Abstract
Challenges in the accurate measurement of sexual behavior in human immunodeficiency virus (HIV) prevention research are well documented and have prompted discussion about whether valid assessments are possible. Audio computer-assisted self-interviewing (ACASI) may increase the validity of self-reported behavioral data. In 2006–2007, Zimbabwean women participated in a randomized, cross-sectional study that compared self-reports of recent vaginal sex and condom use collected through ACASI or face-to-face interviewing (FTFI) with a validated objective biomarker of recent semen exposure (prostate-specific antigen (PSA) levels). Of 910 study participants, 196 (21.5%) tested positive for PSA, an indication of semen exposure during the previous 2 days. Of these 196 participants, 23 (11.7%) reported no sex in the previous 2 days, with no difference in reported sexual activity between interview modes (12.5% ACASI vs. 10.9% FTFI; Fisher's exact test: P = 0.72). In addition, 71 PSA-positive participants (36.2%) reported condom-protected vaginal sex only; their reports also indicated no difference between interview modes (33.7% ACASI vs. 39.1% FTFI; P = 0.26). Only 52% of PSA-positive participants reported unprotected sex during the previous 2 days. Self-report was a poor predictor of recent sexual activity and condom use in this study, regardless of interview mode, providing evidence that such data should be interpreted cautiously.
Keywords: biological markers, condoms, data collection, epidemiologic measurements, HIV, prostate-specific antigen, sexual behavior
The challenges of obtaining accurate measures of self-reported sexual behavior in sexually transmitted infection and human immunodeficiency virus (HIV) prevention research are well documented. Study participants may provide inaccurate reports of their sexual behavior for a variety of reasons, including social desirability bias (e.g., as a result of repeated counseling messages related to condom and study product use), poor recall, concerns about being dropped from the study if they report low levels of sexual activity or product use, or poorly worded questions or response categories (1–4). The need for accurate behavioral data remains important for interpreting results from product effectiveness studies (to differentiate poor adherence from lack of product efficacy) and for assessing product safety (5). Furthermore, reliable behavioral outcomes may be needed to evaluate interventions conducted in settings or among population groups where disease incidence is low or where behavior change is the key outcome of interest (6, 7). Nonetheless, persistent difficulties in collecting accurate behavioral data on sexual activity and condom use have prompted some investigators to question whether the collection of valid behavioral data is even possible.
Interviewer-administered assessments are the standard data collection mode used to ascertain sexual behavior in sexually transmitted infection or HIV prevention research. However, researchers have adopted audio computer-assisted self-interviewing (ACASI) because it has shown promise in increasing the accuracy of self-reported behaviors through the use of a self-administered questionnaire that affords privacy (by not requiring an interviewer or a written record of responses). In addition, ACASI can be designed for low-literacy populations and can accommodate questionnaires with complex skip patterns (8, 9). Evaluations of ACASI to date have been designed as comparisons of responses between data collection modes (e.g., ACASI vs. face-to-face interviewing (FTFI)), with inferences regarding validity being based on the assumption that higher reports of sensitive behaviors are more accurate. Numerous randomized studies, mostly conducted in the United States, have shown higher levels of reported risky sex-related behaviors when ACASI is employed compared with FTFI. However, other studies have found inconsistent results (lower reports of risky behavior with ACASI or no differences between interview modes) depending on the behavior assessed (10–20). Because of the lack of a standard in self-report measures, the use of an objective biomarker of sexual activity is needed for direct evaluation of the effect of ACASI on the validity of behavioral assessments.
We conducted a study among Zimbabwean women to compare the validity of reports of recent vaginal sex (with and without condoms) as measured by ACASI with the validity of reports measured by FTFI. We used the detection of prostate-specific antigen (PSA), a biomarker with high positive predictive value (21), as the reference standard for recent semen exposure and hypothesized that ACASI would yield a lower proportion of discrepant results (e.g., PSA-positive but no recent sexual activity reported) than FTFI.
MATERIALS AND METHODS
Study design
This study consisted of a cross-sectional assessment of recent sexual activity and condom use. It was conducted from December 2006 through June 2007 among a subset of women who had recently completed participation in the Methods for Improving Reproductive Health in Africa (MIRA) Trial. MIRA was a large randomized controlled trial that examined the protective effects of diaphragms plus lubricant gel in decreasing susceptibility to HIV. The trial was conducted among sexually active HIV-negative women aged 18–49 years at 3 sites in South Africa and Zimbabwe (22). Women participated in our study a median of 8.8 months (range, 2.5–20.5) after their final MIRA study visit. Study participants provided a self-obtained vaginal swab that was tested for PSA and were randomized to complete a short behavioral interview administered through either ACASI or FTFI. The interview comprised questionnaire items similar to those completed during the MIRA trial.
Study participants and data collection
Former MIRA Trial participants from the 2 Zimbabwe MIRA study clinics in Chitungwiza and Epworth were eligible for participation. Women learned about the PSA study at their final MIRA study visit, through community outreach, or during post-MIRA clinic visits (e.g., visits made to obtain additional condoms). Women who expressed interest were encouraged to return to the MIRA clinic to consent to and enroll in the PSA study. Only nonpregnant former MIRA participants who had not had a vaginal delivery or third-trimester stillbirth in the previous 6 weeks were eligible for enrollment. All participants gave written informed consent prior to enrollment in the PSA study. Institutional review boards at the University of California, San Francisco, the Medical Research Council of Zimbabwe, and Family Health International approved the study.
Participants were randomized to either the ACASI group or the FTFI group by means of sequentially numbered, sealed opaque envelopes containing randomization assignments. Using their assigned mode of interviewing, participants answered questions about their sexual activity and condom use in the past 7 days. They also were asked about the following problems with condom use: breakage, slippage, and spillage. Participants then provided a self-collected vaginal swab by following instructions developed for a MIRA ancillary study (designed to examine whether diaphragm use conferred protection against human papillomavirus) in which most of the PSA study participants had taken part. Vaginal swabs were air-dried and shipped in batches to a research laboratory at the University of North Carolina, Chapel Hill, for PSA testing.
PSA detection
PSA is produced by the prostate gland and secreted into the urethra during ejaculation. The protein can be detected in vaginal fluid specimens collected after exposure to semen and has high positive predictive value (21, 23, 24). PSA begins to clear from the vaginal fluid promptly after exposure (e.g., PSA is only detectable in an estimated 29% of women 24 hours after known exposure) and is rarely present beyond 48 hours after semen exposure (21). Because of the rapid clearance of PSA, our analysis focused on discrepant reporting among women who tested PSA-positive only (i.e., no report of recent intercourse or condom-protected intercourse only and a PSA-positive test result). We used the Abbott Laboratories IMx assay (Abbott Laboratories, Abbott Park, Illinois) to detect PSA in the vaginal swab specimens. PSA levels greater than 1.0 ng/mL indicate semen exposure within the past 48 hours (21).
For recovery of vaginal secretions, each swab was placed into 3.0 mL of phosphate-buffered saline, incubated at room temperature for 15–30 minutes, and then agitated and pressed against the side of the tube to elute the sample. To avoid including swabs that had not been used to collect vaginal fluid, we scored vaginal swab eluates from self-obtained specimens for the presence of epithelial cells. A small volume of specimen (0.01 mL) was loaded into the chamber of a hemacytometer and examined at 200× magnification in a Nikon Labphot 2 light microscope (Nikon Instruments, Inc., Melville, New York). Epithelial cells were present in all self-obtained vaginal swab eluates. Vaginal specimens were then centrifuged at 250 × g for 10 minutes, and supernatants were removed from cell pellets and stored at −80°C until testing. Supernatants (0.20 mL) from vaginal swab eluates were tested using the IMx PSA assay according to the manufacturer's instructions. The enzyme immunoassay measures PSA concentrations from 0.04 ng/mL to 50 ng/mL. Samples with initial test results greater than 50 ng/mL were diluted 1:100 with phosphate-buffered saline and retested to obtain PSA concentrations.
Analysis
The analysis population consisted of participants who provided a vaginal swab specimen for PSA testing and completed the sexual behavior interview. Data on participant sociodemographic characteristics and sexual history were extracted from the baseline MIRA study visit. We used chi-squared tests to compare the proportions of women who reported having vaginal sex and condom-protected vaginal sex in the past 2 days via ACASI with the proportions who reported those activities via FTFI. To compare self-reported behaviors with PSA test results, we conducted a 1-sided Fisher's exact test, on the basis of our hypothesis that ACASI would yield increased reporting of sensitive behaviors (i.e., no recent vaginal sex and vaginal sex unprotected by a condom) and thus lower the proportion of results that were discrepant with the PSA test results as compared with FTFI. We established our sample size to ensure 80% power to detect a 33% reduction in discordance (reporting no vaginal sex in the previous 2 days but testing PSA-positive) by ACASI and assumed that the discordance in the FTFI group would be 20% (1-sided test; α = 0.05). Our primary endpoint was defined as reporting no sex for the past 2 days yet testing positive for PSA. Secondary endpoints included having a PSA-positive test result yet reporting: 1) only vaginal sex protected by a condom; 2) only vaginal sex protected by a condom that was used without breakage, slippage, or spillage of semen; and 3) no vaginal sex in the previous 7 days. To assess the robustness of our findings to different PSA levels, we evaluated a semiquantitative measure by using 4 categories of PSA concentration as evidence of PSA positivity (1.1–5.0 ng/mL, 5.1–25.0 ng/mL, 25.1–100.0 ng/mL, and >100.0 ng/mL). Our findings for all study endpoints were consistent with those obtained using the dichotomous cutpoint of >1.0 ng/mL (i.e., P values remained nonsignificant); therefore, we present only the results of the primary analysis.
RESULTS
Study participants
The PSA study included 918 former participants from the MIRA Trial (36.7% of the MIRA Zimbabwe sample). Approximately equal numbers of participants were assigned to the ACASI and FTFI groups. Eight women in the ACASI arm did not complete their interviews because of loss of electrical power for several consecutive days at 1 site. Consequently, the analysis population consisted of 910 participants (450 ACASI and 460 FTFI). At the time of entry into the MIRA Trial, most PSA study participants were married (96.6%) and had not completed high school (55.7%). Participants reported a mean of 1.3 lifetime sexual partners (range, 1–5) and a mean age of 18.5 years at first sexual intercourse (range, 10–28). The baseline characteristics of women in the ACASI and FTFI groups were similar (Table 1).
Table 1.
Sociodemographic Characteristics, Reproductive History, and Self-Reported Sexual Behaviora Among 910 Sexually Active Women, by Randomization Group, Prostate-Specific Antigen Study, Zimbabwe, 2006–2007
| ACASI group (n = 450) |
FTFI group (n = 460) |
|||
| No. | % | No. | % | |
| Age, years | ||||
| 18–24 | 131 | 29.1 | 157 | 34.1 |
| 25–34 | 219 | 48.7 | 215 | 46.8 |
| ≥35 | 100 | 22.2 | 88 | 19.1 |
| Education | ||||
| Less than high school | 253 | 56.2 | 254 | 55.2 |
| High school or more | 197 | 43.8 | 206 | 44.8 |
| Earned income in past year | ||||
| Yes | 335 | 74.4 | 360 | 78.3 |
| No | 115 | 25.6 | 100 | 21.7 |
| Married | ||||
| Yes | 438 | 97.3 | 441 | 95.9 |
| No | 12 | 2.7 | 19 | 4.1 |
| Mean lifetime no. of sexual partners (range) | 1.3 (1–5) | 1.3 (1–4) | ||
| Mean age at first sexual intercourse, years (range) | 18.5 (10–28) | 18.5 (13–28) | ||
| Coital frequency, no. of times per week | ||||
| ≤3 | 238 | 52.9 | 255 | 55.4 |
| >3 | 212 | 47.1 | 205 | 44.6 |
| Tested positive for sexually transmitted infectionb | ||||
| Yes | 33 | 7.3 | 30 | 6.5 |
| No | 417 | 92.7 | 430 | 93.5 |
| Tested positive for herpes simplex virus type 2c | ||||
| Yes | 238 | 53.1 | 239 | 52.0 |
| No | 210 | 46.9 | 221 | 48.0 |
| High behaviorial riskd | ||||
| Yes | 107 | 23.8 | 117 | 25.4 |
| No | 343 | 76.2 | 343 | 74.6 |
| Condom use in past 3 months | ||||
| Never | 122 | 27.1 | 131 | 28.5 |
| Sometimes | 199 | 44.2 | 206 | 44.8 |
| Always | 129 | 28.7 | 123 | 26.7 |
| Primary type of contraceptive use at screeninge | ||||
| Tubal ligation, vasectomy, intrauterine device, implants | 11 | 2.4 | 17 | 3.7 |
| Injectable hormones | 63 | 14.0 | 67 | 14.6 |
| Oral contraceptives | 279 | 62.0 | 293 | 63.7 |
| Male or female condoms | 58 | 12.9 | 55 | 12.0 |
| Other/none | 39 | 8.7 | 28 | 6.1 |
| MIRA Trial randomization group | ||||
| Diaphragm, gel, and condom | 216 | 48.0 | 226 | 49.1 |
| Condom-only | 234 | 52.0 | 234 | 50.9 |
Abbreviations: ACASI, audio computer-assisted self-interviewing; FTFI, face-to-face interviewing; MIRA, Methods for Improving Reproductive Health in Africa.
Measured at baseline for the MIRA Trial.
At least 1 positive test for Neisseria gonorrhoeae, Chlamydia trachomatis, or Trichomonas vaginalis at baseline in the MIRA Trial.
Data on herpes simplex virus type 2 status were missing for 2 subjects because of missing laboratory test results.
At least 1 of the following: any exchange of sex for money, food, drugs, or shelter; ≥2 sexual partners within the past 3 months; having vaginal sex under the influence of drugs or alcohol in the past 3 months; ever using a needle for injectable drug use; and ever having anal sex.
Participants were classified on the basis of the most effective method reported.
Self-reported recent sexual activity
Thirty-four percent of the women (n = 310) reported having no vaginal sex in the past 2 days, and 36.4% (n = 331) reported using a condom for all vaginal sex acts during this period. The proportion of women who reported no vaginal sex did not vary between interview modes (34.0% ACASI vs. 34.4% FTFI; P = 0.48), but the proportion who reported condom-protected sex only was lower in the ACASI group than in the FTFI group (32.7% vs. 40.0%; P = 0.01).
PSA detection and discordance between self-reported behaviors and PSA results
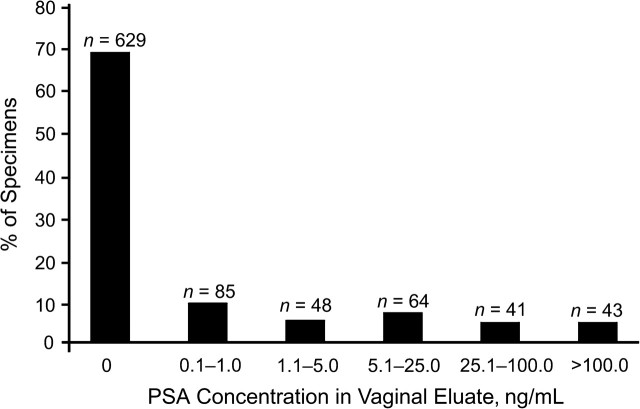
We found 21.5% of participants (n = 196) to be PSA-positive, and the proportions of PSA-positive results did not differ by randomization group (P = 0.25). The median PSA concentration among participants who tested positive (>1 ng/mL) was 15.7 ng/mL (interquartile range, 5.3–82.6). The distribution of positive PSA values is depicted in Figure 1. Twenty-three (11.7%) participants who tested positive for PSA reported having no vaginal sex in the previous 2 days, and we observed no difference between interview modes (12.5% ACASI vs. 10.9% FTFI; P = 0.72; Table 2). Seventy-one (36.2%) participants who tested positive for PSA reported condom-protected sex only, and no difference by interview mode was observed (33.7% ACASI vs. 39.1% FTFI; P = 0.26). Five of these 71 participants experienced condom breakage (n = 2), slippage (n = 1), or semen spillage (n = 2) during this time period; no difference between interview modes was observed. Thus, reports of condom problems also did not explain discordant results in general (the overall level of discordance was reduced from 48% to 45% when these women were excluded) or between the ACASI and FTFI groups. Finally, among the PSA-positive participants, 7.7% (n = 15) reported having no vaginal sex over the prior 7 days. However, the samples were too small for us to make meaningful comparisons between interview modes.
Figure 1.
Distribution of prostate-specific antigen (PSA) concentrations among 910 sexually active women who completed an interview about sexual activity and condom use in the past 7 days, Prostate-Specific Antigen Study, Zimbabwe, 2006–2007. PSA concentrations greater than 1.0 ng/mL were considered as providing evidence of semen exposure within the past 2 days.
Table 2.
Biomarker Validation (by PSA Testinga) of Reports of Sexual Activity and Condom Use Among Sexually Active Womenb, Prostate-Specific Antigen Study, Zimbabwe, 2006–2007
| Reported Sexual Activity During the Past 2 Days | Randomization Group |
Total (n = 196) |
P Valuec | ||||
| ACASI (n = 104) |
FTFI (n = 92) |
||||||
| No. | % | No. | % | No. | % | ||
| No vaginal sex | 13 | 12.5 | 10 | 10.9 | 23 | 11.7 | 0.72 |
| Sex protected by a male or female condom only | 35 | 33.7 | 36 | 39.1 | 71 | 36.2 | 0.26 |
| Total | 48 | 46.2 | 46 | 50.0 | 94 | 48.0 | 0.35 |
Abbreviations: ACASI, audio computer-assisted self-interviewing; FTFI, face-to-face interviewing; PSA, prostate-specific antigen.
PSA concentrations greater than 1.0 ng/mL were considered as providing evidence of semen exposure within the past 2 days.
Analysis was restricted to the subset of women (n = 196) who tested positive for PSA (>1 ng/mL) in vaginal eluate.
1-sided Fisher's exact test.
DISCUSSION
In this study, we found substantial levels of discrepant reporting of recent vaginal sex and sex with a condom relative to the presence of a validated marker of recent semen exposure. Nearly half of participants with biologic evidence of recent semen exposure reported either that they had not had sex (12%) or that they had had condom-protected sex only (36%) during the previous 2 days. Direct comparison of self-reports between interview modes in the absence of the biomarker suggested that ACASI yielded lower reports of consistent condom use during recent sexual activity—ostensibly an indication that ACASI had a modest effect on improving the accuracy of self-reports. Nevertheless, as compared with FTFI, ACASI did not improve the level of concordance between self-reports and the PSA biomarker results.
The high level of discrepant reporting in this study, regardless of interview mode, highlights the question of whether sufficiently valid self-reported behavioral data can be obtained in HIV prevention research or in other studies that rely on reports of sexual activity. Our findings corroborate those from several studies, including 2 that found high levels of discrepancy between reports of condom use and PSA positivity among female sex workers in Kenya and Madagascar (25, 26); 1 that found a substantial discrepancy between reports of condom use and the presence of Y chromosome in the vagina (another biomarker of recent sexual behavior) among adolescents in the United States (27); and another that identified detectable spermatozoa in a substantial proportion of women reporting consistent condom use in a microbicide clinical trial in South Africa (28). The consistency of apparent overreporting of condom use or underreporting of sexual activity (even with ACASI data collection (27)), as corroborated by the presence of a biomarker of recent sexual activity, is worthy of further exploration. These results point to several possible explanations, including perceived stigma or social desirability bias associated with admitting nonadherence to HIV prevention strategies (e.g., condom use, partner reduction, or product use in a microbicide trial) that may prompt intentional misreporting (29); poor recall, even of recent sexual activity; complex sexual behavior that cannot be captured using standard quantitative instruments (30); or poorly worded questions that can be misinterpreted by study participants.
Given these seemingly intractable problems with behavioral assessments of sexual activity, particularly condom use, should we simply conclude that such data cannot be collected at all? In part this depends upon the research questions being investigated and the availability of adequate biomarkers for assessing the behaviors of interest. A biomarker like PSA detects only recent semen exposure and constitutes a measure distinct from those that assess condom-use behaviors more thoroughly. Because of user errors and mechanical failures, some exposure to semen does occur with condom use (31–33), although that did not seem to influence results here. Future in-depth qualitative interviews (which we were not able to conduct) with study participants for whom discrepancies between PSA test results and self-reported sexual behavior were identified might aid in the interpretation of these findings and guide subsequent decisions on questionnaire wording and on the influence of technological innovations like ACASI.
Several issues should be noted in interpreting our findings. First, although PSA has proven to be a valid biomarker of recent semen exposure (21), low levels of PSA could in fact reflect false-positive results due to specimen contamination (34). However, the proportions of discrepant responses overall, and between ACASI and FTFI, were comparable when we examined a semiquantitative measure of PSA that permitted comparison at 4 PSA concentrations, indicating that our results are robust to the PSA cutpoint chosen. Second, because our measure of consistent and correct condom use was incomplete (in that we did not evaluate the timing of use and whether condoms may have been applied following onset of genital contact or removed prior to ejaculation), we might have overestimated the level of discrepant results for a minority of participants who may have used condoms but did so incorrectly. Previous studies suggest that these problems may be common (35); however, delayed application of condoms is unlikely to explain our findings, because PSA levels in preejaculate are below the threshold we used for defining PSA positivity (36).
In contrast, it is far more likely that we underestimated the overall prevalence of discrepant results (and possible misreporting) in our study population because of the rapid decay curve for PSA (21), which generally is not detectable after 24 hours for the majority of samples. As a result, some participants who reported no sexual activity over the last 2 days may have had recent intercourse but with enough lead time for their PSA result to be negative. Even if this had occurred, it is unlikely that this underestimation would differ between the randomized groups, and thus it cannot explain the lack of difference between interview modes. Finally, because reporting of sexual history and other sensitive behaviors probably varies across populations and settings, our findings may have limited generalizability for measuring the utility of ACASI as compared with FTFI, especially given that women in the study had all previously participated in an HIV prevention trial.
In this study, ACASI did not improve the validity of reports of recent sexual activity and condom use over FTFI based on comparison of self-reports with a validated biomarker of semen exposure. Overall levels of discordance between self-reports of sexual behavior and the presence of a PSA biomarker were high. These findings underscore the limitations of relying exclusively on self-reported sexual behavioral data and highlight the need for cautious interpretation of behavioral data on condom use collected using current approaches. Objective biologic measures of sexual activity and product use (37) that can be employed in research that requires valid assessments of sexual activity are urgently needed.
Acknowledgments
Author affiliations: Women's Global Health Imperative, RTI International, San Francisco, California (Alexandra M. Minnis, Ariane van der Straten, Nancy S. Padian); School of Public Health, University of California, Berkeley, Berkeley, California (Alexandra M. Minnis, Nancy S. Padian); Family Health International, Research Triangle Park, North Carolina (Markus J. Steiner); Division of Reproductive Health, Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia (Maria F. Gallo, Lee Warner, Maurizio Macaluso); Division of Infectious Diseases, School of Medicine, University of North Carolina, Chapel Hill, North Carolina (Marcia M. Hobbs); Center for AIDS Prevention Studies, Department of Medicine, University of California, San Francisco, San Francisco, California (Ariane van der Straten); and UZ-UCSF Research Programme in Women's Health, University of Zimbabwe, Harare, Zimbabwe (Tsungai Chipato).
This work was supported by the US Agency for International Development (cooperative agreement GPO-A-OO-05-00022-00); the Contraceptive and Reproductive Health Technologies and Research Utilization Program; and the Bill and Melinda Gates Foundation (grant 21082).
The authors acknowledge the leadership of the study coordinators in Zimbabwe: Agnes Chidanyika, Precious Moyo, and Constancia Watadzaushe; Kate Clouse, the US-based study coordinator; and the MIRA Trial team members, who facilitated field implementation of this study. They also thank Jennifer Balkus for programming the ACASI questionnaire and Tiffany Bailey for editing the final manuscript.
Conflict of interest: none declared.
Glossary
Abbreviations
- ACASI
audio computer-assisted self-interviewing
- FTFI
face-to-face interviewing
- HIV
human immunodeficiency virus
- MIRA
Methods for Improving Reproductive Health in Africa
- PSA
prostate-specific antigen
References
- 1.Fenton KA, Johnson AM, McManus S, et al. Measuring sexual behaviour: methodological challenges in survey research. Sex Transm Infect. 2001;77(2):84–92. doi: 10.1136/sti.77.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham CA, Crosby RA, Sanders SA, et al. Assessment of condom use in men and women. Annu Rev Sex Res. 2005;16:20–52. [PubMed] [Google Scholar]
- 3.O'Sullivan LF. Challenging assumptions regarding the validity of self-report measures: the special case of sexual behavior. J Adolesc Health. 2008;42(3):207–208. doi: 10.1016/j.jadohealth.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Weinhardt LS, Forsyth AD, Carey MP, et al. Reliability and validity of self-report measures of HIV-related sexual behavior: progress since 1990 and recommendations for research and practice. Arch Sex Behav. 1998;27(2):155–180. doi: 10.1023/a:1018682530519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss HA, Wasserheit JN, Barnabas RV, et al. Persisting with prevention: the importance of adherence for HIV prevention. Emerg Themes Epidemiol. 2008;5:8. doi: 10.1186/1742-7622-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross DA, Changalucha J, Obasi AI, et al. Biological and behavioural impact of an adolescent sexual health intervention in Tanzania: a community-randomized trial. AIDS. 2007;21(14):1943–1955. doi: 10.1097/QAD.0b013e3282ed3cf5. [DOI] [PubMed] [Google Scholar]
- 7.Pronyk P, Hargreaves JR, Kim JC, et al. Effect of a structural intervention for the prevention of intimate-partner violence and HIV in rural South Africa: a cluster randomised trial. Lancet. 2006;368(9551):1973–1983. doi: 10.1016/S0140-6736(06)69744-4. [DOI] [PubMed] [Google Scholar]
- 8.Mensch BS, Hewett PC, Erulkar AS. The reporting of sensitive behavior by adolescents: a methodological experiment in Kenya. Demography. 2003;40(2):247–268. doi: 10.1353/dem.2003.0017. [DOI] [PubMed] [Google Scholar]
- 9.Langhaug L, Cheung YB, Pascoe SJ, et al. Comparing four questionnaire delivery methods for collection of self-reported sexual behavior data in rural Zimbabwean youth. (Abstract 0-038) Presented at the 17th Meeting of the International Society for Sexually Transmitted Diseases Research, Seattle, Washington, July 29–August 1, 2007. [Google Scholar]
- 10.Hewett PC, Mensch BS, Erulkar AS. Consistency in the reporting of sexual behaviour by adolescent girls in Kenya: a comparison of interviewing methods. Sex Transm Infect. 2004;80(suppl 2):ii43–ii48. doi: 10.1136/sti.2004.013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaya, Hindin MJ, Ahmed S. Differences in young people's reports of sexual behaviors according to interview methodology: a randomized trial in India. Am J Public Health. 2008;98(1):169–174. doi: 10.2105/AJPH.2006.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le LC, Blum RW, Magnani R, et al. A pilot of audio computer-assisted self-interview for youth reproductive health research in Vietnam. J Adolesc Health. 2006;38(6):740–747. doi: 10.1016/j.jadohealth.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Macalino GE, Celentano DD, Latkin C, et al. Risk behaviors by audio computer-assisted self-interviews among HIV-seropositive and HIV-seronegative injection drug users. AIDS Educ Prev. 2002;14(5):367–378. doi: 10.1521/aeap.14.6.367.24075. [DOI] [PubMed] [Google Scholar]
- 14.Metzger DS, Koblin B, Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. Am J Epidemiol. 2000;152(2):99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- 15.Minnis AM, Muchini A, Shiboski S, et al. Audio computer-assisted self-interviewing in reproductive health research: reliability assessment among women in Harare, Zimbabwe. Contraception. 2007;75(1):59–65. doi: 10.1016/j.contraception.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Morrison-Beedy D, Carey MP, Tu X. Accuracy of audio computer-assisted self-interviewing (ACASI) and self-administered questionnaires for the assessment of sexual behavior. AIDS Behav. 2006;10(5):541–552. doi: 10.1007/s10461-006-9081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simoes AA, Bastos FI, Moreira RI, et al. A randomized trial of audio computer and in-person interview to assess HIV risk among drug and alcohol users in Rio De Janeiro, Brazil. J Subst Abuse Treat. 2006;30(3):237–243. doi: 10.1016/j.jsat.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.van Griensven F, Naorat S, Kilmarx PH, et al. Palmtop-assisted self-interviewing for the collection of sensitive behavioral data: randomized trial with drug use urine testing. Am J Epidemiol. 2006;163(3):271–278. doi: 10.1093/aje/kwj038. [DOI] [PubMed] [Google Scholar]
- 19.Villarroel MA, Turner CF, Rogers SM, et al. T-ACASI reduces bias in STD measurements: the National STD and Behavior Measurement Experiment. Sex Transm Dis. 2008;35(5):499–506. doi: 10.1097/OLQ.0b013e318165925a. [DOI] [PubMed] [Google Scholar]
- 20.Hewett PC, Mensch BS, Ribeiro MC, et al. Using sexually transmitted infection biomarkers to validate reporting of sexual behavior within a randomized, experimental evaluation of interviewing methods. Am J Epidemiol. 2008;168(2):202–211. doi: 10.1093/aje/kwn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macaluso M, Lawson L, Akers R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999;59(3):195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 22.Padian NS, van der Straten A, Ramjee G, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007;370(9583):251–261. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaviacic M, Ablin RJ. The use of prostate specific antigen as a criterion for condom effectiveness [letter] Am J Epidemiol. 2005;162(7):704–705. doi: 10.1093/aje/kwi265. [DOI] [PubMed] [Google Scholar]
- 24.Macaluso M, Lawson ML, Warner DL. Macaluso et al. reply [letter] Am J Epidemiol. 2005;162(7):705–706. [Google Scholar]
- 25.Gallo MF, Behets FM, Steiner MJ, et al. Prostate-specific antigen to ascertain reliability of self-reported coital exposure to semen. Sex Transm Dis. 2006;33(8):476–479. doi: 10.1097/01.olq.0000231960.92850.75. [DOI] [PubMed] [Google Scholar]
- 26.Gallo MF, Behets FM, Steiner MJ, et al. Validity of self-reported ‘safe sex’ among female sex workers in Mombasa, Kenya—PSA analysis. Int J STD AIDS. 2007;18(1):33–38. doi: 10.1258/095646207779949899. [DOI] [PubMed] [Google Scholar]
- 27.Rose E, DiClemente RJ, Wingood GM, et al. The validity of teens’ and young adults’ self-reported condom use. Arch Pediatr Adolesc Med. 2009;163(1):61–64. doi: 10.1001/archpediatrics.2008.509. [DOI] [PubMed] [Google Scholar]
- 28.Gopolang FP. A comparison between self-reported condom use and presence of spermatozoa using TV in pouch and wet mount, among women enrolled in the Phase III Carraguard™ clinical trial in Cape Town. (Abstract 520) Presented at Microbicides 2008, New Delhi, India, February 24–27, 2008. [Google Scholar]
- 29.Turner AN, De Kock AE, Meehan-Ritter A. Many vaginal microbicide participants acknowledged they had misreported sensitive sexual behavior in face-to-face interviews. J Clin Epidemiol. 2009;62(7):759–765. doi: 10.1016/j.jclinepi.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Pool R. Increasing the accuracy of adherence data in the Microbicides Development Programme 301 trial. (Abstract 368) Presented at Microbicides 2008, New Delhi, India, February 24–27, 2008. [Google Scholar]
- 31.Galvão LW, Oliveira LC, Díaz J, et al. Effectiveness of female and male condoms in preventing exposure to semen during vaginal intercourse: a randomized trial. Contraception. 2005;71(2):130–136. doi: 10.1016/j.contraception.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Bahamondes L, Diaz J, Marchi NM, et al. Prostate-specific antigen in vaginal fluid after exposure to known amounts of semen and after condom use: comparison of self-collected and nurse-collected samples. Hum Reprod. 2008;23(11):2444–2451. doi: 10.1093/humrep/den283. [DOI] [PubMed] [Google Scholar]
- 33.Macaluso M, Blackwell R, Jamieson D, et al. Efficacy of the male latex condom and of the female polyurethane condom as barriers to semen during intercourse: a randomized clinical trial. Am J Epidemiol. 2007;166(1):88–96. doi: 10.1093/aje/kwm046. [DOI] [PubMed] [Google Scholar]
- 34.Steiner MJ, Feldblum PJ, Padian N. Invited commentary: condom effectiveness—will prostate-specific antigen shed new light on this perplexing problem? Am J Epidemiol. 2003;157(4):298–300. doi: 10.1093/aje/kwf213. [DOI] [PubMed] [Google Scholar]
- 35.Warner L, Newman DR, Kamb ML, et al. Problems with condom use among patients attending sexually transmitted disease clinics: prevalence, predictors, and relation to incident gonorrhea and chlamydia. Am J Epidemiol. 2008;167(3):341–349. doi: 10.1093/aje/kwm300. [DOI] [PubMed] [Google Scholar]
- 36.Ndovi TT, Parsons T, Choi L, et al. A new method to estimate quantitatively seminal vesicle and prostate gland contributions to ejaculate. Br J Clin Pharmacol. 2007;63(4):404–420. doi: 10.1111/j.1365-2125.2006.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauck C, Straten A. Using objective markers to assess participant behavior in HIV prevention trials of vaginal microbicides. J Acquir Immune Defic Syndr. 2008;49(1):64–69. doi: 10.1097/QAI.0b013e318183a917. [DOI] [PubMed] [Google Scholar]