Abstract
Background
Spontaneous renal artery dissection (SRAD) is a rare entity of unknown etiology. We aimed to study the clinical course and outcomes and compare the characteristics of patients with SRAD with those of the general population.
Methods
All cases of isolated renal artery dissection diagnosed at the University of Michigan Hospitals between January 2000 and July 2012 were identified by the ICD-9 code. Cases were matched by age, gender and race with individuals from the 2009–2010 National Health and Nutrition Examination Survey (NHANES). Characteristics and awareness of comorbid conditions were compared. Information about the clinical course after diagnosis was retrieved from the case group to ascertain their outcomes.
Results
Overall, 17 patients with SRAD with a mean age of 38.6 years (SD = 8.3) were identified. Eleven patients were male and 14 were white. The most common presenting symptom was excruciating sudden-onset flank pain ipsilateral to the site of dissection. Fibromuscular dysplasia, Ehlers–Danlos and polyarteritis nodosa were present in 4, 4 and 1 patients, respectively. After adjusting in a multivariable model, the case group was more likely to report history of hypertension, cancer and connective tissue disorders (P < 0.001), and less likely to have obesity (BMI ≥30 kg/m2) compared with the general population. Supportive medical treatment, endovascular intervention and surgery were required in 8, 5 and 4 cases, respectively. After discharge from the hospital, hypertension was adequately controlled in all the patients but one.
Conclusion
SRAD may be part of a syndrome having multi-organ involvement. With appropriate medical or surgical management, long-term clinical outcome appears favorable.
Keywords: Ehlers–Danlos syndrome, fibromuscular dysplasia, nail–patella syndrome, Poland syndrome, renal artery dissection
INTRODUCTION
The first case of spontaneous renal artery dissection (SRAD) was reported in 1944 [1]. For several decades after the first reported case, the literature remained limited to case reports and small case series describing this rare entity. The roles of different therapeutic options including supportive medical management, endovascular angioplasty and surgical operations were also described in some of these reports. No study to date has examined the clinical characteristics of these patients in a systematic manner and compared these with those of the general population or a standard control group. In order to determine the clinical characteristics of patients with SRAD, we aimed to compare the characteristics of the patients with the radiologic diagnosis of SRAD in our medical center with age, gender and race-matched individuals from the general population. In continuum, we illustrate the clinical course and compare the renal outcomes and control of blood pressure by treatment strategies in this group of patients.
METHODS
This is a Health Insurance Portability and Account Act (HIPAA)-compliant, retrospective, case–control study. Patients with the diagnosis of ‘renal artery dissection’ (RAD) using the International Classification of Diseases (ICD)-9 443.23 code were identified by searching all inpatient and outpatient medical records at the University of Michigan from 1 January 2000 to 31 July 2012. Institutional review board approval was obtained for conduct of this study.
A diagnosis of RAD was established by conventional angiography, CT angiogram or both in all the patients. The radiologic criteria for diagnosis included luminal irregularity associated with aneurysmal dilatation or secular dissection with segmental stenosis, extension of dissection distal to the first renal artery bifurcation, ‘cuffing’ at branch points and variable degrees of reversibility documented by subsequent arteriographic images [2]. All the images were reviewed by two of the authors (F.A. and B.S.) and the diagnoses were verified. The inclusion criteria in the case group were age older than 18 and RAD with or without kidney infarction. The exclusion criteria included the following: blunt or deceleration traumatic injury or severe stretching of the renal artery, iatrogenic dissection of renal arteries by surgical or catheter manipulation and extension of an aortic dissection into the renal arteries.
Demographic data, comorbid conditions and other relevant clinical information were abstracted for each patient by review of medical records. Blood pressure and serum creatinine at the time of presentation and at the last follow-up were recorded. Connective tissue disorders included patients exhibiting fibromuscular dysplasia (FMD), Ehler–Danlos syndrome, polyarteritis nodosa, gout or arthritis. The control group was composed of individuals from the general population matched by age, sex and gender, selected from the 2009–2010 National Health and Nutrition Examination Survey (NHANES) publically available datasets. Comparable information including awareness of comorbid conditions was obtained for the control group. Definitions of comorbidities and the variables obtained from the NHANES dataset are summarized in Appendix 1.
Statistical analysis
The 2003–2004 guidelines for analysis of NHANES datasets set forth by the National Center for Health Statistics, Centers for Disease Control and Prevention based the weighing methodology applied in the analysis [3]. For descriptive purposes, counts and percentages are used to compare categorical variables. Mean ± standard deviation is applied for presentation of normally distributed variables. To test categorical variables in the two groups, the chi-square test and to compare continuous variables the t-test is used, respectively. The two-sided Fisher exact test was applied when the expected frequency in at least 25% of the cells in the contingency tables is less than five. Ordinal regression analysis is used to identify the comorbidities independently associated with SRAD. IBM SPSS Statistics version 20 (Chicago, IL) was used for the analysis.
RESULTS
Seventeen patients with SRAD were identified. The demographic characteristics of the patients are shown in Table 1. Accordingly, the mean (SD) of age was 38.6 (8.3) years. There were 11 male (64.7%). Fourteen patients (82.4%) were white and the others were black or multiracial. The distribution of smoking status, weight and height was not significantly different in patients compared with age-, gender- and race-matched individuals from the general population. Patients with SRAD had significantly higher mean serum creatinine, systolic and diastolic blood pressure at presentation (P ≤ 0.01). BMI in SRAD patients was slightly lower (P = 0.046). Among the comorbid conditions, the distribution of awareness from diabetes, coronary artery disease, stroke, chronic pulmonary diseases, liver diseases, cancer, leukemia, lymphoma, and positive HIV) was not significantly different from that in matched individuals in general population. History of hypertension, CHF and connective tissue disorders was more common in SRAD patients compared with the general population (P ≤ 0.011). After adjusting for other comorbidities, hypertension, connective tissue disorders and cancer were more likely to be present in association with SRAD. Similarly, risk of SRAD was higher in normal-range BMI (20–24 kg/m2) as well as overweight (25–29 kg/m2) compared with the obese category (≥30 kg/m2) (Table 2).
Table 1.
Distribution of general characteristics and chronic comorbidities in patients with isolated dissection of renal arteries compared with age- and gender-matched individuals from the 2009–2010 NHANES. Values are mean ± SD or percentages
| Variable | Case | Control | P value |
|---|---|---|---|
| n | 17 | 27760610 | |
| Age (year) | 38.6 ± 8.3 | 37.5 ± 9.5 | 0.635 |
| Male gender (%) | 64.7% | 64.6% | 0.991 |
| Race | 0.950 | ||
| White (%) | 82.4% | 84.6% | |
| Black (%) | 5.9% | 5.9% | |
| Others (%) | 11.8% | 9.5% | |
| Smoking | 0.309 | ||
| Never smoked (%) | 41.2% | 59.2% | |
| Ex-smoker (%) | 23.5% | 17.7% | |
| Current smoker (%) | 35.3% | 23.2% | |
| Weight (kg) | 82.7 ± 14.7 | 85.3 ± 20.7 | 0.624 |
| Height (kg) | 1.74 ± 0.09 | 1.72 ± 0.10 | 0.395 |
| BMI (kg/m2) | 26.8 ± 3.5 | 28.7 ± 6.3 | 0.046 |
| BMI category | 0.253 | ||
| <20 kg/m2 | 0 | 4.2% | |
| 20–24 kg/m2 | 33.3% | 24.8% | |
| 25–29 kg/m2 | 53.3% | 36.9% | |
| ≥30 kg/m2 | 13.3% | 34.1% | |
| Systolic BP (mmHg) | 150.5 ± 22.1 | 116.8 ± 13.1 | <0.001 |
| Diastolic BP (mmHg) | 86.7 ± 12.6 | 71.1 ± 11.6 | <0.001 |
| Baseline creatinine (mg/dL) | 1.16 ± 0.43 | 0.87 ± 0.19 | 0.013 |
| Baseline eGFR (mL/min) | 77.0 ± 26.0 | 100.0 ± 20.0 | <0.001 |
| Comorbidities | |||
| Hypertension (%)a | 88.2% | 20.8% | <0.001 |
| Diabetes (%)a | 0% | 5.7% | 0.621 |
| CAD (%)a | 0% | 2.1% | 1.0 |
| CHF (%)a | 5.9% | 0.1% | 0.01 |
| Stroke (%)a | 0% | 0.9% | 1.0 |
| Pulmonary (%)a | 5.9% | 15.3% | 0.498 |
| Liver disease (%)a | 5.9% | 4.1% | 0.510 |
| Arthritis, gout, connective tissue dis. (%)a | 35.3% | 12.0% | 0.011 |
| Cancer (%)a | 11.8% | 2.6% | 0.073 |
| Leukemia (%)a | 0% | 0.3% | 1.0 |
| Lymphoma (%)a | 0% | 0.3% | 1.0 |
| HIV positive (%)a | 0% | 0.4% | 1.0 |
SD, standard deviation; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CHF, congestive heart failure; HIV, human immunodeficiency virus.
aFisher exact test applied.
Table 2.
Multivariable analysis of the factors independently associated with spontaneous dissection of renal arteries
| Variables | Coefficient | Standard error | 95% CI | P value |
|---|---|---|---|---|
| Hypertension | 6.5 | 1.5 | 3.5 to 9.5 | <0.001 |
| Cancer | 5.5 | 1.4 | 2.8 to 8.1 | <0.001 |
| Connective tissue dis. | 3.5 | 0.7 | 2.0 to 4.9 | <0.001 |
| BMI < 20 kg/m2 | −13.5 | 1440.3 | −2836 to 2809 | 0.999 |
| BMI 20–24 kg/m2 | 5.4 | 1.4 | 2.7 to 8.1 | <0.001 |
| BMI 25–29 kg/m2a | 3.8 | 1.1 | 1.6 to 6.1 | 0.001 |
CI, confidence interval; BMI, body mass index.
aBMI ≥ 30 kg/m2 is reference category in BMI subgroups.
The individualized characteristics, clinical course and outcomes of each patient were revealing (Table 3). The most frequently observed presenting symptom was pain (flank pain in 10 cases and abdominal pain in 2 cases). One patient presented with CHF exacerbation and flank pain, one with headache and two were asymptomatic. The two SRAD patients who presented with headache also had coexisting dissection of vertebral arteries. The pain was spontaneous sudden and severe in 12 patients (70.6%). In one patient the pain started after weight lifting, and in two patients it was associated with gross hematuria. Connective tissue disorders were observed in nine patients including FMD in four patients, Ehlers–Danlos syndrome in four patients and polyarteritis nodosa in one patient. Poland syndrome was observed in one patient. One patient with FMD also had nail patella syndrome. Seven patients had radiographic features of renal artery aneurysm (Figures 1–3), including all four patients with Ehlers–Danlos, two patients with FMD and the patient with Poland syndrome syndrome. One patient with FMD and the patient with Poland syndrome had simultaneous dissection of vertebral arteries. Two patients with FMD also had malignant melanoma. Two patients including one with Ehlers–Danlos syndrome had recurrent bilateral dissection within a 7-year period.
Table 3.
Presenting symptoms, associated diseases and clinical course in patients with RAD
| # | Age (year) | gender | Chief complaint | Characteristics of the pain and other symptoms | Site | Associated diseases/findings | Intervention, clinical course, outcome |
|---|---|---|---|---|---|---|---|
| 1 | 38 | M | R flank pain | Spontaneous sudden onset, severe | R | – | Supportive medical therapy, stabilized, alive at 119 Mo |
| 2 | 38 | F | L flank pain | Spontaneous sudden onset, severe | L | HTN | L aortorenal arterial bypass, stabilized, alive at 39 Mo |
| 3 | 26 | F | Increase in creatinine | Asymptomatic | R | HTN, FMD | R aortorenal arterial bypass, stabilized, alive at 117 Mo |
| 4 | 49 | M | R flank pain | Spontaneous sudden onset, severe | R | HTN, history of spontaneous L RAD 7 years earlier. | R aortorenal arterial bypass, stabilized, alive at 13 Mo |
| 5 | 40 | M | CHF exacerbation | Spontaneous sudden onset, severe | L | HTN, CHF, Poland syndrome, aneurismal dissection of vertebral artery | Supportive medical therapy, stabilized, alive at 23 Mo |
| 6 | 30 | M | L flank pain | Spontaneous sudden onset, severe, recurrent epistaxis | L | HTN, FMD, Nail Patella, renal artery aneurysm | Supportive medical therapy, stabilized, alive at 17 Mo |
| 7 | 42 | M | R flank pain | Spontaneous sudden onset, severe | R | – | Supportive medical therapy, stabilized, alive at 18 Mo |
| 8 | 47 | F | LLQ pain (abdominal) | Spontaneous sudden onset, severe | L | HTN, cryptogenic liver cirrhosis, status post orthotopic liver transplantation | Thrombolysis and supportive care, stabilized, alive at 33 Mo |
| 9 | 51 | M | L flank pain | Spontaneous sudden onset, severe with gross hematuria | L | HTN, Ehlers–Danlos, renal artery aneurysm, gout | Supportive medical therapy, stabilized, alive at 19 Mo |
| 10 | 45 | M | R flank pain | Sudden onset severe pain and hematuria post weight lifting | R | HTN, FMD | Supportive medical therapy, stabilized, alive at 73 Mo |
| 11 | 27 | M | R flank pain | Spontaneous sudden onset, severe | R | HTN, Ehlers–Danlos, leak from renal artery aneurysm | Embolization of R accessory renal artery, stabilized, alive at 48 Mo |
| 12 | 41 | F | Headache | Headache, blurry vision | L | HTN, FMD, renal artery aneurysm, carotids & L vertebral artery dissection | Intensive care, stabilized, alive at 41 Mo |
| 13 | 26 | F | Uncontrolled hypertension | Asymptomatic | R | HTN, complicated by renal artery stenosis | R nephrectomy, alive at 38 Mo |
| 14 | 33 | F | Harrington rod placement | Developed L flank pain on post-operative day 12 | R | HTN, Ehlers–Danlos, leak from renal artery aneurysm | Embolization and coiling, died at 40 Mo with cerebral hemorrhage |
| 15 | 46 | M | R flank pain | Spontaneous sudden onset, severe | R | HTN, polyarteritis nodosum | Embolization, progressed to ESRD, transplanted in 5 years |
| 16 | 32 | M | Abdominal pain | Spontaneous sudden onset, severe | R,L | HTN, Ehlers–Danlos, renal artery aneurysm | Embolization and coiling, recurrent dissection in 3 and 7 years |
| 17 | 45 | M | L flank pain | Spontaneous sudden onset, severe | L | HTN | Supportive medical therapy |
M, male; F, female; R, right; L, left; Mo, month; HTN, hypertension; FMD, fibromuscular dysplasia; CHF, congestive heart failure; LLQ, left lower quadrant.
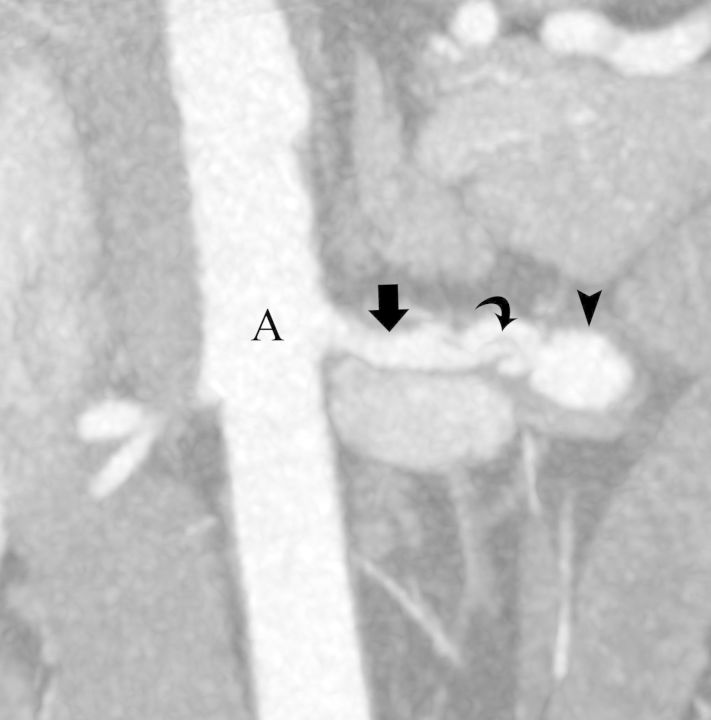
FIGURE 1:
(Case#5) A 40-year-old male with congestive heart failure and Poland syndrome. Coronal maximum intensity projection image from a CT angiography of the abdominal aorta (A) shows dissection flap (curved arrow) and aneurysmal dilatation (arrow head) of the left renal artery (arrow).
FIGURE 2:
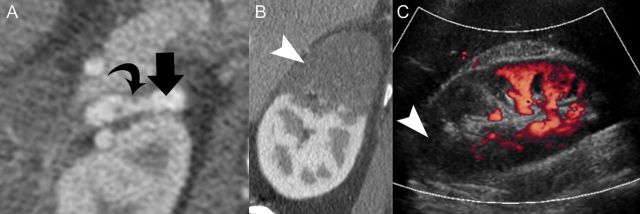
(Case #7) A 42-year-old male with no significant past medical history. Magnified axial image (A) from an intravenous contrast-enhanced CT renal angiography shows subtle internal luminal strands (curved arrow) indicating a branch artery (arrow) dissection at the right renal hilum. Sagittal reformatted image (B) of the right kidney shows geographic low attenuation of the upper pole (arrow head). Power Doppler evaluation (C) of the right kidney also shows absent perfusion (arrow head) of the upper pole with preservation blood flow in the lower pole.
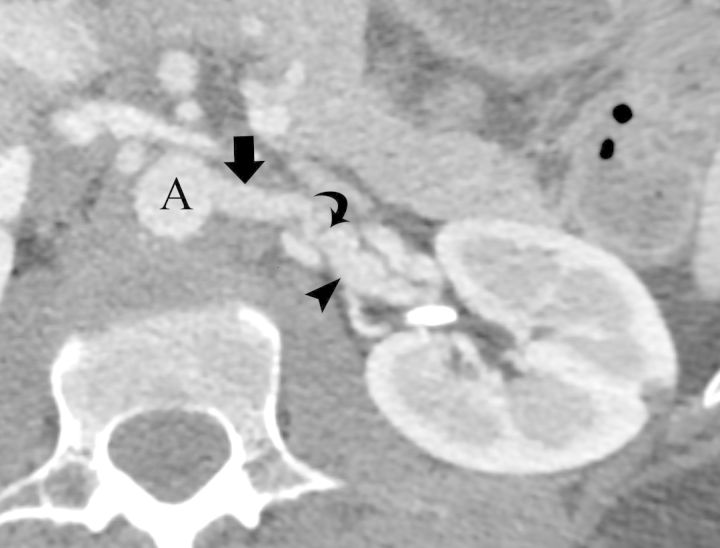
FIGURE 3:
(Case #12) A 41-year-old female with fibromuscular dysplasia. Axial image from a CT angiography of the abdominal aorta (A) shows dissection flap (curved arrow) and aneurysmal dilatation (arrow head) of the left renal artery (arrow).
Supportive medical management including analgesics, antihypertensive medications and anticoagulation was administered to eight of the SRAD patients. Endovascular procedures were performed in five patients. One patient with radiographic evidence of total occlusion of left renal artery due to formation of thrombosis underwent thrombolytic therapy followed by systematic heparinization as a bridge to subsequent oral anticoagulation with warfarin. Three patients with Ehlers–Danlos syndrome who had a leak from dissection of renal artery aneurysm underwent selective arterial embolization by interventional radiology service with immediate stabilization. Four cases underwent surgical operation with aortorenal arterial bypass surgery. Of the four bypass procedures, one required unilateral nephrectomy at the site of RAD due to extension of dissection to hilum of the kidney making the arterial reconstruction impossible (Figure 4). All the patients were stabilized and discharged after recovery from acute presentation.
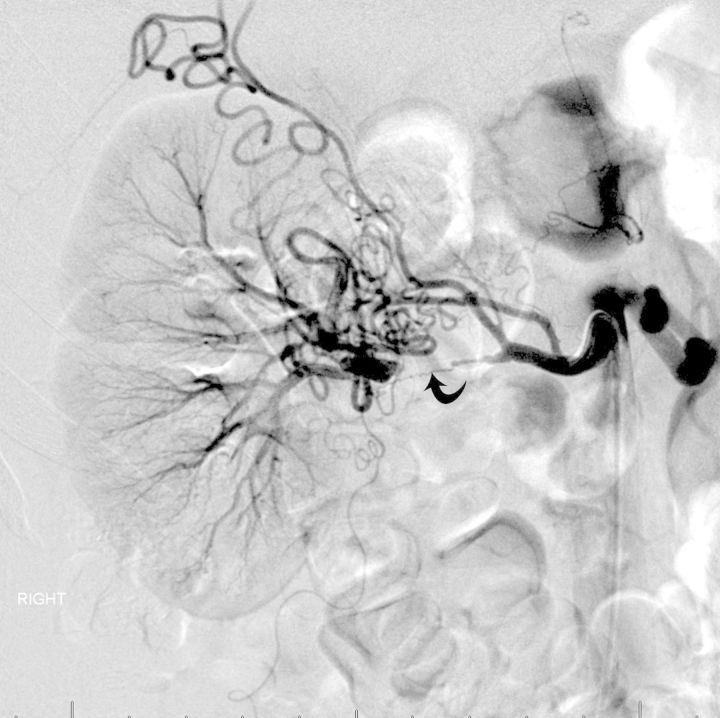
FIGURE 4:
(Case#13) A 26-year-old female with a history of hypertension presented with severe right-sided main RAD. Failed attempt for surgical revascularization required nephrectomy.
The blood pressure and serum creatinine in the patients subjected to different modalities of therapy are listed in Table 4. Median follow-up was 16, 32 and 4 months in medical, endovascular and surgical methods of therapy, respectively. Accordingly, systolic blood pressure decreased with follow-up, but has not reached statistical significance in any of the subgroups compared with their baseline. One patient in the subgroup of radiographic endovascular treatment developed acute kidney injury, which progressed to end-stage kidney disease, and underwent kidney transplantation after 5 years. Serum creatinine with follow-up has not been different clinically from baseline in medical or surgical treatment subgroups. Blood pressure was eventually controlled with follow-up in all patients except in one (case 14). One patient with Ehlers–Danlos syndrome (case 14) died after 40 months because of cerebral hemorrhage.
Table 4.
Follow-up information in patients with RAD by different therapeutic strategies including medical, radiographic endovascular and surgical operation
| Treatment strategy | n | Duration, median (IQR), months | SBP at presentation, mmHg | SBP at follow-up, mmHg | DBP at presentation, mmHg | DBP at follow-up, mmHg | Creatinine at presentation, mg/dL | Creatinine at follow-up, mg/dL |
|---|---|---|---|---|---|---|---|---|
| Medical | 8 | 16.2 (1.3, 50.6) | 154 ± 8 | 122 ± 15 | 87 ± 11 | 79 ± 9* | 1.1 ± 0.3 | 1.0 ± 0.2 |
| Endovascular | 5 | 32.0 (25.0, 42.5) | 152 ± 28 | 131 ± 25 | 84 ± 10 | 80 ± 13 | 1.1 ± 0.4 | 3.1 ± 3.8 |
| Surgical | 4 | 4.4 (0.2, 10.1) | 145 ± 37 | 127 ± 10 | 91 ± 21 | 82 ± 6 | 1.4 ± 0.7 | 1.4 ± 0.7 |
IQR, inter-quartile range; SBP, systolic blood pressure; DBP, diastolic blood pressure.
*P = 0.01.
DISCUSSION
This is a large case series of patients with SRAD and the first study that systematically compares the characteristics of patients with spontaneous RAD with those of matched controls from the general population. In this study, SRAD is associated with middle-age, a history of hypertension and flank pain, elevated creatinine and uncontrolled blood pressure at presentation. In patients with SRAD CHF, cancer and connective tissue may also be present at a significantly higher rate as expected in this age-group.
The etiology of SRAD is unclear. In addition to its association with connective tissue disorders such as FMD, Ehlers–Danlos syndrome and Marfan's syndrome in a few case reports, SRAD is also reported in association with malignant hypertension, atherosclerosis, blunt trauma and strenuous exercise in several other case reports and small series [4–18]. Cocaine abuse and extracorporeal shock wave lithotripsy were reported in rare cases [19, 20]. Arterial dysplasia and abnormalities involving vasa vasorum have been viewed as predisposing factors for spontaneous peripheral arterial dissections [21]. Arterial dysplasia is further classified to intimal fibroplasia (5%), medial hyperplasia (1%), medial fibroplasia (84%) and perimedial dysplasia [22]. Rupture of vaso vasorum may result in hemorrhage with subsequent intramural hematoma, which may lead to medial ischemia and a further compromise in vessel wall integrity. In a study of 316 dissected vessels from 196 patients with arterial fibrodysplasia, medial dysplasia was observed in up to 85% of cases, but only two were associated with RADs, suggesting rarity of SRAD in arterial fibrodysplasia [23]. Unusual physical stress can lead to traction of renal arteries and abnormalities in the integrity of connective tissue may present predisposing factors that can result in rapture of vasa vasorum and subsequent cascade of events such as intramural hematoma and SRAD.
In the present study, SRADs were observed in two different categories. One is an isolated form without any other significant comorbidity and the other one is being part of a syndrome with multisystem involvement including association with Ehlers–Danlos syndrome in four patients, FMD in four patients, Poland syndrome, polyarteritis nodosa and nail–patella syndrome each in one patient suggesting possibility of disorders in connective tissue. Although the two groups are distinct by the above-mentioned comorbidities, the underlying etiologic factors may be identical, including genetic predisposition and interactions with environmental factors. As Ehlers–Danlos syndrome and FMD are rare diseases in the general population, their clustering in our patients strongly suggests that they are risk factors for development of SRAD.
In an earlier observation from 1960 to 1976, two of four patients with SRAD exhibited with renal artery FMD and other two had arteriosclerosis of the renal arteries [24]. Two of the patients with FMD also were diagnosed with malignant melanoma. An association of FMD with neoplastic disorders such neurofibromatosis, carotid body tumor, cardiac fibroelastoma, renal cell carcinoma, pheochromocytoma and spinal hemangioma has been the subject of a few case reports [25–30]. Larger studies are needed to identify possible associations of connective disorders and cancer with SRAD.
Sustained elevated blood pressure may contribute to the development of arterial dissection by potentiating arteriosclerosis and medial degeneration [24], although hypertension itself may be secondary to renal ischemia following the SRAD. Depending on the severity, extent and main artery versus branch involvement, SRAD may cause renal ischemia of varying degree, renin-mediated renovascular hypertension and renal infarction [31–34].
In our study, as compared to obese patients, risk of SRAD was higher in individuals with normal BMI and overweight patients. Although this finding may just be a chance finding, protective effect of obesity may be explained by coexisting sedentary lifestyle imposing less stretch to renal arteries. We also noted bilateral dissection of the renal arteries over a span of 7 years, which suggests that unilateral SRAD may be a risk factor for the subsequent dissection of the contralateral renal artery.
The most frequent presenting features were uncontrolled hypertension and severe spontaneous sudden-onset flank pain ipsilateral to the site of dissection. Other series have reported additional symptoms, including groin and/or testicular pain, headache, nausea, vomiting, fever, dysuria, hematuria and blurry vision [6, 33, 35]. These symptoms are unspecific and may also be present in patients with nephrolithiasis, a very common disease. Among the laboratory findings, increased serum creatinine, leukocytosis and markedly increased level of serum lactate dehydrogenase (LDH) may reflect renal parenchymal cell death [36, 37].
In an exhaustive review of published reports, up to 29% of all cases (42 cases) were diagnosed during autopsy, and the rest were diagnosed based on arteriography or during surgery following an abnormal but non-diagnostic angiography [33]. There is general agreement that the definite diagnosis is most commonly achieved by catheter arteriography. However, recent advances in multi-detector CT and magnetic angiography make renal artery evaluation possible in a robust and reliable way. It is also a relatively more efficient, safe and inexpensive procedure than invasive catheter angiography [11, 38, 39]. In our report, duplex ultrasound was applied in four cases in conjunction with definite diagnostic modalities, and could show a non-specific pattern of interruption in segmental blood flow in two cases (Figure 2).
Treatment options for SRAD include supportive medical management, endovascular procedures and open surgical operations [8, 32, 40–43]. Medical management is the preferred method of management, when further invasive procedures can be avoided. This includes pain management, control of hypertension, systemic anticoagulation and management of coexistent symptoms [7, 35, 43]. There is still controversy surrounding the role of anticoagulation. Pellerin et al. argue for a beneficial role of thrombosis of the false lumen preventing the risk of occlusion of the true lumen, which may be hampered by systemic anticoagulation [40]. On the other hand, there is general agreement for control of hypertension with a goal of systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg. In the absence of acute kidney injury or rise of serum creatinine to <30%, angiotensin-converting enzyme inhibition or angiotensin receptor blockade may be beneficial particularly in the presence of evidence for survival benefit in renovascular atherosclerosis [44].
Endovascular procedures are described in several reports and series [40, 45–48]. In a small series of only three patients with RAD and obstruction of the artery in the setting of trauma, a self-expanding stent was deployed through a guide wire with immediate restoration of blood flow and stable kidney function at 23–30 months of follow-up [45]. In cases of dissection with luminal thrombosis, thrombolysis was performed, followed by stent placement and hemodynamic stabilization [46, 47]. In another series of 16 consecutive patients with SRAD with a mean age of 42 years, all patients underwent endovascular stenting irrespective of severity of dissection [40]. The authors have reported normal blood pressure at 8.6 years of follow-up with normal-level plasma creatinine and no sign of restenosis or occlusion in any of the patients.
In the setting of aneurysm formation of the renal artery and/or rupture or leak from the aneurysm particularly in the presence of renovascular hypertension and in women of child-bearing age, renal artery embolization has been successfully employed [49, 50].
Surgical repair as the definitive treatment was reported in few prior studies [8, 9, 32, 42]. Some of the proposed criteria for surgical intervention include presence of correctable dissection which causes hemodynamically significant occlusion of the main or major segmental renal arteries, uncontrolled renovascular hypertension resistant to medical treatment and significantly deteriorating renal function [35]. In a case series of 22 patients with a mean age of 41 years, Lacombe reported 17 repairs in 16 patients along with 8 nephrectomies [8]. Hypertension resolved in 9 patients (41%) and improved in 11 (50%). No death was reported during surgery, but with long-term follow-up one late thrombosis of repaired polar artery and one spontaneous dissection of the contralateral artery were observed. In the remaining eight patients, late angiography revealed hemodynamic stability with long-term follow-up. In another series of nine treated patients, there were 10 bypass procedures, including 5 complex branch reconstruction performed with 100% immediate patency in reconstructed arteries, adequate blood pressure control and restoration of normal renal function with long-term follow-up [9]. There were two deaths, each due to subarachnoid hemorrhage and septic meningitis. Muller et al. reported a series of 25 surgically repaired dissection patients of whom 22 patients had SRAD and the other 3 had after trauma. Hypertension resolved or improved in 86% of patients who did not have kidney injury prior to the operation, while this rate was only 38% in patients with preoperative kidney injury. Preservation of kidney function was noted in 82% of the revascularized kidneys, but in three kidneys late renal artery occlusion developed. Overall, our choice of selecting the surgical approach for management of SRAD is reserved for severe cases where medical management alone was insufficient to treat symptoms or preserve renal function. The renal outcomes and control of blood pressure in our study are similar to those of the other case series which have shown a relative preservation of renal function and control of blood pressure in the long-term. The overall outcome is a function of general comorbid conditions.
This is the first systematic study of RAD comparing its characteristics with those of the general population. In that regard, several novel relationships with the above details are noted. There are several limitations in our study. The sample size in the case group is small and conclusions should be drawn with caution. For this reason, we chose the entry criteria for stepwise inclusion in the structure of the multivariable model to be set at 0.01. The results may not be generalizable to other settings. Since the search for the cases was based on the application of ICD9 codes, the search strategy might have missed some cases of RAD.
In conclusion, SRAD may be seen in association with hypertension, CHF, cancer and rare connective tissue disorders. Therefore, its occurrence should prompt investigation for rare diseases such as Ehlers–Danlos syndrome and FMD. The diagnosis can be established in most cases using high-resolution CT and magnetic angiography. Medical management appears to be a reasonable choice in some patients, but severity and extent of the dissection as the degree of hemodynamic instability in the territory of the dissected artery determine the need for intervention. With appropriate intervention and follow-up, the clinical course of the disease may remain favorable.
FUNDING
F.A. is supported by the grant 5T32DK7378-32.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1.Bumpus HC., Jr A case of renal hypertension. Trans Am Assoc Genitourin Surg. 1945;37:135–140. [PubMed] [Google Scholar]
- 2.Hare WS, Kincaid-Smith P. Dissecting aneurysm of the renal artery. Radiology. 1970;97:255–263. doi: 10.1148/97.2.255. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Analytic and Reporting Guidelines. Hyattsville, MD: Centers for Disease Control and Prevention; 2006. http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf . [Google Scholar]
- 4.Conway R, Bergin D, Coughlan RJ, et al. Renal infarction due to spontaneous renal artery dissection in Ehlers-Danlos syndrome type IV. J Rheumatol. 2012;39:199–200. doi: 10.3899/jrheum.111034. doi:10.3899/jrheum.111034. [DOI] [PubMed] [Google Scholar]
- 5.Kolhe N, Downes M, O'Donnell P, et al. Renal artery dissection secondary to medial hyperplasia presenting as loin pain haematuria syndrome. Nephrol Dial Transplant. 2004;19:495–497. doi: 10.1093/ndt/gfg496. doi:10.1093/ndt/gfg496. [DOI] [PubMed] [Google Scholar]
- 6.Alamir A, Middendorf DF, Baker P, et al. Renal artery dissection causing renal infarction in otherwise healthy men. Am J Kidney Dis. 1997;30:851–855. doi: 10.1016/s0272-6386(97)90094-9. doi:10.1016/S0272-6386(97)90094-9. [DOI] [PubMed] [Google Scholar]
- 7.Edwards BS, Stanson AW, Holley KE, et al. Isolated renal artery dissection, presentation, evaluation, management, and pathology. Mayo Clin Proc. 1982;57:564–571. [PubMed] [Google Scholar]
- 8.Lacombe M. Isolated spontaneous dissection of the renal artery. J Vasc Surg. 2001;33:385–391. doi: 10.1067/mva.2001.111736. doi:10.1067/mva.2001.111736. [DOI] [PubMed] [Google Scholar]
- 9.Smith BM, Holcomb GW, III, Richie RE, et al. Renal artery dissection. Ann Surg. 1984;200:134–146. doi: 10.1097/00000658-198408000-00004. doi:10.1097/00000658-198408000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyer RW, Daily PO. Renal artery dissection associated with Gz acceleration. Aviat Space Environ Med. 2004;75:284–287. [PubMed] [Google Scholar]
- 11.Dobrilovic N, Bennett S, Smith C, et al. Traumatic renal artery dissection identified with dynamic helical computed tomography. J Vasc Surg. 2001;34:562–564. doi: 10.1067/mva.2001.116302. doi:10.1067/mva.2001.116302. [DOI] [PubMed] [Google Scholar]
- 12.Iqbal FM, Goparaju M, Yemme S, et al. Renal artery dissection following marathon running. Angiology. 2009;60:122–126. doi: 10.1177/0003319707310278. doi:10.1177/0003319707310278. [DOI] [PubMed] [Google Scholar]
- 13.Lee JT, White RA. Endovascular management of blunt traumatic renal artery dissection. J Endovasc Ther. 2002;9:354–358. doi: 10.1177/152660280200900315. doi:10.1583/1545-1550(2002)009<0354:EMOBTR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Memon S, Cheung BY. Long-term results of blunt traumatic renal artery dissection treated by endovascular stenting. Cardiovasc Intervent Radiol. 2005;28:668–669. doi: 10.1007/s00270-004-0296-x. doi:10.1007/s00270-004-0296-x. [DOI] [PubMed] [Google Scholar]
- 15.Payne SR, Snell ME. Traumatic renal artery dissection. Urology. 1988;31:335–337. doi: 10.1016/0090-4295(88)90094-5. doi:10.1016/0090-4295(88)90094-5. [DOI] [PubMed] [Google Scholar]
- 16.Sharples EJ, Sobeh M, Matson M, et al. Renal artery dissection after blunt abdominal trauma: a rare cause of acute cortical necrosis. Am J Kidney Dis. 2002;40:E11. doi: 10.1053/ajkd.2002.34937. doi:10.1053/ajkd.2002.34937. [DOI] [PubMed] [Google Scholar]
- 17.Springer F, Schmehl J, Heller S, et al. Delayed endovascular treatment of renal artery dissection and reno-vascular hypertension after blunt abdominal trauma. Cardiovasc Intervent Radiol. 2011;34:1094–1097. doi: 10.1007/s00270-011-0133-y. doi:10.1007/s00270-011-0133-y. [DOI] [PubMed] [Google Scholar]
- 18.Thomas MC, Walker RJ, Packer S. Running repairs: renal artery dissection following extreme exertion. Nephrol Dial Transplant. 1999;14:1258–1259. doi: 10.1093/ndt/14.5.1258. doi:10.1093/ndt/14.5.1258. [DOI] [PubMed] [Google Scholar]
- 19.Edmondson DA, Towne JB, Foley DW, et al. Cocaine-induced renal artery dissection and thrombosis leading to renal infarction. WMJ. 2004;103:66–69. [PubMed] [Google Scholar]
- 20.Orhan O, Kultigin T, Osman K, et al. An exceedingly rare cause of secondary hypertension: bilateral renal artery dissection possibly secondary to extracorporeal shock-wave lithotripsy (ESWL) Intern Med. 2011;50(21):2633–2636. doi: 10.2169/internalmedicine.50.5351. doi:10.2169/internalmedicine.50.5351. [DOI] [PubMed] [Google Scholar]
- 21.Meyers DS, Grim CE, Keitzer WF. Fibromuscular dysplasia of the renal artery with medial dissection. A case simulating polyarteritis nodosa. Am J Med. 1974;56:412–416. doi: 10.1016/0002-9343(74)90624-x. doi:10.1016/0002-9343(74)90624-X. [DOI] [PubMed] [Google Scholar]
- 22.Stanley JC, Wakefield TW. Arterial fibrodysplasia. In: Rutherford RB, editor. Vascular Surgery. 6th edn. Philadelphia: PA, Saunders; 2005. pp. 431–452. [Google Scholar]
- 23.Stanley JC, Gewertz BL, Bove EL, et al. Arterial fibrodysplasia. Histopathologic character and current etiologic concepts. Arch Surg. 1975;110:561–566. doi: 10.1001/archsurg.1975.01360110107018. [DOI] [PubMed] [Google Scholar]
- 24.Gewertz BL, Stanley JC, Fry WJ. Renal artery dissections. Arch Surg. 1977;112:409–414. doi: 10.1001/archsurg.1977.01370040061009. doi:10.1001/archsurg.1977.01370040061009. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan A, Krishnamurthy G, Fontalvo-Herazo L, et al. Spectrum of renal findings in pediatric fibromuscular dysplasia and neurofibromatosis type 1. Pediatr Radiol. 2011;41:308–316. doi: 10.1007/s00247-010-1854-9. doi:10.1007/s00247-010-1854-9. [DOI] [PubMed] [Google Scholar]
- 26.Han DK, Fishman EW, Walkup MH, et al. A rare case of familial carotid body tumor in a patient with bilateral fibromuscular dysplasia. J Vasc Surg. 2010;52:746–750. doi: 10.1016/j.jvs.2010.04.028. doi:10.1016/j.jvs.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez-Caballero PE, Servia M, Marsal-Alonso C. Fibromuscular dysplasia associated with cardiac fibroelastoma and blepharophimosis. Rev Neurol. 2009;49:614–615. [PubMed] [Google Scholar]
- 28.Madersbacher S, Ponholzer A, Franz K, et al. Synchronous bilateral renal cell cancer with a single ovarian metastasis and a fibromuscular dysplasia of the renal artery. Aktuelle Urol. 2007;38:52–54. doi: 10.1055/s-2006-932159. doi:10.1055/s-2006-932159. [DOI] [PubMed] [Google Scholar]
- 29.Stoll G. A combination of multiple cerebral aneurysms, fibromuscular dysplasia and multiple spinal hemangioblastomas. A random or a dysontogenetically explicable coincidence? Rofo. 1991;155:194–196. doi: 10.1055/s-2008-1033245. doi:10.1055/s-2008-1033245. [DOI] [PubMed] [Google Scholar]
- 30.de Mendonca WC, Espat PA. Pheochromocytoma associated with arterial fibromuscular dysplasia. Am J Clin Pathol. 1981;75:749–754. doi: 10.1093/ajcp/75.5.749. [DOI] [PubMed] [Google Scholar]
- 31.Ando T, Ohno H, Hirata Y, et al. Spontaneous recovery from renal infarction resulting from renal artery dissection. Int J Urol. 2005;12:405–408. doi: 10.1111/j.1442-2042.2005.01062.x. doi:10.1111/j.1442-2042.2005.01062.x. [DOI] [PubMed] [Google Scholar]
- 32.van Rooden CJ, van Baalen JM, van Bockel JH. Spontaneous dissection of renal artery: long-term results of extracorporeal reconstruction and autotransplantation1. J Vasc Surg. 2003;38:116–122. doi: 10.1016/s0741-5214(02)75453-0. doi:10.1016/S0741-5214(02)75453-0. [DOI] [PubMed] [Google Scholar]
- 33.Beroniade V, Roy P, Froment D, et al. Primary renal artery dissection. Presentation of two cases and brief review of the literature. Am J Nephrol. 1987;7:382–389. doi: 10.1159/000167504. doi:10.1159/000167504. [DOI] [PubMed] [Google Scholar]
- 34.Debie B, Hammer F, Dahan K, et al. Rupture of the renal artery and renal parenchyma in a pregnant woman with vascular form of Ehlers-Danlos syndrome. Prog Urol. 2005;15:303–305. [PubMed] [Google Scholar]
- 35.Stawicki SP, Rosenfeld JC, Weger N, et al. Spontaneous renal artery dissection: three cases and clinical algorithms. J Hum Hypertens. 2006;20:710–718. doi: 10.1038/sj.jhh.1002045. doi:10.1038/sj.jhh.1002045. [DOI] [PubMed] [Google Scholar]
- 36.Bolderman R, Oyen R, Verrijcken A, et al. Idiopathic renal infarction. Am J Med. 2006;119:356.e9–356.e12. doi: 10.1016/j.amjmed.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 37.Winzelberg GG, Hull JD, Agar JW, et al. Elevation of serum lactate dehydrogenase levels in renal infarction. JAMA. 1979;242:268–269. doi:10.1001/jama.1979.03300030040019. [PubMed] [Google Scholar]
- 38.Muroya T, Koga S, Maemura K. Chronic renal artery dissection with aneurysm formation treated by stent implantation with coil embolization with detailed intravascular ultrasound evaluation. Catheter Cardiovasc Interv. 2012;81:574–577. doi: 10.1002/ccd.24308. [DOI] [PubMed] [Google Scholar]
- 39.Casciani E, Polettini E, Masselli G, et al. Spontaneous renal artery dissection diagnosed by unenhanced magnetic resonance angiography: case report. Urol Int. 2012;89:486–488. doi: 10.1159/000339751. [DOI] [PubMed] [Google Scholar]
- 40.Pellerin O, Garcon P, Beyssen B, et al. Spontaneous renal artery dissection: long-term outcomes after endovascular stent placement. J Vasc Interv Radiol. 2009;20:1024–1030. doi: 10.1016/j.jvir.2009.04.069. doi:10.1016/j.jvir.2009.04.069. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-Kerim A, Cassagnes L, Alfidja A, et al. Management of isolated non-traumatic renal artery dissection: report of four cases. Acta Radiol. 2012;53:401–405. doi: 10.1258/ar.2012.110303. doi:10.1258/ar.2012.110303. [DOI] [PubMed] [Google Scholar]
- 42.Muller BT, Reiher L, Pfeiffer T, et al. Surgical treatment of renal artery dissection in 25 patients: indications and results. J Vasc Surg. 2003;37:761–768. doi: 10.1067/mva.2003.171. doi:10.1067/mva.2003.171. [DOI] [PubMed] [Google Scholar]
- 43.Ramamoorthy SL, Vasquez JC, Taft PM, et al. Nonoperative management of acute spontaneous renal artery dissection. Ann Vasc Surg. 2002;16:157–162. doi: 10.1007/s10016-001-0154-0. doi:10.1007/s10016-001-0154-0. [DOI] [PubMed] [Google Scholar]
- 44.Chrysochou C, Foley RN, Young JF, et al. Dispelling the myth: the use of renin-angiotensin blockade in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27:1403–1409. doi: 10.1093/ndt/gfr496. doi:10.1093/ndt/gfr496. [DOI] [PubMed] [Google Scholar]
- 45.Chabrot P, Cassagnes L, Alfidja A, et al. Revascularization of traumatic renal artery dissection by endoluminal stenting: three cases. Acta Radiol. 2010;51:21–26. doi: 10.3109/02841850903473314. doi:10.3109/02841850903473314. [DOI] [PubMed] [Google Scholar]
- 46.Jeon YS, Cho SG, Hong KC. Renal infarction caused by spontaneous renal artery dissection: treatment with catheter-directed thrombolysis and stenting. Cardiovasc Intervent Radiol. 2009;32:333–336. doi: 10.1007/s00270-008-9465-7. doi:10.1007/s00270-008-9465-7. [DOI] [PubMed] [Google Scholar]
- 47.Lupattelli T, Basile A, Iozzelli A, et al. Thrombolytic therapy followed by stenting for renal artery dissection secondary to blunt trauma. Emerg Radiol. 2005;11:164–166. doi: 10.1007/s10140-004-0390-z. doi:10.1007/s10140-004-0390-z. [DOI] [PubMed] [Google Scholar]
- 48.Peynircioglu B, Piskinkaya S, Ozer C, et al. Isolated spontaneous renal artery dissection: diagnosis and endovascular management. Diagn Interv Radiol. 2011;17:101–104. doi: 10.4261/1305-3825.DIR.2786-09.1. [DOI] [PubMed] [Google Scholar]
- 49.Somarouthu B, Rabinov J, Waichi W, et al. Stent-assisted coil embolization of an intraparenchymal renal artery aneurysm in a patient with neurofibromatosis. Vasc Endovascular Surg. 2011;45:368–371. doi: 10.1177/1538574411403327. doi:10.1177/1538574411403327. [DOI] [PubMed] [Google Scholar]
- 50.Mavili E, Donmez H, Ozcan N, et al. Transarterial embolization for renal arterial bleeding. Diagn Interv Radiol. 2009;15:143–147. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.