P., Vogt P. K., Beemon K., Lai M. Avian RNA tumor viruses: mechanism of recombination and complexity of the genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):847–857. doi: 10.1101/sqb.1974.039.01.099. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Mahy B. W., Bishop J. M., Varmus H. E. Ethidium bromide inhibits appearance of closed circular viral DNA and integration of virus-specific DNA in duck cells infected by avian sarcoma virus. Nature. 1975 Feb 13;253(5492):507–511. doi: 10.1038/253507a0. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS. I. CHARACTERIZATION OF THE HELPER VIRUS. Virology. 1964 Apr;22:591–601. doi: 10.1016/0042-6822(64)90081-9. [DOI] [PubMed] [Google Scholar]
- Hampe A., Eladari M. E., Gobet M., Boiron M. Analyse par homochromatographie du RNA 70 S extrait d'un oncornavirus murin. C R Acad Sci Hebd Seances Acad Sci D. 1973 Mar 26;276(13):2093–2095. [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T. Recovery of a new virus from apparently normal chick cells by infection with avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1797–1803. doi: 10.1073/pnas.67.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H., Horst J., Vogt P. K. Avian tumor virus RNA: a comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2266–2270. doi: 10.1073/pnas.70.8.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Coffin J. M., Haroz R. K., Bromley P. A., Weissmann C. Quantitative determination and location of newly synthesized virus-specific ribonucleic acid in chicken cells infected with Rous sarcoma virus. J Virol. 1973 May;11(5):761–774. doi: 10.1128/jvi.11.5.761-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quade K., Smith R. E., Nichols J. L. Evidence for common nucleotide sequences in the RNA subunits comprising Rous sarcoma virus 70 S RNA. Virology. 1974 Sep;61(1):287–291. doi: 10.1016/0042-6822(74)90263-3. [DOI] [PubMed] [Google Scholar]
- Steinberg C. M., Stahl F. W. Interference in circular maps. J Cell Physiol. 1967 Oct;70(2 Suppl):4–12. [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Deng C. T., Bishop J. M. Synthesis, structure and function of avian sarcoma virus-specific DNA in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):987–996. doi: 10.1101/sqb.1974.039.01.113. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C., Parsons J. T., Coffin J. W., Rymo L., Billeter M. A., Hofstetter H. Studies on the structure and synthesis of Rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1043–1056. doi: 10.1101/sqb.1974.039.01.120. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Bell J. G., Beamand J. A. Genetic recombination among temperature-sensitive mutnats of Rous sarcoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):897–905. doi: 10.1101/sqb.1974.039.01.104. [DOI] [PubMed] [Google Scholar]
- Wyke J. A. Temperature sensitive mutants of avian sarcoma viruses. Biochim Biophys Acta. 1975 Jul 11;417(2):91–121. doi: 10.1016/0304-419x(75)90001-3. [DOI] [PubMed] [Google Scholar]