Abstract
Background
Safe, effective interventions to improve cancer-related fatigue (CRF) are needed because it remains a prevalent, distressing, and activity-limiting symptom. Based on pilot data, a phase III trial was developed to evaluate the efficacy of American ginseng on CRF.
Methods
A multisite, double-blind trial randomized fatigued cancer survivors to 2000mg of American ginseng vs a placebo for 8 weeks. The primary endpoint was the general subscale of the Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF) at 4 weeks. Changes from baseline at 4 and 8 weeks were evaluated between arms by a two-sided, two-sample t test. Toxicities were evaluated by self-report and the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) provider grading.
Results
Three hundred sixty-four participants were enrolled from 40 institutions. Changes from baseline in the general subscale of the MFSI-SF were 14.4 (standard deviation [SD] = 27.1) in the ginseng arm vs 8.2 (SD = 24.8) in the placebo arm at 4 weeks (P = .07). A statistically significant difference was seen at 8 weeks with a change score of 20 (SD = 27) for the ginseng group and 10.3 (SD = 26.1) for the placebo group (P = .003). Greater benefit was reported in patients receiving active cancer treatment vs those who had completed treatment. Toxicities per self-report and CTCAE grading did not differ statistically significantly between arms.
Conclusions
Data support the benefit of American ginseng, 2000mg daily, on CRF over an 8-week period. There were no discernible toxicities associated with the treatment. Studies to increase knowledge to guide the role of ginseng to improve CRF are needed.
Effective and safe interventions to prevent and treat cancer-related fatigue (CRF) are needed because it remains one of the most prevalent, distressing, and activity-limiting symptoms survivors can experience, in both the short and long term (1–6). The prevalence of fatigue in patients undergoing chemotherapy is reported to be between 59% and 96% and, in patients receiving radiation therapy, between 65% and 100% (4,7). Fatigue can persist 5 to 10 years after diagnosis and treatment (2,3). CRF profoundly and negatively affects patients’ quality of life and interferes with routine daily functioning (5,6). Furthermore, fatigue accounts for a substantial amount of the variance in overall quality of life, with over 40% of variance attributed to fatigue (1).
There is a lack of evidence to support the efficacy of pharmacologic interventions. Psychostimulants have been the most commonly studied pharmacologic intervention for CRF. Eight placebo-controlled randomized trials evaluating methylphenidate or related agents have been completed with all but one trial (8) being negative (9–15). Despite popularity, the newer psychostimulants, such as modafanil, have also not yet been found to be effective in randomized controlled trials (16). In addition, other central nervous system agents (eg, donepezil, paroxetine) have had negative results to date (17,18).
Dietary supplements are a popular self-administered remedy among patients for symptoms that have no known effective treatment; CRF is no exception. Coenzyme Q 10, L-Carnitine, guarana, and ginseng have been used for fatigue and subsequently studied. Based on their role in cellular energy production, both coenzyme Q 10 and L-Carnitine were evaluated in placebo-controlled trials for CRF; they were found to be no more helpful than placebos (19,20). Guarana has supportive data from a phase II placebo-controlled trial (21), warranting further research.
Although many herbs have been touted through folklore and through traditional use as remedies for fatigue, none has probably enjoyed as much worldwide reputation and interest as ginseng. Within the context of traditional Chinese medicine, ginseng is generally viewed as an “adaptogen,” a substance that can help restore balance to the body by bringing it back to a point of homeostasis (22). There are two major species of ginseng, Asian (Panax ginseng) and American (Panax quinquefolius) (22,23). Both have a common mixture of active ingredients, the most important being ginsensosides. Between species of ginseng, there are varying amounts, strengths, and varieties of ginsensosides (22,24,25).
Substantial objective evidence supporting that ginseng may be helpful for fatigue comes from preclinical data. Specifically, in vitro data demonstrate anti-inflammatory and cortisol modulating effects (26–28) consistent with the currently established physiology of CRF. Animal studies have reported improved endurance and swimming duration time with ginseng, specifically ginsenosides Rb1 and Rg1, both of which are present in both Asian and American ginseng (25,29,30,31).
Two pilot trials have been completed in cancer survivors. One small study, only published in abstract form to date, reported positive effects of Asian ginseng in patients receiving chemotherapy (32). A subsequent larger pilot trial was conducted within the North Central Cancer Treatment Group, which randomized 290 patients, receiving or having completed cancer treatment, to one of three doses of American ginseng—750mg, 1000mg, and 2000 mg—vs a placebo for 8 weeks. The primary outcome was CRF as measured by the Brief Fatigue Inventory (BFI), with a secondary fatigue measure of the Vitality subscale of the SF-36. Area under the curve analysis for the summed six items of activity interference from the BFI (higher being better) was 460 units for placebo, 467 for 750mg of ginseng, 480 for 1000mg of ginseng, and 551 for 2000mg of ginseng at 8 weeks. Likewise, mean changes from baseline in the vitality subscale (higher being better) were 7.3 and 7.8 for placebo and the 750-mg dose of ginseng, respectively, versus 14.6 and 10.5 for the 1000- and 2000-mg doses of ginseng, respectively. The improvements in the 1000- and 2000-mg doses of ginseng were seen at 4 weeks and were maintained at 8 weeks. The two highest doses of ginseng (1000 and 2000mg/day) outperformed the 750mg/day dose and placebo in every one of the eight predetermined study endpoints (33).
Based on these encouraging pilot data, the purpose of our trial was to evaluate, using a double-blind design, the efficacy of 2000mg/day of American ginseng (Panax quinquefolius) as therapy for CRF and to evaluate its toxicities.
Methods
Eligibility
Participants were randomized to receive 2000mg of Wisconsin ginseng (a common type of American ginseng) or a placebo, with twice a day dosing (around breakfast and at about lunch or noon) over 8 weeks. Eligible participants included adult men and women with CRF defined as a score of 4 or more on an 11-point scale where 0 is “no fatigue” and 10 is “as bad as it can be.” The fatigue had to have been present for 1 month or more before study entry. Participants with all cancers, other than brain or CNS lymphoma, undergoing or having undergone curative intent treatment, were eligible, but participants had to have been diagnosed within the past 2 years. Other causes of fatigue were ruled out, and participants could not have had pain or insomnia rated 4 or higher on an 11-point scale. Use of systemic steroids, opioids, prior/current ginseng, or other agents for fatigue were cause for exclusion. Participants could be getting cancer treatment or have completed treatment, but they could not be scheduled to change treatment status during the 8-week trial. All participating sites received local approval from their institutional review boards, written informed consent was obtained from participants, and the study was registered (NCT00719563).
Randomization
Randomization was accomplished by computer using dynamic allocation with an established algorithm that balances the marginal distribution. This algorithm has been used in all of our cooperative group studies (34) and controlled for the following factors: baseline fatigue, initial vs recurrent disease, current treatment with radiation and/or chemotherapy, hematologic vs solid tumor malignancy, and months of cancer treatment.
Endpoints
The primary endpoint was the Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF) (35). The general subscale is a six-item subscale to measure the subjective experience of fatigue. The six-item subscale addresses the degree to which various descriptors of fatigue have been experienced in the past week. The items include feeling “pooped, worn out, fatigued, sluggish, run down and tired.” Answers are on a 5-point scale, ranging from 0, “not at all,” to 4, “extremely.” Confirmatory factor analysis has provided data that the items are a good fit in the subscales. Item loadings ranged from 0.88 to 0.90 for the general fatigue subscale. The alpha coefficient for the general fatigue subscale was 0.96 (35).
Secondary outcomes included the Profile of Mood States (POMS) (36), specifically the fatigue-inertia and vigor-activity subscales, as well as the BFI (37). Data were collected at baseline (before starting ginseng/placebo) and at 4 and 8 weeks. Side effects were collected by self-report questionnaires, where participants rated severity of side effects on a 0 to 10 scale at baseline and every week. In addition, providers graded side effects per CTCAE during assessment calls or visits every other week. Numeric analog scales ranging from 0 to 10 measuring fatigue, pain, and sleep were completed weekly.
Intervention
The intervention was supplied in 500-mg opaque capsules and consisted of pure ground root of Wisconsin ginseng from one production lot or a matching placebo containing rice powder. The ginseng contained 3% ginsensosides and was evaluated for quality and potency by an independent company. An Investigational New Drug Application (IND 73088) was in place. The ginseng and placebo were donated by the Ginseng Board of Wisconsin (Wausau, WI) and were manufactured using good manufacturing practices by Beehive Botanicals (Hayward, WI).
Statistical Analysis
The primary outcome was the change from baseline in the general subscale of the MFSI-SF at 4 weeks. This analysis was repeated at 8 weeks. Differences between arms at 4 and 8 weeks were carried out with a two-sample, two-sided t test. For all outcomes, scores were converted to a 100-point scale, with higher numbers indicating less fatigue, to facilitate comparisons between scales. One hundred fifty patients per arm provided 90% power to detect a difference of 38% of the standard deviation, for a low to moderate effect size (38). This effect size was chosen as the minimal difference likely to be clinically important while affording a sample size large enough to accommodate a subset analysis related to whether or not participants were currently receiving anticancer treatment or had completed treatment. Powering our study on the pilot trial effect size (Cohen’s d of 0.56) would have made this important subanalysis not possible. Correlation coefficients were calculated for fatigue (MFSI general subscale) with pain and with sleep (using the single item 0–10 scales) at baseline to evaluate whether sleep or pain was confounding CRF. The primary outcome analysis was repeated after separating the population into two groups: those who were receiving cancer treatment and those who had completed such. This was a planned subset analysis to look at these two groups based on the fact that the trajectory of fatigue is different during vs after treatment. Chronic posttreatment fatigue tends to be stable over time, whereas treatment-related fatigue tends to worsen during therapy and improve during the month or two after the completion of therapy (6,39,40). Finally, simple percentage reductions from baseline were calculated for each participant using the MFSI general subscale, and an overall χ2 analysis was performed.
Missing Data
Multiple and single imputation methods were used to handle missing data. We have repeated our analysis using multiple imputations by replicating eight sets of imputations. All results demonstrate consistent findings. The results presented are based on all available data required to calculate the primary endpoint without imputation.
Results
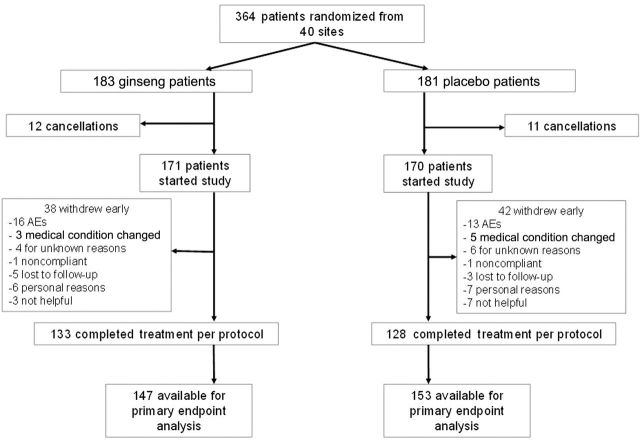
Between October 2008 and July 2011, 364 patients were enrolled from 40 different sites, mostly community cancer centers. All analyses were based on data frozen on November 29, 2011. A CONSORT diagram is shown as Figure 1. Seventy-eight percent of the participants completed all study interventions. Patient characteristics of those who started the study treatment were well balanced between arms (Table 1). There were no statistically significant differences in any of the fatigue measures between arms at baseline. All MFSI subscales are shown in Table 1. There were also not any statistically significant differences in demographic characteristics or fatigue scores between those who cancelled their participation after consent and randomization but before beginning the study treatment (cancellations) and those who began the study drug. Spearman correlation coefficients for the general fatigue subscale and pain were 0.08 (P = .13) and 0.01 (P = .80) between fatigue and sleep, respectively.
Figure 1.
CONSORT diagram. AE = adverse event.
Table 1.
Participant characteristics*
| Characteristic | Ginseng (n = 171) | Placebo (n = 170) | P |
|---|---|---|---|
| Age, y, mean (SD) | 55.3 (12.7) | 55.9 (11.8) | .79 |
| Sex, female, No. (%) | 138 (81) | 128 (75) | .23 |
| Race, No. (%) | .35 | ||
| White | 155 (91) | 157 (92) | |
| Black or African American | 10 (6) | 8 (5) | |
| Native Hawaiian/Pacific Islander | 2 (1) | 0 (0) | |
| Asian | 1 (1) | 3 (2) | |
| American Indian or Alaska Native | 1 (1) | 2 (1) | |
| Ethnicity, not Hispanic or Latino, No. (%) | 164 (96) | 166 (98) | .72 |
| Menopausal status, No. (%) | .76 | ||
| Pre | 37 (22) | 31 (18) | |
| Post/natural-surgical | 95 (56) | 90 (53) | |
| Not applicable (Male) | 33 (19) | 42 (25) | |
| Time since current cancer diagnosis, No. (%) | .84 | ||
| <180 days | 63 (37) | 64 (38) | |
| 180–360 days | 47 (28) | 42 (25) | |
| >360 days | 61 (36) | 64 (38) | |
| >1 primary cancer | 40 (23) | 36 (21) | .62 |
| Type of cancer, No. (%) | .54 | ||
| Breast | 110 (64) | 96 (57) | |
| Colon | 20 (12) | 17 (10) | |
| Prostate | 6 (4) | 8 (5) | |
| Hematologic | 8 (5) | 9 (5) | |
| Gynecologic | 5 (3) | 7 (4) | |
| Combination/unknown/other | 22 (13) | 33 (19) | |
| Currently receiving treatment, No. (%) | 83 (49) | 83 (49) | .96 |
| Current endocrine therapy, No. (%) | .46 | ||
| Tamoxifen | 23 (14) | 22 (13) | |
| Aromatase inhibitor | 27 (16) | 33 (19) | |
| Antiandrogen | 2 (1) | 5 (3) | |
| Other | 7 (4) | 3 (2) | |
| None | 112 (66) | 107 (63) | |
| Sleep aids, No. (%) | .07 | ||
| Yes | 43 (25) | 29 (17) | |
| If taking sleep aids, how frequent?, No. (%) | .27 | ||
| Daily | 18 (42) | 16 (55) | |
| Intermittent | 25 (58) | 13 (45) | |
| Exercising regularly, no, No. (%) | 98 (58) | 98 (59) | .85 |
| Baseline MFSI-SF General, mean (SD) | 39.0 (23.1) | 41.2 (23.5) | .44 |
| Baseline MFSI-SF Physical, mean (SD) | 76.3 (19.1) | 76.4 (19.7) | .86 |
| Baseline MFSI-SF Mental, mean (SD) | 74.0 (18.1) | 73.7 (19.4) | .96 |
| Baseline MFSI-SF Emotional, mean (SD) | 78.4 (18.3) | 76.9 (19.8) | .56 |
| Baseline MFSI-SF Vigor, mean (SD) | 38.3 (17.4) | 38.6 (18.4) | .88 |
| Baseline MFSI-SF total score, mean (SD) | 61.2 (12.4) | 61.1 (15.4) | .86 |
* Percentages may not equal 100% due to rounding or missing data. MFSI-SF = Multidimensional Fatigue Symptom Inventory–Short Form; SD = standard deviation.
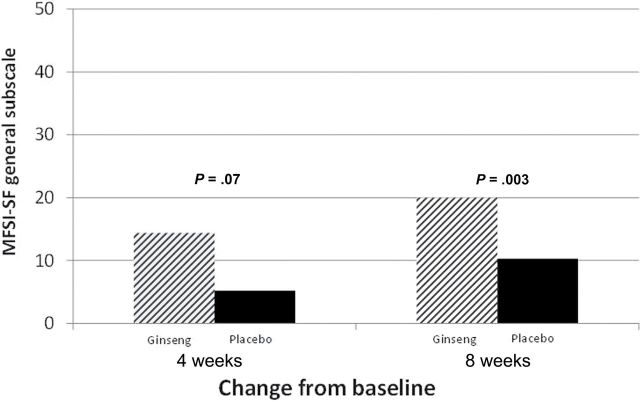
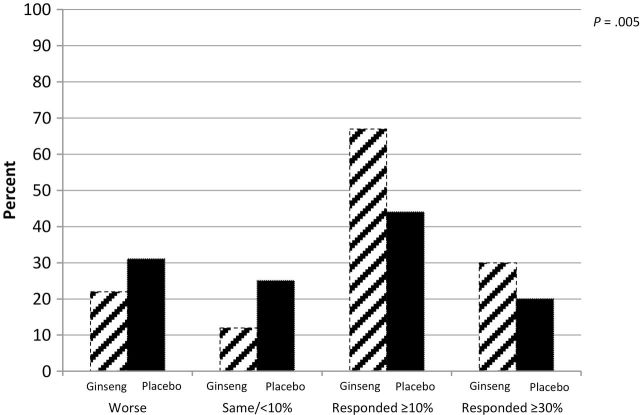
The primary endpoint of change from baseline in the general subscale of the MFSI-SF at 4 weeks was 14.4 (standard deviation [SD] = 27.1) in the ginseng arm (n = 147) and 8.2 (SD = 24.8) in the placebo arm (n = 153) (P = .07). At 8 weeks, there was statistically significant improvement in fatigue for those on ginseng (n = 138) vs those on placebo (n = 133), with change scores of 20 (SD = 27) vs 10.3 (SD = 26.1), respectively (P = .003) (Figure 2). The magnitude in response for the group as a whole is shown in Figure 3. More participants had a positive response to the ginseng and more had a strong clinical benefit (≥30% improvement) from ginseng compared with placebo.
Figure 2.
Multidimensional Fatigue Symptom Inventory–Short Form general fatigue subscale change from baseline at 4 and 8 weeks. Differences between arms at 4 and 8 weeks were carried out with a two-sample, two-sided t test. All statistical tests were two-sided.
Figure 3.
Eight-week response to ginseng vs placebo (percentage of participants) per Multidimensional Fatigue Symptom Inventory–Short Form general subscale determined by χ2 test. All statistical tests were two-sided.
Secondary endpoints included the other subscales of the MFSI-SF, as well as the fatigue-inertia and vigor-activity subscales of the POMS. Change from baseline for these is shown in Table 2. Statistically significant improvements in fatigue were reported in the ginseng group over the placebo group for the physical subscale and total score of the MFSI-SF and the fatigue/inertia subscale of the POMS. The BFI total score and activity interference did not demonstrate statistically significant differences between the arms; however, individual items of worst fatigue and fatigue “now” were significantly different at 8 weeks, favoring the ginseng arm (data not shown).
Table 2.
Secondary endpoints change from baseline*
| Variable (range: 0–100, higher is better) | Data Point | Ginseng (SD) | Placebo (SD) | P |
|---|---|---|---|---|
| MFSI-SF Physical | 4 weeks† | 1.6 (15.9) | −0.4 (14.7) | .39 |
| 8 weeks‡ | 3.0 (17.9) | −1.7 (18.2) | .004 | |
| MFSI-SF Mental | 4 weeks† | 2.0 (15.2) | 0.6 (16.1) | .41 |
| 8 weeks‡ | 2.8 (16.5) | 3.4 (15.2) | .80 | |
| MFSI-SF Emotional | 4 weeks† | 0.5 (16.1) | 0.5 (16.7) | .99 |
| 8 weeks‡ | 3.0 (17.4) | 2.3 (17.4) | .68 | |
| MFSI-SF Vigor | 4 weeks† | 1.8 (19.0) | 0.4 (15.5) | .70 |
| 8 weeks‡ | 4.6 (20.5) | 2.5 (17.6) | .71 | |
| MFSI-SF total score | 4 weeks† | 4.1 (13.4) | 2.1 (12.9) | .21 |
| 8 weeks‡ | 6.7 (14.0) | 3.7 (14.6) | .02 | |
| POMS | ||||
| Fatigue inertia | 4 weeks§ | 14.5 (25) | 7.7 (23.6) | .08 |
| 8 weeks|| | 18.6 (24.8) | 10.2 (26.1) | .008 | |
| Vigor activity | 4 weeks§ | 5 (18.7) | 3.9 (17.3) | .79 |
| 8 weeks|| | 8.2 (19.8) | 6.4 (19.8) | .47 | |
* MFSI = Multidimensional Fatigue Symptom Inventory–Short Form; POMS = Profile of Mood States; SD = standard deviation.
† n = 147 in the ginseng arm and n = 152 in the placebo arm at 4 weeks.
‡ n = 138 in the ginseng arm and n = 132 in the placebo arm at 8 weeks.
§ n = 139 in the ginseng arm and n = 142 in the placebo arm at 4 weeks.
|| n = 132 in the ginseng arm and n = 128 in the placebo arm at 8 weeks.
When the population was divided into two groups, those receiving cancer treatment vs those who had completed treatment, and the primary analysis was repeated within each of those groups, participants undergoing cancer therapy assigned to the ginseng arm had statistically significant improvement in fatigue at 4 and 8 weeks compared with those in the placebo arm (Figure 4).
Figure 4.
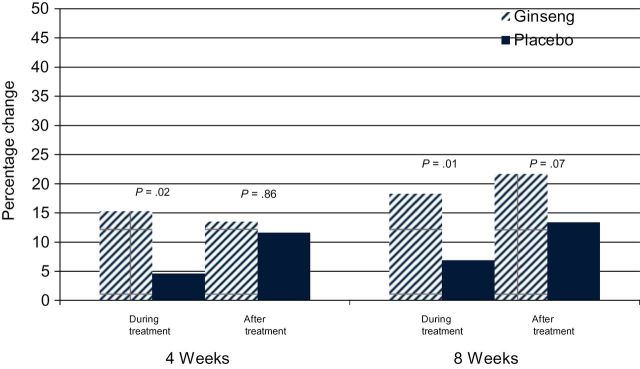
Percentage change from baseline for general subscale of Multidimensional Fatigue Symptom Inventory–Short Form at 4 and 8 weeks by current vs postcancer treatment determined by χ2 test. All statistical tests were two-sided. The 4 week data were analyzed with a Wilcoxon signed rank test, and the 8 week data were analyzed with equal variance t-tests.
Toxicities/Side Effects
Only five toxicities greater than 1% incidence were attributed to study treatment, and these were not statistically significantly different between arms per CTCAE grading by study personnel. These toxicities were agitation, anxiety, insomnia, nausea, and vomiting (Table 3). Patient-reported toxicities, controlling for baseline, were also not statistically significantly different between the arms, over the 8 weeks of treatment. Scores changed little over the course of the study (no more than 5 points out of 100) for nausea, vomiting, nervousness, anxiety, trouble sleeping, and loose stools. Only loose stools at 4 weeks (–0.8) and pain at 8 weeks (–0.3) were worse than baseline, and these occurred only in the placebo group. All other symptoms improved over the course of the study.
Table 3.
National Cancer Institute’s Common Terminology Criteria for Adverse Events treatment-related grade 2 to 3 toxicity greater than 1% incidence
| Adverse event | Ginseng (n = 168) | Placebo (n = 169) | Wilcoxon P |
|---|---|---|---|
| Nausea, No. (%) | .24 | ||
| 2: Moderate | 5 (3) | 3 (2) | |
| Vomiting, No. (%) | .21 | ||
| 2: Moderate | 2 (1) | 2 (1) | |
| Insomnia, No. (%) | .63 | ||
| 2: Moderate | 9 (5) | 10 (6) | |
| 3: Severe | 1 (1) | 1 (1) | |
| Anxiety, No. (%) | .44 | ||
| 2: Moderate | 4 (2) | 5 (3) | |
| Agitation | .83 | ||
| 2: Moderate | 2 (1) | 4 (2) | |
Discussion
Data from this study support that American ginseng has activity against CRF but that clinically meaningful results may not be realized until 2 months after starting ginseng. Clinically meaningful was defined as a difference of at least 10 points on a 0 to 100 scale based on previously published work (41). Participants currently receiving radiation and/or chemotherapy had statistically significantly better general fatigue scores at both 4 and 8 weeks in the ginseng arm vs the placebo arm. Better results in those receiving cancer treatment may indicate that ginseng may be a better preventive agent than treatment intervention, despite the fact that all patients had to have a certain level of fatigue to enter the trial. This is based on the fact that although it would be expected for fatigue to increase throughout cancer treatment, fatigue scores actually decreased (improved) during treatment. However, this issue requires further study. These data also provide further support that vigor and fatigue are conceptually and experientially different constructs, as the vigor subscales in the MFSI-SF and POMS were not significantly improved, whereas the fatigue-inertia subscale in the POMS and general physical fatigue subscales in the MFSI-SI were positively impacted.
Strengths of this study include that it was a randomized, double-blind trial involving 40 different clinical sites, most of which are community cancer centers. In addition, based on the correlation coefficients between fatigue, sleep, and pain, enrollment effectively targeted fatigue and not fatigue secondary to sleep disturbance or pain. An important limitation of this study is that it evaluated the use of ginseng only out to 8 weeks. Long-term or continued efficacy is not known.
It is curious that two of the fatigue measures were sensitive to changes over the course of the study from the intervention (POMS fatigue-inertia and MFSI general subscale), whereas the BFI was not. We cannot know, with certainty, why this was. The BFI, however, does use a 0 to 10 scale with descriptive anchors at the beginning and end, whereas the POMS and MFSI use a response scale that is shorter and delineated with descriptors throughout. It may be that patients were not able to distinguish between such closely spaced numbers without descriptors.
The mechanism by which American ginseng may be able to moderate fatigue is evidenced by preclinical data. Several investigators have established a consistent link between CRF and inflammation and have provided data to support dysregulation of the hypothalamic pituitary adrenal axis (42–46). These data suggest that chronic fatigue in cancer is associated with an inability for the hypothalamic pituitary adrenal axis to regulate inflammatory processes and that concentrations of inflammatory cytokines remain elevated instead of reachieving homeostasis (42–46). Preclinical data evaluating the biologic activity of ginseng have demonstrated the ability of ginseng to downregulate inflammatory pathways (47), decrease inflammation (26–28), and modulate cortisol and the impact of chronic stress on the hypothalamic pituitary adrenal axis (27).
Based on the provider- and patient-reported toxicity data in this trial, there were no discernible side effects from ginseng. In addition, there are other data to corroborate ginseng’s safety. The first is a recent report investigating herbs for possible inhibition of the cytochrome P450 system. American ginseng (P. quinquefolius) was one of a few herbs found to be noninhibitory (48). Second, there have been contradictory reports of ginseng’s ability to proliferate breast cancer cells as well as the thought that it might be estrogenic. Preclinical research sheds insight into this contradiction. Characteristics and properties of ginsenosides depend on the processing; certain extraction methods can result in estrogenic properties. Specifically, ginseng derived from methanol extraction, as opposed to water extraction, does exhibit estrogenic properties and has been found to proliferate cancer cells in breast cell lines in vitro (49–51). Ginseng products not derived from methanol extraction methods, but instead from water extraction or pure ground root, do not have estrogenic properties (51). In fact, preclinical data have demonstrated breast cancer cell inhibition by water-extracted American ginseng in both estrogen-sensitive and -insensitive cell lines (50).
Does ginseng interfere with the activity of chemotherapeutic agents? There are preclinical data demonstrating that American ginseng does not interfere with tamoxifen, doxorubicin, cyclophosphamide, paclitaxel, 5-fluorouracil, and methotrexate; rather ginseng was synergistic with these agents against MCF-7 breast cancer cell lines, inhibiting growth (47,51,52). It must be noted, however, that this is not definitive proof because there have not been studies done in humans to answer this question.
Because ginseng is not a regulated drug by the Food and Drug Administration and is a plant that is subject to all of the variables relevant for any agricultural crop, the potency of important ginsenosides is variable (24), and standardization regarding manufacturing processes and quality are not well established or proactively enforced. The pilot ginseng trial was fortunate enough to get a crop that contained 5% ginsensosides (33), where our phase III trial only had 3% ginsenoside content. Despite some increase in dose to compensate, it is possible that the effect of ginseng on fatigue may have been more profound had our dose and/or potency been higher.
In summary, although this study provides support for the use of American ginseng to ameliorate CRF, more research is necessary to understand its role and how to maximize its positive effects. It would, however, be reasonable for a cancer survivor to try American ginseng for fatigue, taking into consideration that there are no other pharmacologic agents known to be effective. Attention should be paid to the type of ginseng purchased, as mentioned above. In addition, it will be important to further explore the biologic activity of American ginseng with respect to CRF and to work toward a safe, standardized, potent product that is accessible to all who wish to try it.
Funding
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-60276, CA-35195, CA35090, CA35101, CA-35269, CA-37417, CA-35448, CA-35267, CA-63849, CA-35113, CA-35103, CA-35415, CA-35119, CA-63844, CA-35431 and the Breast Cancer Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Additional participating institutions include: Siouxland Hematology-Oncology Associates, Sioux City, IA (Donald Wender, MD); Toledo Community Hospital Oncology Program (Rex B. Mowat, MD); Medical College of Georgia, Augusta, GA (Anand P. Jillella MD); Iowa Oncology Research Association CCOP, Des Moines, IA (Robert J. Behrens, MD); Rapid City Regional Hospital, Inc, Rapid City, SD (Richard Charles Tenglin, MD); Columbus CCOP, Columbus, OH (J. Philip Kuebler, MD, PhD); Michigan Cancer Research Consortium, Ann Arbor, MI (Philip J. Stella, MD); Meritcare Hospital CCOP, Fargo, ND (Preston D. Steen, MD); Geisinger Clinic & Medical Center CCOP, Danville, PA (Albert M. Bernath Jr, MD); Montana Cancer Consortium CCOP, Billings, MT (Benjamin T. Marchello, MD); Sioux Community Cancer Consortium, Sioux Falls, SD (Miroslaw Muzurczak, MD); Lehigh Valley Hospital, Allentown, PA (Suresh Nair, MD); Colorado Cancer Research Program, Denver, CO (Eduardo R. Pajon Jr, MD); Mayo Clinic Arizona, Scottsdale, AZ (Michele Y. Halyard, MD); Medcenter One Health Systems, Bismarck, ND (Edward J. Wos, DO); Carle Cancer Center CCOP, Urbana, IL (Kendrith M. Rowland Jr, MD); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA (Martin Wiesenfeld, MD); Hematology & Oncology of Dayton, Inc, Dayton, OH (Howard M. Gross, MD); Essentia Duluth CCOP, Duluth, MN (Daniel A. Nikcevich, MD); Altru Health Systems, Grand Forks, ND (Grant Seeger, MD); Grand Rapids Clinical Oncology Program, Grand Rapids, MI (Martin J. Bury, MD); Grand Rapids Clinical Oncology Program, Grand Rapids, MI; St. Vincent Regional Cancer Center CCOP, Green Bay, WI (Anthony J. Jaslowski, MD); Hawaii Minority-Based CCOP (William S. Loui, MD); Heartland Cancer Research CCOP, St. Louis, MO (Alan P. Lyss, MD); Edward Comprehensive Cancer Center, Huntington, WV (Maria Rosalia B. Tri Tirona, MD); Marshfield Clinical Research Foundation, Minocqua, WI (Matthias Weiss, MD); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN (Patrick J. Flynn, MD); Missouri Valley Cancer Consortium, Omaha, NE (Gamini S. Soori, MD); University of New Mexico, Albuquerque, NM (Zoneddy R. Dayao, MD); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN (Robin T. Zon, MD); Cancer Care Associates, Tulsa, OK (Alan M. Keller, MD); Illinois Oncology Research Assn. CCOP, Peoria, IL (John W. Kugler, MD); Columbia River Oncology Program, Portland, OR (Janet C. Ruzich, MD); CentraCare Clinic, St. Cloud, MN (Donald J. Jurgens, MD); Cancer Research for the Ozarks, Springfield, MO (Robert L. Carolla, MD).
References
- 1. Arndt V, Stegmaier C, Ziegler H, et al. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer. 2006;107(10)2496–2503 [DOI] [PubMed] [Google Scholar]
- 2. Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4)751–758 [DOI] [PubMed] [Google Scholar]
- 3. Harrington CB, Hansen JA, Moskowitz M, et al. It’s not over when it’s over: long-term symptoms in cancer survivors—a systematic review. Int J Pych Med. 2010;40(2)163–181 [DOI] [PubMed] [Google Scholar]
- 4. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10 [DOI] [PubMed] [Google Scholar]
- 5. Molassiotis A, Zheng Y, Denton-Cardew L, et al. Symptoms experienced by cancer patients during the first year from diagnosis: patient and informal caregiver ratings and agreement. Palliat Support Care. 2010;8(3)313–324 [DOI] [PubMed] [Google Scholar]
- 6. Purcell A, Fleming J, Bennett S, et al. A multidimensional examination of correlates of fatigue during radiotherapy. Cancer. 2010;116(2)529–537 [DOI] [PubMed] [Google Scholar]
- 7. Fulton C, Knowles G. Cancer fatigue. Eur J Cancer Care (Engl). 2000;9(3)167–171 [PubMed] [Google Scholar]
- 8. Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009;38(5)650–662 [DOI] [PubMed] [Google Scholar]
- 9. Bruera E, Valero V, Driver L, et al. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2006;24(13)2073–2078 [DOI] [PubMed] [Google Scholar]
- 10. Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28(23)3673–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Auret KA, Schug SA, Bremner AP, et al. A randomized, double-blind, placebo-controlled trial assessing the impact of dexamphetamine on fatigue in patients with advanced cancer. J Pain Symptom Manage. 2009;37(4) 613–621 [DOI] [PubMed] [Google Scholar]
- 12. Butler JM, Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol. 2007;69(5)1496–1501 [DOI] [PubMed] [Google Scholar]
- 13. Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16(6)577–583 [DOI] [PubMed] [Google Scholar]
- 14. Bruera E, Yennurajalingam S, Perez-Cruz PE, et al. Methylphenidate (MP) and nursing telephone intervention (NTI) for cancer-related fatigue (CRF) in advanced cancer patients: a double-blind randomized phase II trial [published online ahead of print May 20, 2013]. J Clin Oncol. [Google Scholar]
- 15. Escalante C, Meyers C, Reuben J, et al. A randomized, double-blind, placebo-controlled crossover trial of a sustained release methylphenidate in cancer-related fatigue. J Clin Oncol. 2012;30(Suppl.):abstract 9072. [Google Scholar]
- 16. Morrow CJ, Ghattas M, Smith C, et al. Src family kinase inhibitor saracatinib (AZD0530) impairs oxaliplatin uptake in colorectal cancer cells and blocks organic cation transporters. Cancer Res. 2010;70(14)5931–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruera E, El Osta B, Valero V, et al. Donepezil for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2007;25(23)3475–3481 [DOI] [PubMed] [Google Scholar]
- 18. Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21(24)4635–4641 [DOI] [PubMed] [Google Scholar]
- 19. Cruciani RA, Dvorkin E, Homel P, et al. L-carnitine supplementation in patients with advanced cancer and carnitine deficiency: a double-blind, placebo-controlled study. J Pain Symptom Manage. 2009;37(4)622–631 [DOI] [PubMed] [Google Scholar]
- 20. Lesser GJ, Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q(10) to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol. 2013;11(1):31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campos MPdO, Riechelmann R Martins LC et al. Guarana (Paullinia cupana) improves fatigue in breast cancer patients undergoing systemic chemotherapy. J Altern Complem Med. 2011;17(6)505–512 [DOI] [PubMed] [Google Scholar]
- 22. Natural Standard Monograph, Ginseng. http://naturalstandard.com/naturalstandard/monographs
- 23. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11)1685–1693 [DOI] [PubMed] [Google Scholar]
- 24. Cui J. Identification and quantification of ginsenosides in various commercial ginseng preparations. Eur J Pharm Sci. 1995;3 77–85 [Google Scholar]
- 25. Tadano T, Nakagawasai O, Niijima F, et al. The effects of traditional tonics on fatigue in mice differ from those of the antidepressant imipramine: a pharmacological and behavioral study. Am J Chinese Med. 2000;28(1)97–104 [DOI] [PubMed] [Google Scholar]
- 26. Jin Y, Hofseth AB, Cui X, et al. American ginseng suppresses colitis through p53-mediated apoptosis of inflammatory cells. Cancer Prev Res (Phila). 2010;3(3)339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang A, Hao H, Zheng X, et al. Peripheral anti-inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti-depression efficacy. J Neuroinflammation. 2011;8 100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang CS, Ko SR, Cho BG, et al. The ginsenoside metabolite compound K, a novel agonist of glucocorticoid receptor, induces tolerance to endotoxin-induced lethal shock. J Cell Mol Med. 2008;12(5A)1739–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang LC, Lee TF. Effect of ginseng saponins on exercise performance in non-trained rats. Planta Med. 1998;64(2)130–133 [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Sakuma T, Asafu-Adjaye E, et al. Determination of ginsenosides in plant extracts from Panax ginseng and Panax quinquefolius L. by LC/MS/MS. Anal Chem. 1999;71(8)1579–1584 [DOI] [PubMed] [Google Scholar]
- 31. Banerjee U, Izquierdo JA. Antistress and antifatigue properties of Panax ginseng: comparison with piracetam. Acta Physiol Lat Am. 1982;32(4):277–285 [PubMed] [Google Scholar]
- 32. Younus J, Collins A, Wang X, et al. A double blind placebo controlled pilot study to evaluate the effects of ginseng on fatigue and quality of life in adult chemo-naive cancer patients. J Clin Oncol. 2003;22(4)733 [Google Scholar]
- 33. Barton DL, Soori GS, Bauer BA, et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer. 2010;18(2)179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Therneau TM. How many stratification factors are “too many” to use in a randomization plan? Control Clin Trials. 1993;14(2)98–108 [DOI] [PubMed] [Google Scholar]
- 35. Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27(1)14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Curran S, Andrykowsky M, Studts J. Short form of the Profile of Mood States (POMS-SF): psychometric information. Psychol Assess. 1995;7(1)80–83 [Google Scholar]
- 37. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5)1186–1196 [DOI] [PubMed] [Google Scholar]
- 38. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 39. Dhruva A, Dodd M, Paul SM, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nursing. 2010;33(3)201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Donovan KA, Jacobsen PB, Andrykowski MA, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Mange. 2004;28(4)373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sloan JA, Frost MH, Berzon R, et al. The clinical significance of quality of life assessments in oncology: a summary for clinicians. Support Care Cancer. 2006;14(10)988–998 [DOI] [PubMed] [Google Scholar]
- 42. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67(2)277–280 [DOI] [PubMed] [Google Scholar]
- 43. Bower JE, Ganz PA, Aziz N, et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4)604–611 [DOI] [PubMed] [Google Scholar]
- 44. Bower JE, Ganz PA, Dickerson SS, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30(1)92–100 [DOI] [PubMed] [Google Scholar]
- 45. Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21(4)413–427 [DOI] [PubMed] [Google Scholar]
- 46. Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29(3)333–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. King ML, Murphy LL. American ginseng (Panax quinquefolius L.) extract alters mitogen-activated protein kinase cell signaling and inhibits proliferation of MCF-7 cells. J Exp Ther Oncol. 2007;6(2)147–155 [PubMed] [Google Scholar]
- 48. Budzinski JW, Foster BC, Vandenhoek S, et al. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7(4)273–282 [DOI] [PubMed] [Google Scholar]
- 49. Duda RB, Taback B, Kessel B, et al. pS2 expression induced by American ginseng in MCF-7 breast cancer cells. Ann Surg Oncol. 1996;3(6)515–520 [DOI] [PubMed] [Google Scholar]
- 50. Duda RB, Kang SS, Archer SY, et al. American ginseng transcriptionally activates p21 mRNA in breast cancer cell lines. J Korean Med Sci. 2001;16(Suppl):S54–S60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. King ML, Adler SR, Murphy LL. Extraction-dependent effects of American ginseng (Panax quinquefolium) on human breast cancer cell proliferation and estrogen receptor activation. Integr Cancer Ther. 2006;5(3)236–243 [DOI] [PubMed] [Google Scholar]
- 52. Duda RB, Zhong Y, Navas V, et al. American ginseng and breast cancer therapeutic agents synergistically inhibit MCF-7 breast cancer cell growth. J Surg Oncol. 1999;72(4)230–239 [DOI] [PubMed] [Google Scholar]