Respiratory tract infections (RTIs) are very common worldwide. We prospectively demonstrate an association between low serum 25-hydroxyvitamin D level and increased risk of laboratory-confirmed viral upper RTI in children. Future studies should evaluate the role of supplementation to reduce RTIs.
Keywords: vitamin D, serum 25-hydroxyvitamin D, upper respiratory tract infection, cold
Abstract
Background. Vitamin D may be important for immune function. Studies to date have shown an inconsistent association between vitamin D and infection with respiratory viruses. The purpose of this study was to determine if serum 25-hydroxyvitamin D (25(OH)D) was associated with laboratory-confirmed viral respiratory tract infections (RTIs) in children.
Methods. Serum 25(OH)D levels were measured at baseline and children from Canadian Hutterite communities were followed prospectively during the respiratory virus season. Nasopharyngeal specimens were obtained if symptoms developed and infections were confirmed using polymerase chain reaction. The association between serum 25(OH)D and time to laboratory-confirmed viral RTI was evaluated using a Cox proportional hazards model.
Results. Seven hundred forty-three children aged 3–15 years were followed between 22 December 2008 and 23 June 2009. The median serum 25(OH)D level was 62.0 nmol/L (interquartile range, 51.0–74.0). A total of 229 participants (31%) developed at least 1 laboratory-confirmed viral RTI. Younger age and lower serum 25(OH)D levels were associated with increased risk of viral RTI. Serum 25(OH)D levels <75 nmol/L increased the risk of viral RTI by 50% (hazard ratio [HR], 1.51; 95% confidence interval [CI], 1.10–2.07, P = .011) and levels <50 nmol/L increased the risk by 70% (HR, 1.67; 95% CI, 1.16–2.40, P = .006).
Conclusions. Lower serum 25(OH)D levels were associated with increased risk of laboratory-confirmed viral RTI in children from Canadian Hutterite communities. Interventional studies evaluating the role of vitamin D supplementation to reduce the burden of viral RTIs are warranted.
Viral upper respiratory tract infections (RTIs) are very common worldwide. Despite their perceived benign nature, the burden of disease is significant in terms of morbidity and economic loss [1]. Viral respiratory infections may also be associated with mortality in certain patient populations [2, 3]. Unfortunately, treatment remains unsatisfactory and is often focused on symptom relief. As a result, prevention remains a key strategy in reducing the burden of these infections.
There has been increasing interest in the role of vitamin D in respiratory infections. Vitamin D has been shown to have important roles for both innate [4–6] and adaptive immune responses [7–9]. In particular, vitamin D has been linked to innate immune responses in lung epithelial cells [10, 11]. In addition, antimicrobial peptides induced by vitamin D may have antiviral effects with activity shown against herpes simplex virus type 1 [12], adenovirus [13], human immunodeficiency virus (HIV) [14], and vaccinia virus [15].
Several observational studies have evaluated the role of serum 25-hydroxyvitamin D (25(OH)D) concentration in respiratory tract infections [16–25]. However, all these studies have important limitations including short follow-up period [16], small sample size [16–20, 24, 25], case-control design [18–20], or cross-sectional design with retrospective ascertainment of symptoms [21, 22] and failure to obtain laboratory confirmation of self-reported illness [16, 21–23]. The studies in adults have shown an association between lower vitamin D levels and increased respiratory infections (self-reported) [21, 22, 24] and absence from work due to respiratory symptoms [23]. However, pediatric studies have focused predominantly on lower RTIs (chest radiography–confirmed pneumonia or bronchiolitis) [17–20, 25]. The relationship between serum 25(OH)D concentration and upper RTIs in children has not been evaluated.
The purpose of this study was to determine if serum 25(OH)D levels are associated with subsequent risk of laboratory-confirmed viral upper RTIs in children and adolescents.
METHODS
Population and Study Design
We conducted a prospective cohort study of children and adolescents participating in a cluster randomized controlled trial evaluating the effect of influenza vaccination of children on viral infection rates in Hutterite communities, the results of which have been published elsewhere [26]. Hutterite communities (colonies) are rural, self-governing, and self-sufficient communal-living groups of Anabaptists. In the RCT, children with no underlying chronic medical conditions between 3 and 15 years of age (n = 947) from 46 colonies were randomly assigned by colony to receive either inactivated seasonal influenza vaccine (A/Brisbane/59/2007[H1N1]-like virus, A/Brisbane/10/2007[H3N2]-like virus, B/Florida/4/2006-like virus; Vaxigrip) or hepatitis A vaccine (Avaxim-Pediatric, Sanofi Pasteur). Children with underlying conditions (n = 65) and other children (n = 174) were followed but not randomized. All participants were followed regularly with twice-weekly assessments by research nurses over the influenza season, defined by the start date (1 laboratory-confirmed influenza case for 2 consecutive weeks) and stop date (no laboratory-confirmed influenza cases for 2 consecutive weeks). This period was from 28 December 2008 to 23 June 2009.
Covariables of interest were age, sex, presence of asthma, presence of other underlying conditions, influenza vaccination, and 25(OH)D level (nmol/L). Serum 25(OH)D levels were collected on children at baseline unless bloodwork was refused. Serum from venous blood was frozen at −80°C until batched analysis was performed according to the manufacturer's instructions using the DiaSorin LIAISON chemiluminescence assay. Serum 25(OH)D levels were taken between 16 October 2008 and 28 April 2009 with most taken between 16 October 2008 and 31 December 2008 (n = 724, 97%).
Outcome
The primary outcome was laboratory-confirmed viral infection defined by a positive nasopharyngeal swab polymerase chain reaction (PCR) result. Copan flocked nasopharyngeal swabs (Copan Italia, Brescia, Italy) were collected in universal transport medium (Copan Italia) if 2 or more of the following symptoms were present: fever (≥38°C), cough, nasal congestion, sore throat, headache, sinus problems, muscle aches, fatigue, ear ache or infection, or chills. Specimens were first tested for influenza A (including pH1N1) and B using the Centers for Disease Control and Prevention Human Influenza Virus Real-time Reverse Transcription PCR Detection and Characterization Panel [27]. Negative specimens were then tested for influenza (A, B), coronavirus (229E, NL63, OC43), enterovirus (including rhinovirus), parainfluenza (1–4), respiratory syncytial virus (RSV) (A, B), and human metapneumovirus by xTAG Respiratory Virus Panel multiplex PCR (Luminex, Austin, Texas).
Statistical Analysis
Survival analysis was used to assess the relationship between time to laboratory-confirmed viral respiratory infection and serum 25(OH)D level. Univariable analyses were conducted to obtain unadjusted hazard ratios (HRs), and significance was determined using the log-rank test. Vitamin D levels were analyzed as both a continuous variable (log-transformed to correct positive skew) and dichotomized based on both the American Academy of Pediatrics (AAP [≥50 nmol/L]) [28] and Canadian Paediatric Society (CPS [≥75 nmol/L]) [29] recommendations. Age was also analyzed both as a continuous variable and categorized into 3 groups (<5 years, 5–9 years, 10–15 years). A Cox proportional hazards model was used to estimate adjusted hazard ratios (aHRs), and the model was adjusted for clustering at the colony level. Variables with a P value < .1 were considered for inclusion in the multivariable model, and the final model was determined using a stepwise backwards elimination method. It was decided a priori to adjust the final model for age and sex. The proportional hazards assumption was evaluated using Shoënfeld residual test, graphically using Shoënfeld residuals and log-log curves and examination of variables for time dependence. Overall model fit was assessed using Cox-Snell residuals and deviance residual plots.
A recurrent events analysis was conducted to examine the relationship between 25(OH)D and rate of occurrence of respiratory tract infection adjusted for the biologically plausible covariables specified above. A counting process model was used to treat recurrent events as identical. The model was adjusted for clustering at the colony level.
All estimates are presented with 95% confidence intervals (CIs). P values <.05 was considered statistically significant. All analyses were conducted using Stata Statistical Software release 12 (StataCorp, College Station, Texas).
RESULTS
Baseline characteristics are summarized in Table 1. Of 1186 children (aged ≤15 years) in the RCT, 947 were randomized to influenza or hepatitis A vaccine, and 743 (63%) children from 43 colonies had serum 25(OH)D levels and were included in the study. There were no appreciable differences between participants with serum 25(OH)D levels and those without. The mean age was 9.3 years (SD, 4.3 years); 47.5% were male. The median serum 25(OH)D level was 62.0 nmol/L (interquartile range, 51.0–74.0); 565 (76%) participants had levels <75 nmol/L, 152 (20.5%) had levels <50 nmol/L, and 4 (0.5%) had levels <25 nmol/L. The colony latitudes ranged from 49.2°N to 54.8°N (median, 50.0°N).
Table 1.
Baseline Characteristics of Children With and Without Serum 25-Hydroxyvitamin D Levels and Colony Characteristics
| Characteristic | Vitamin D Levels Availablea (n = 743) |
|---|---|
| Age, mean (SD) | 9.3 (3.4) |
| Male sex | 353 (47.5) |
| Comorbidities | |
| ≥1 comorbidityb | 18 (2.4) |
| Asthma | 11 (1.5) |
| Serum 25(OH)D level, nmol/L, median (IQR) | 62.0 (51.0–74.0) |
| No. with serum 25(OH)D levels: | |
| <25 nmol/L | 4 (0.5) |
| <50 nmol/L | 152 (20.5) |
| <75 nmol/L | 565 (76) |
| Cluster size, mean (SD) | |
| All residents per colony | 71.1 (25.2) |
| Enrolled children in study | 17.4 (8.0) |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; IQR, interquartile range; SD, standard deviation.
a Categorical variables presented as No. (%).
b Comorbidity: heart/lung disease (including asthma), blood disorder, swallowing/choking disorder, aspirin use, chronic metabolic condition, kidney/liver disease, immunodeficiency.
Participants were followed between 22 December 2008 and 23 June 2009 for a median of 156 days (range, 17–176 days). Ten participants (1%) withdrew from the study in the first month. A total of 229 (31%) participants developed at least 1 laboratory-confirmed respiratory tract infection. The most common infection was influenza (n = 101, 14%), followed by enterovirus/rhinovirus (n = 81, 11%), RSV (n = 35, 5%), parainfluenza (n = 33, 4%), coronavirus (n = 26, 3%), and human metapneumovirus/metapneumovirus (n = 7, 1%). Coinfection occurred in 6 participants. Repeated infections occurred in 46 participants; 44 participants (6%) had 2 infections and 2 participants (0.5%) had 3 infections.
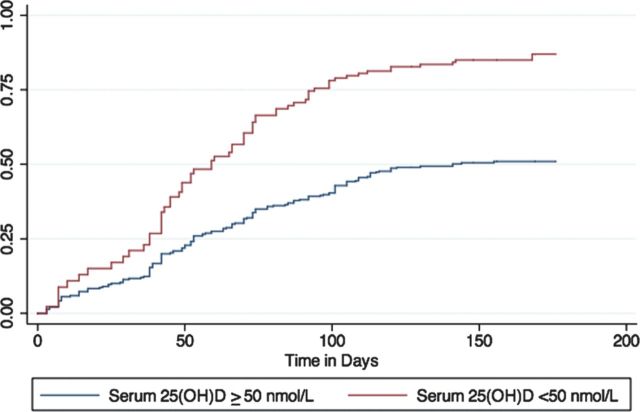
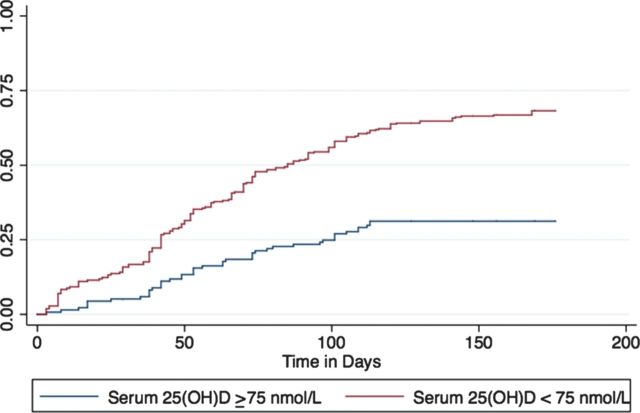
In univariable analyses, age and serum 25(OH)D level were associated with RTI (Table 2). In multivariable analysis accounting for clustering at the colony level, serum 25(OH)D level was independently associated with viral RTI (Table 2). For every 1-unit increase in log serum 25(OH)D level (corresponding to a 2.72-fold increase in serum 25(OH)D level), the hazard of developing a respiratory tract infection decreased by 50% (aHR, 0.52; 95% CI, .35–.79, P = .002). When levels were dichotomized based on AAP and CPS recommendations, levels <50 nmol/L (aHR, 1.67; 95% CI, 1.16–2.40, P = .006) and <75 nmol/L (aHR, 1.51; 95% CI, 1.10–2.07, P = .011), were both associated with increased risk of infection (Figures 1 and 2).
Table 2.
Predictors of Laboratory-Confirmed Viral Respiratory Tract Infection (Univariable and Multivariable Analyses)
| Univariable Analyses |
Multivariable Analyses |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Agea | ||||
| Per 1-y increase | 0.93 (.88–.99) | .017 | 0.92 (.87–.97) | .005 |
| Age groups | ||||
| <5 y | 2.08 (1.31–3.30) | .002 | ||
| 5–9 y | 1.14 (.77–1.68) | .502 | ||
| 10–15 y | Reference | |||
| Male sex | 0.73 (.53–1.03) | .070 | 0.74 (.52–1.04) | .082 |
| ≥1 comorbidity | 1.07 (.51–2.25) | .867 | ||
| Asthma | 0.81 (.31–2.09) | .660 | ||
| Vaccination | ||||
| Influenza (vs hepatitis A) | 0.74 (.37–1.49) | .401 | ||
| Serum 25(OH)D level, nmol/La | ||||
| Per 1-unit change in log | 0.61 (.40–.92) | .019 | 0.52 (.35–.79) | .002 |
| levels (dichotomized) | ||||
| <25 nmol/L vs ≥25 nmol/L | 0.72 (.13–3.94) | .700 | ||
| <50 nmol/L vs ≥50 nmol/L | 1.54 (1.07–2.21) | .021 | ||
| <75 nmol/L vs ≥75 nmol/L | 1.35 (1.01–1.82) | .043 | ||
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; HR, hazard ratio.
a Analyzed as either continuous variable and dichotomized.
Figure 1.
Time to laboratory-confirmed viral infection by serum 25-hydroxyvitamin D level (<50 nmol/L vs ≥50 nmol/L), adjusted for age and sex. Abbreviation: 25(OH)D, 25-hydroxyvitamin D.
Figure 2.
Time to laboratory-confirmed viral infection by serum 25-hydroxyvitamin D level (<75 nmol/L vs ≥75 nmol/L), adjusted for age and sex. Abbreviation: 25(OH)D, 25-hydroxyvitamin D.
Age was also independently associated with viral RTI in the multivariable analysis. Children aged <5 years were at highest risk of RTI compared to those aged 5–9 years (aHR, 1.92; 95% CI, 1.26–2.88, P = .002) and 10–15 years (aHR, 2.40; 95% CI, 1.48–3.87, P < .001). There were no interactions found between serum 25(OH)D level and either age and sex.
When multiple viral RTI events were taken into account, age and log serum 25(OH)D levels were associated with increased rate of occurrence of respiratory tract infection (Table 3). Serum 25(OH)D level was an important predictor both as a continuous variable (aHR, 0.57; 95% CI, .39–.83, P = .003) and when dichotomized based on levels <50 nmol/L (aHR, 1.49; 95% CI, 1.11–2.02, P = .008) and <75 nmol/L (aHR, 1.50; 95% CI, 1.11–2.03, P = .009). Younger age conferred an increased risk of viral RTI with a hazard ratio of 1.45 for every 5-year decrease in age (aHR, 1.45; 95% CI, 1.09–1.94, P = .011).
Table 3.
Serum 25-Hydroxyvitamin D Level as a Predictor of Recurrent Respiratory Virus Infections, Adjusted for Age and Sex
| Variable | Adjusted HR (95% CI) | P Value |
|---|---|---|
| Age | ||
| Per 1-y change | 0.93 (.88–.98) | .011 |
| Per 5-y change | 0.69 (.52–.92) | .011 |
| Male sex | 0.80 (.59–1.07) | .135 |
| Serum 25(OH)D level, nmol/La | ||
| Per 1-unit change in log | 0.57 (.39–.83) | .003 |
| levels (dichotomized) | ||
| <50 nmol/L vs ≥50 nmol/L | 1.49 (1.11–2.02) | .008 |
| <75 nmol/L vs ≥75 nmol/L | 1.50 (1.11–2.03) | .009 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; HR, hazard ratio.
a Analyzed as either continuous variable and dichotomized.
DISCUSSION
We found a statistically significant association between serum 25(OH)D and laboratory-confirmed viral RTIs in children from Hutterite communities in Canada. Lower serum 25(OH)D levels were associated with increased risk of RTI after adjusting for age and sex. Younger age was also associated with increased risk.
This study provides novel information on the effects of vitamin D status on laboratory-confirmed viral upper RTIs in children and adolescents. Previous studies have shown that children with rickets are at increased risk of lower respiratory tract disease or pneumonia [30–32]. More recently, several observational studies have shown an association between low serum 25(OH)D levels and risk of lower respiratory tract infection in children in India [17], Bangladesh [20], and Turkey [18]. Our study extends these results, suggesting that vitamin D status may also be important for susceptibility to viral upper RTIs in children and adolescents, a finding consistent with the adult literature [21–24]. If confirmed with intervention trials, our findings may have important public health implications given the frequency of viral upper RTIs and their associated morbidity and the prevalence of low vitamin D levels.
We found that serum 25(OH)D level was an important predictor of RTI both as a continuous variable (per 1-unit change in log 25(OH)D), and also when dichotomized into levels <50 nmol/L (AAP recommendations) and levels <75 nmol/L (CPS recommendations). Therefore, although maintaining levels >50 nmol/L is important to prevent rickets, [33] higher levels may be needed for prevention of viral RTI. This was suggested in a recent study involving healthy adults, where a serum 25(OH)D concentration of >38 ng/mL (approximately 95 nmol/L) was associated with a reduced incidence of acute viral respiratory tract infection [24]. Further studies in children are needed to determine the optimal level required for immune function.
Other Canadian studies have failed to show an association between vitamin D status and risk for hospitalization for acute lower respiratory infection in children <5 years of age [19, 25]. Although differences in vitamin D levels were not found in these studies, a higher proportion of participants with acute lower respiratory infection were found to have vitamin D receptor polymorphisms associated with reduced receptor expression in one study [34]. We did find an association between serum 25(OH)D level and upper RTI in children <5 years of age, which may be related to differences in population (urban vs rural, genetic differences such as vitamin D receptor polymorphisms), study design, serum 25(OH)D measurements, or outcome (upper vs lower RTI).
The role of serum 25(OH)D level and individual respiratory viruses has not been studied. It has been postulated that lower vitamin D levels may explain the seasonal variation in influenza [35]. In the case of RSV infection, studies have shown that in RSV-infected human airway epithelial cells, vitamin D induces Ikβα, an NF-kβ inhibitor, in airway epithelium and decreases RSV induction of inflammatory genes [11]. Although we were unable to look at serum 25(OH)D level and the risk of individual respiratory viruses, this area warrants further research as serum 25(OH)D level may impact viruses differently.
Our study has several limitations. First, we obtained only 1 vitamin D measurement for each participant and therefore do not have the exact level around the time of RTI. However, serum 25(OH)D measurements were taken around the same time in October and November and reflect the vitamin D status at the start of the respiratory infection season prior to the development of viral RTI. We believe this estimate is more appropriate than estimates taken at the time of illness because the impact of acute viral illness on serum 25(OH)D levels has not been studied. These levels were also taken at a time just after levels tend to peak in the Northern Hemisphere [36]. Therefore, it is unlikely that vitamin D levels would have rebounded by early spring and had an appreciable impact on infections that occurred later in the follow-up period. In addition, 10 participants had serum for 25(OH)D levels obtained after the follow-up period had started. Sensitivity analysis removing these estimates also did not affect the results. A third limitation was that data were not collected on sources of vitamin D intake for each individual participant. However, there are no data demonstrating that vitamin D supplementation or the use of fortified foods is part of routine practice in Hutterite communities, and therefore we do not feel that this would affect our analysis. Finally, this study was conducted in Hutterite children and adolescents and may not be generalizable to other populations. Studies evaluating other pediatric populations are warranted.
CONCLUSIONS
We found that children and adolescents with lower vitamin D levels were at increased risk for laboratory-confirmed viral upper RTI. Current recommendations regarding target serum 25(OH)D levels may be too low to prevent viral upper RTI. This study provides evidence in support of future interventional trials examining the efficacy of vitamin D supplementation on viral RTIs in children and adolescents.
Notes
Acknowledgments. M. Science receives salary support from the Canadian Institutes for Health Research. M. L. holds the Michael DeGroote Institute for Infectious Disease Research Chair.
Financial support. This work was supported by the Canadian Institutes for Health Research (grant number MCT-88113) and the National Institute for Allergy and Infectious Diseases (grant number 1 U01 AI 76208-01A1).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–94. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 2.Quach C, Piche-Walker L, Platt R, Moore D. Risk factors associated with severe influenza infections in childhood: implication for vaccine strategy. Pediatrics. 2003;112(3 Pt 1):e197–201. doi: 10.1542/peds.112.3.e197. [DOI] [PubMed] [Google Scholar]
- 3.Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia, PA: Churchhill Livingstone, 2010. [Google Scholar]
- 4.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 5.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Oberg F, Botling J, Nilsson K. Functional antagonism between vitamin D3 and retinoic acid in the regulation of CD14 and CD23 expression during monocytic differentiation of U-937 cells. J Immunol. 1993;150(8 Pt 1):3487–95. [PubMed] [Google Scholar]
- 7.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 9.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin D3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 10.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–9. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184:965–74. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res. 2005;30:385–94. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon YJ, Romanowski EG, Shanks RM, Yates KA, Hinsley H, Pereira HA. CAP37-derived antimicrobial peptides have in vitro antiviral activity against adenovirus and herpes simplex virus type 1. Curr Eye Res. 2009;34:241–9. doi: 10.1080/02713680802714066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–5. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 15.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–7. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 16.Porojnicu AC, Moroti-Constantinescu R, Laslau A, et al. Vitamin D status in healthy Romanian caregivers and risk of respiratory infections. Public Health Nutr. 2012;15:2157–62. doi: 10.1017/S1368980012000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 18.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63:473–7. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 19.Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D status is not associated with the risk of hospitalization for acute bronchiolitis in early childhood. Eur J Clin Nutr. 2009;63:297–9. doi: 10.1038/sj.ejcn.1602946. [DOI] [PubMed] [Google Scholar]
- 20.Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99:389–93. doi: 10.1111/j.1651-2227.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- 21.Berry DJ, Hesketh K, Power C, Hypponen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106:1433–40. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- 22.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–7. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 24.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44:981–8. doi: 10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- 26.Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA. 2010;303:943–50. doi: 10.1001/jama.2010.250. [DOI] [PubMed] [Google Scholar]
- 27.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 28.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 29.Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12:583–98. [PMC free article] [PubMed] [Google Scholar]
- 30.Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–4. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 31.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr. 2004;50:364–8. doi: 10.1093/tropej/50.6.364. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui TS, Rai MI. Presentation and predisposing factors of nutritional rickets in children of Hazara Division. J Ayub Med Coll Abbottabad. 2005;17:29–32. [PubMed] [Google Scholar]
- 33.Greer FR. 25-Hydroxyvitamin D: functional outcomes in infants and young children. Am J Clin Nutr. 2008;88:529S–33. doi: 10.1093/ajcn/88.2.529S. [DOI] [PubMed] [Google Scholar]
- 34.Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. J Infect Dis. 2008;197:676–80. doi: 10.1086/527488. [DOI] [PubMed] [Google Scholar]
- 35.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–8. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]