Abstract
Background. Major impediments to development of vaccines and drugs for Plasmodium vivax malaria are the inability to culture this species and the extreme difficulty in undertaking clinical research by experimental infection.
Methods. A parasite bank was collected from a 49-year-old woman with P. vivax infection, characterized, and used in an experimental infection study.
Results. The donor made a full recovery from malaria after collection of a parasite bank, which tested negative for agents screened for in blood donations. DNA sequence analysis of the isolate indicated that it was clonal. Two subjects inoculated with the isolate became polymerase chain reaction positive on days 8 and 9, with onset of symptoms and positive blood smears on day 14, when they were treated with artemether-lumefantrine, with rapid clinical and parasitologic response. Transcripts of the parasite gene pvs25 that is expressed in gametocytes, the life cycle stage infectious to mosquitoes, were first detected on days 11 and 12.
Conclusions. This experimental system results in in vivo parasite growth, probably infectious to mosquitoes. It offers the opportunity to undertake studies previously impossible in P. vivax that will facilitate a better understanding of the pathology of vivax malaria and development of antimalarial drugs and vaccines.
Trial Registration. ANZCTR: 12612001096842.
Keywords: Plasmodium vivax, malaria, experimental infection
More than 2.4 billion persons are at risk of infection with the apicomplexan parasite Plasmodium vivax, with an estimated 80–360 million clinical cases occurring each year. Although often considered benign, P. vivax infection can cause severe disease in nonimmune individuals, resulting in significant morbidity and even mortality [1]. It is becoming clear that public health interventions, including long-lasting insecticide-treated bed nets, improved access to accurate diagnosis, and deployment of highly effective artemisinin combination therapy are resulting in significant falls in the burden of Plasmodium falciparum infection. However, the fall is less pronounced for P. vivax [2], and indeed elimination of P. vivax is likely to be far more difficult than elimination of P. falciparum [2].
The development of a method to maintain P. falciparum in continuous culture in 1976 [3] enabled fundamental research on the biology of this parasite, including a better understanding of the pathogenesis of falciparum malaria, and facilitated drug and vaccine development. Continuous in vitro culture has also greatly facilitated clinical research, by enabling the conduct of controlled human malaria infection (CHMI) studies [4]. This can be achieved by the bite of infected laboratory-bred mosquitoes [5] or by injection of cryopreserved sporozoites [6]. An alternative is induced blood-stage infection, achieved by intravenous injection of well-defined P. falciparum–infected erythrocytes [7].
The inability to undertake continuous culture of P. vivax has significantly hampered both laboratory and experimental infection studies of this Plasmodium species. Induced infection with P. vivax, either by the bite of an infected mosquito or by intravenous injection of infected blood, was the preferred therapy for neurosyphilis until the development of penicillin [8]. Because of a number of ethical and safety concerns, experimental P. vivax blood-stage infection for study of immunity [9] or drug development [10] ceased >50 years ago. The only experimental method available for controlled human P. vivax infection studies has been by feeding mosquitoes on infected patients, and then using these mosquitoes to infect human volunteers [11]. This procedure is logistically challenging, not readily available, and hampered by problems of reproducibility. On each occasion the infecting strain of P. vivax will probably be different. Furthermore, using this method of infection, latent liver infection with hypnozoites must be eliminated by treatment with primaquine, an approach that is not reliable, with relapses having been reported in controlled infection studies despite using appropriate doses of primaquine in subjects with normal levels of G6PD [12]. In this article we report the establishment of a system to undertake induced blood-stage malaria with a well-characterized strain of P. vivax.
METHODS
A 49-year-old woman returned to Brisbane, Australia in February 2012 after a trip to the Solomon Islands where she had participated in volunteer work. She had not taken antimalarial prophylaxis. She had a history of splenectomy in childhood, a consequence of trauma; her blood group was A, Rh negative, Duffy positive. Thirteen days after her return to Australia she developed symptoms of malaria, including fevers, chills, headaches, rigors, lethargy, and generalized myalgia, and she presented to the hospital 2 days later. At initial review her peripheral blood films were positive for P. vivax at a density below the threshold for quantitative estimation by blood film (≤10 parasites/µL); infection with any of the 3 other species of Plasmodium endemic in the Solomon Islands (P. falciparum, P. malariae, or P. ovale) was excluded by polymerase chain reaction (PCR).
On admission to hospital, the patient was febrile (39.5°C) and tachycardic (pulse rate, 120/min), with a blood pressure of 100/57 mm Hg. After she provided written informed consent, 200 mL of blood was collected into a leukodepletion blood collection pack (Pall Medical). Supportive therapy, including fluid therapy, and oral antimalarial treatment with artemethur-lumefantrine (Coartem) was promptly commenced in accordance with Australian guidelines [13]. Within 24 hours her fever had subsided and her condition improved. Thick films were clear of parasites when checked 48 hours after hospital admission.
The patient's hospital stay was complicated by transient acute kidney injury, with a serum creatinine level peaking at 226 µmol/L; this subsequently returned to normal. Concomitant infections were excluded using standard serologic and PCR screening tests for bloodborne virus infection as undertaken with healthy blood donors by the Australian Red Cross Blood Service [14] and mandated by the Australian Therapeutic Goods Administration [15] and the United States FDA [16, 17]. This was supplemented by serologic testing for arboviruses potentially present in the Pacific. Serologic testing demonstrating past infection with cytomegalovirus and Epstein-Barr virus. Follow-up serologic testing 6 months later did not reveal any change in serostatus for the infectious agents tested for.
The donated blood unit was stored for approximately 8 hours at 4°C under controlled conditions prior to being processed. Storage and processing of the blood was in accordance to Good Manufacturing Practice (GMP) guidelines. The unit was leukocyte depleted using the inline leukodepletion filter that was a component of the blood collection pack, red cells were separated from plasma by centrifugation, and the blood was then cryopreserved using a method described elsewhere [18]. Briefly, a ratio of 2 volumes of Glycerolyte 57 to 1 volume of infected cell pellet was used. The first 20% of the Glycerolyte 57 volume was added dropwise with gentle agitation, and the suspension was incubated for 5 minutes at room temperature before the remaining Glycerolyte 57 was added. Aliquots were dispensed into 1mL cryogenic vials, frozen in a controlled rate freezer at a temperature of −165°C. The cooling rate of the controlled freezer was 4°C/min. Aliquots are stored in vapour phase in a dedicated liquid nitrogen tank in a secure environment. This process was performed in accordance with GMP guidelines. The leukodepleted blood tested negative for bacterial contamination with a validated blood culture technique, and for Epstein-Barr and Cytomegalovirus provirus by PCR.
Inoculum Preparation
To prepare inocula for experimental infection studies, aliquots of the P. vivax cell bank were thawed, washed, and resuspended in injectable saline solution according to a method described elsewhere [18], with the modification that the final red cell pellet was resuspended in normal saline solution rather than Roswell Park Memorial Institute medium. One mL of the resuspended cells was set aside for quantification of parasitemia by PCR, and the remainder of the dose dispensed aseptically into a 2-mL syringe and stored on ice until administration. The number of parasite genome equivalents that were injected into the subjects was determined using the quantitative PCR method described below.
Administration of the Inoculum
Two healthy volunteers, both blood group A, Duffy positive, were recruited to undertake this study. Details of the inclusion and exclusion criteria are available in the online trial registration [19]. Both were inoculated by intravenous injection.
DNA Sequencing of the Parasite
Bulk genomic DNA was isolated from frozen leukocyte-depleted blood samples. After standard Illumina library preparation, DNA sequencing was carried out on an Illumina Hi-Seq2000. The method used for bioinformatics analysis is described in the Supplementary Material. Data for the sample sequenced in this study is available in the National Center for Biotechnology Information Sequence Read Archive (SRX217056). Single-nucleotide variant (SNV) discovery was conducted on the 10 publicly available P. vivax genomes (North Korea I, SRP000316; Mauritania I, SRP000493; Brazil I, SRP007883; India VII, SRP007923; IQ07, SRP003406; SA94–SA98, SRA047163).
PCR Quantification of P. vivax Parasitemia
A consensus Plasmodium species RT-PCR method described elsewhere [20] was modified to make use of TaqMan hydrolysis probe chemistry. This PCR assay was designed to amplify a conserved 199–base pair (bp) target of the multicopy and a highly conserved 18S ribosomal RNA gene. The assay is described in detail in the Supplementary Material. Parasites were quantified from 500 µL of packed red cells, using P. falciparum control standards described elsewhere [21]. Each sample was tested in duplicate during the study. After completion of the study, all samples were retested in triplicate. When coefficients of variation varied by >20%, samples were tested again.
Quantification of Gametocytemia
The level of P. vivax gametocytes in blood was quantified by RT-PCR to measure the level of a messenger RNA transcript expressed in mature gametocytes, psv25 [22]. The assay is described in detail in the Supplementary Material. Nucleic acid extraction was undertaken as described above, and genomic DNA removed using RNA-free DNase treatment (Ambion). Quantification was performed in triplicate, and the average recorded. To control for potential DNA contamination, another PCR reaction was performed after heat inactivation of the reverse-transcriptase enzyme. RNA standards were prepared using the Riboprobe System Transcription kit (Promega) from a 267-bp fragment of the pvs25 messenger RNA sequence cloned into the pGEM-T Easy plasmid vector (Promega) that had been linearized by digestion with NotI (New England BioLabs). Ten-fold serial dilutions (7.22 × 109−7.22 copies/µL) were PCR amplified in each test run and used to generate a standard curve.
Ethics
Informed consent was obtained from the patient and volunteers. Human experimentation guidelines of the responsible Human Research Ethics Committees were followed in the conduct of this research. The collection of Plasmodium-infected blood from patients attending Royal Brisbane and Womens and Princess Alexandra Hospitals, Brisbane Australia, was approved by the responsible human research ethics committee (HREC/10/QRBW/379). The preparation and the preservation of the infected blood bank and the phase 1 clinical trial encompassing experimental infection was approved by the Human Research Ethics Committee of the Queensland Institute of Medical Research (p1478). The trial was registered with the Australia and New Zealand Clinical Trials Registry (registration 12612001096842).
RESULTS
Clinical Course
Two subjects were inoculated on consecutive days with 270 µL of reconstituted blood. Retrospective quantitative PCR testing of aliquots of the inocula indicated that they had been injected with approximately 13 000 genome-equivalents of P. vivax. Symptoms consistent with malaria began to be reported from day 11 in subject 1 and day 14 in subject 2, including headache, malaise and myalgia. These symptoms progressed to chills and sweats on the evening of day 13 in subject 1 and on day 14 in subject 2. At this stage, both subjects were admitted to the inpatient facility and treated promptly with artemether-lumefantrine. In the first 12 hours after treatment, the clinical features of malaria increased. Subject 1 became nauseated and vomited, reported headache, and experienced another episode of sweats in the ensuing 12 hours. Subject 2 developed rigors and a headache, both of which resolved with symptomatic treatment. The maximal temperature elevations recorded for subjects 1 and 2 were 39.4°C and 39°C, respectively.
In both subjects, all symptoms of malaria had resolved within 24 hours of commencing antimalarial therapy. For subject 1, mild nontender splenomegaly was palpable on the morning of day 15 through the morning of day 16, with complete resolution by day 28. When reviewed at day 28, both subjects had remained well after discharge, with no subsequent adverse events reported. Serologic follow-up 90 days after completion of the study did not reveal any change in serostatus for bloodborne viruses tested at enrollment.
Expected mild hematologic derangements were observed for both subjects, with mild transient neutropenia (1.3 × 109/L in subject 1 and 1.4 × 109/L in subject 2) and thrombocytopenia (78 × 109/L in subject 1 and 134 × 109/L in subject 2), both of which resolved within 7 days. Biochemistry results were not significantly deranged at any stage of the study.
Course of Parasitemia
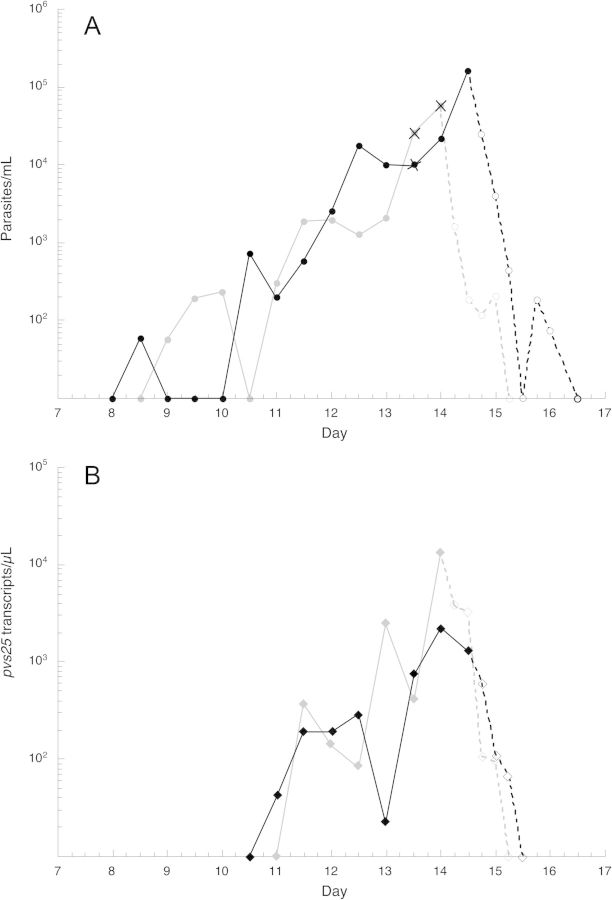
Parasites were first detected by PCR on days 9 and 8 for subjects 1 and 2, respectively; parasitemia peaked on the day of treatment (day 14 morning and evening, respectively) at 54 and 224 parasites/µL, respectively (Figure 1A). Parasites were rapidly cleared from the blood after commencement of treatment as determined by PCR, at 30 and 36 hours, respectively (Figure 1A). Thick blood films were positive at only 2 time points: the afternoon of day 13 (10 parasites/µL) in both subjects; and the morning of day 14 in subject 1 (44 parasites/µL).
Figure 1.
The course of Plasmodium vivax parasitemia (A; circles) and pvs25 transcript levels (B; diamonds) in subjects 1 (gray) and 2 (black). Solid and dashed lines represent pre- and posttreatment findings, respectively. Data points marked with X represent time points when blood smears were positive for malaria parasites.
Available evidence indicates that the life cycle stage of malaria parasites transmitted to mosquitoes is present in the blood early in the course of vivax malaria. The presence of a parasite gene transcript that is expressed only in mature gametocytes, pvs25, was measured using quantitative reverse-transcriptase PCR (RT-PCR). This marker of mature gametocytes was first detected in the blood of the subjects on days 11 and 12, at 2 and 4 days after the first appearance of parasites by PCR, respectively, with a course paralleling that of parasitemia (Figure 1B).
DNA Sequence of the Isolate
Whole-genome sequencing resulted in coverage of the genome at 24X, with 74.3% of the genome covered by ≥5 high-quality bases. The parasite isolate was typed at 47 436 likely SNV positions (Table 1). These markers were spaced, on average, 476 bases apart across the genome and evenly across all chromosomes (Table 1; Supplementary Figure 1A). Of the SNV positions genotyped, 41.5% were located in coding regions, compared with 54.6% of the genome consisting of coding regions (Table 1); of the 20 826 SNVs located in coding regions, 59.7% were nonsynonymous bp changes (Table 1). The parasite strain contained mutations at 13 199 of the 47 436 assayed SNVs, with 5744 located in coding regions, of which 3459 were nonsynonymous bp changes (Table 1). The SNVs were spread evenly throughout the P. vivax genome and spaced an average of 1707 bp apart (Supplementary Figure 1; Supplementary Table 1). Comparison of the genetic profile of this P. vivax isolate with publicly available P. vivax sequencing data at the same 47 436 positions [23–25] demonstrated that that this strain from the South Pacific is distinct from currently sequenced P. vivax strains. Of the publicly available strains, it is most closely related to those from Cambodia (Supplementary Figure 2) [25].
Table 1.
SNVs in the Genotyping Set and Found in the PvA1
| Genotyping Set | PvA1 | |
|---|---|---|
| Total SNVsab | 47 436 | 13 199 |
| In coding regions | 20 826 | 5774 |
| Synonymous | 8399 | 2315 |
| Nonsynonymous | 12 427 | 3459 |
| In introns | 3592 | 947 |
| In putative promoters | 11 556 | 3203 |
| In UTRs | 2829 | 756 |
| In intergenic regions | 11 441 | 3349 |
| Base pairs per SNV | 476 | 1707 |
| SNVs in coding regions, % | 41.5 | 41.2 |
| Proportion of genome consisting of coding regions, %c | 54.6 | 54.6 |
| Homozygous SNVs, No. | … | 11 665 |
| Heterozygous SNVs, No. | … | 1514 |
| Heterozygous SNVs, % | … | 11.47 |
| Fws statistic | … | 0.92 |
| Heterozygous SNVs in variable regions, % | … | 41.81 |
| Proportion of genome consisting of variable regions, % | … | 11.97 |
Abbreviations: SNVs, single-nucleotide variants; UTR, untranslated region.
a Includes 93 multiallelic SNVs.
b Includes multiple effects caused by a single SNV.
c Genome size, 22 627 091 base pairs; coding regions, 12 348 368 base pairs.
As the population of malaria parasites in a single host is very often genetically polymorphic owing to meiotic recombination of genetically distinct male and female gametes in the mosquito midgut, we investigated the clonality of this isolate. Scrutiny of “heterozygote” SNVs identified in this haploid organism indicated that initial patient infection was likely to be clonal, with only 11.5% of the genotyped SNVs labeled as heterozygotes (Table 1; Supplementary Figure 1A). In contrast, a polyclonal infection would have >40% of the genotyped SNVs labeled as heterozygotes (Supplementary Figure 2B). Furthermore, 41.8% of these “heterozygous” bases were located in regions of the parasite genome where current sequencing technology is less accurate owing to repetitive sequences, namely, subtelomeric regions and internal variable gene families that comprise only 12% of the P. vivax genome (Table 1; Supplementary Figure 1A). In a polyclonal infection, it would be expected that the percentage of “heterozygous” calls in these regions would approximate the percentage of the genome that these regions encompass. The clustered heterozygous SNVs identified here are therefore likely to be sequencing and/or alignment errors. In addition, the Fws statistic, which calculates the parasite diversity observed within a host, compared with the parasite diversity in the population as a whole [26, 27], is 0.91 in this isolate (perfect clonal, 1.0), further supporting the hypothesis that the P. vivax infection in the initial donor was clonal.
Particular attention was paid to 49 SNVs present in 5 known and putative drug resistance genes, namely, pvdhfr (PVX_089950), pvdhps (PVX_123230), pvcrt (PVX_087980), pvmdr (PVX_080100), and pvmrp (PVX_097025) (Table 2) [28–31]. This isolate possessed genetic changes at 17 of 49 genotyped positions, with pvdhps being the only resistance gene not having any SNVs (Table 2) [32, 33]. Full-length analysis of the 4 genes containing SNVs revealed 2 additional nonsynonymous SNVs in pvdhfr, namely, F57L and T61M. In addition, the amino acid change at position 117 was found to be the more resistant S-to-T mutation [34–36]. The parasite strain is therefore a pvdhfr quadruple mutant (F57L, S58R, T61M, and S117 T), a genotype, described elsewhere in Indonesia and Papua New Guinea, that is highly resistant to the antifolate drug pyrimethamine [34, 35].
Table 2.
Genetic Polymorphisms in Putative Drug Resistance Genes of Plasmodium vivax
| Gene | Chromosome | Polymorphism | Amino Acid | Reference | PvA1 |
|---|---|---|---|---|---|
| pvcrt | 1 | T331151C | Intron | T | C |
| PVX_087980 | T332453C | Intron | T | C | |
| A332874C | Intron | A | C | ||
| A333509G | Intron | A | G | ||
| T333513G | Intron | T | G | ||
| A333518G | Intron | A | G | ||
| T333544C | Intron | T | C | ||
| C333689T | D328 | C | T | ||
| A333837G | Intron | A | G | ||
| pvmrp | 2 | C154391T | V1478I | C | T |
| PVX_097025 | |||||
| pvdhfr | 5 | C964760G | F57L | C | G |
| PVX_089950 | C964763G | S58R | C | G | |
| C964771T | T61M | C | T | ||
| G964939C | S117T | G | C | ||
| pvmdr1 | 10 | T363169A | Y976F | T | A |
| PVX_080100 | G363223A | T958M | G | A | |
| T363374G | M908L | T | G | ||
| C364004T | G698S | C | T | ||
| T364509C | T529 | T | C | ||
| pvdhps | 14 | None | |||
| PVX_123230 |
DISCUSSION
Observational studies of malariotherapy for syphilis undertaken in the preantibiotic era have provided key insights into the biology and pathogenesis of malaria [8]. Subsequent CHMI studies with P. falciparum are becoming increasingly recognized as essential tools for clinical research. In recent years, sporozoite-induced CHMI studies with P. falciparum have led to major advances in the understanding of immunity [37] and are leading toward vaccine development [38]. Similarly, blood-stage infection studies have facilitated the development of antimalarial drugs [39]. The successful characterization and in vivo testing of a P. vivax parasite bank, as reported here, provide a system to expand these approaches in the modern era to study of P. vivax.
Major challenges in developing this strategy have been addressing the ethical and safety issues in the conduct of experimental infections of healthy volunteers, whether with malaria or with other pathogens [40, 41]. The safety record of CHMI studies is excellent. The published safety experience with sporozoite-induced P. falciparum infections performed between 1986 and 2010 [42–44] includes 613 volunteers, with no serious adverse events reported, apart from a cardiac event occurring in a 20-year-old healthy female volunteer 5 days after she received curative therapy with artemether-lumefantrine [45]. For P. vivax, the conduct of experimental infection by the bite of infected mosquitoes has been reported in a total of 33 volunteers in 2 studies [11, 46]. Although the safety record of induced blood-stage infection with P. falciparum is less substantial, a total of 134 volunteers in 10 studies have been infected by intravenous injection with a single isolate of P. falciparum, with no serious adverse events [39].
Blood-stage P. vivax infection offers a number of logistical and safety advantages over sporozoite-induced infection. For instance, detailed characterization of the parasite isolate by DNA sequence analysis can be performed before in vivo study. This enables assessment of likely drug sensitivity of the isolate and to characterize the parasite antigens that are believed to be under immune selection. In the current challenge model whole-genome sequencing was applied to investigate markers of drug sensitivity, putative targets of immunity, clonality, and the genetic structure of this isolate. The findings indicated that the isolate is clearly related to yet distinct from the publicly available P. vivax DNA sequences from Southeast Asia, and that it contains a quadruple-mutant genotype in pvdhfr, highly suggestive of pyrimethamine resistance, with a set of amino acid changes previously identified in parasites from Indonesia and Papua New Guinea. In addition, the genotyping data is indicative of a clonal infection.
The course of parasitemia observed in this study is consistent with earlier reports of induced blood-stage infection with P. vivax, in which blood-stage infection was induced by injection of ≤100 live parasites [47] or with a larger number of thawed cryopreserved parasites (approximately 107) [9], with a prepatent period by blood smear positivity and symptom onset on days 13–14. There is a growing literature on the PCR-determined in vivo growth kinetics of P. falciparum after sporozoite- or blood-stage–induced infection [21, 48] but none to date in P. vivax infection. This study provides the first description of the course of P. vivax parasitemia as described by PCR.
Our studies also demonstrate the appearance of the gametocytes in the blood as early as 3 days after the onset of PCR-confirmed parasitemia and >2 days before the onset of symptoms or slide positivity. Although we were unable to determine the infectivity to mosquitos of these sexual stages, the findings are consistent with previous reports documenting the infectiousness of patients with early P. vivax infection. Records of volunteers with induced P. vivax infection for malariotherapy for syphilis showed that 39.3% of mosquitoes that fed on patients with similar submicroscopic gametocytemia became infected [49]. Further studies are needed to confirm the infectiousness to mosquitoes of these subjects, but our results suggest that the system may have application in studying P. vivax transmission and investigating suitable vaccination and treatment strategies.
In conclusion, we describe an experimental system of P. vivax infection that offers the potential to accelerate the development of drugs and vaccines for P. vivax malaria. The logistic challenges and financial costs of field efficacy studies of vaccines and drugs are even greater for P. vivax than for P. falciparum malaria owing to the confounding effect of relapse. Therefore, the ability to undertake experimental study of P. vivax malaria in a dedicated clinical trial setting offers a significant resource, enhancing clinical safety and enabling the design and conduct of well-controlled clinical research necessary for the development of drugs and vaccines to control P. vivax.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We would like to thank Dennis Shanks for serving as medical monitor and Ivor Harris from the Australian Army Malaria Institute for reading the blood slides; Jutta Marfurt, and Ric Price from the Menzies School of Health Research for providing reference material for calibrating the PCR assays; Marcelo Ferreria of Cidade Universitaria, Sao Paulo, Brazil for providing the pvs25 reference plasmid; Kate Thorpe, RN, of QPharm; and the patient and two volunteers in this study.
Financial support. This work was supported by the National Health and Medical Research Council (practitioner fellowship to J. M. C.), the government of Queensland (health research fellowship to J. M. C.), and the Office of Health and Medical Research, Queensland Department of Health.
Potential conflicts of interest. The authors do not have a commercial or other association that might pose a conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 2.Feachem RG, Phillips AA, Hwang J, et al. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–78. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 4.Duncan CJ, Draper SJ. Controlled human blood stage malaria infection: current status and potential applications. Am J Trop Med Hyg. 2012;86:561–5. doi: 10.4269/ajtmh.2012.11-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chulay JD, Schneider I, Cosgriff TM, et al. Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am J Trop Med Hyg. 1986;35:66–8. doi: 10.4269/ajtmh.1986.35.66. [DOI] [PubMed] [Google Scholar]
- 6.Epstein JE, Tewari K, Lyke KE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science. 2011;334:475–80. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Q, Lawrence G, Reed C, et al. Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am J Trop Med Hyg. 1997;57:495–500. doi: 10.4269/ajtmh.1997.57.495. [DOI] [PubMed] [Google Scholar]
- 8.Snounou G, Perignon JL. Malariotherapy: insanity at the service of malariology. Adv Parasitol. 2013;81:223–55. doi: 10.1016/B978-0-12-407826-0.00006-0. [DOI] [PubMed] [Google Scholar]
- 9.Tobie JE, Abele DC, Hill GJ, Contacos PG, Evans CB. Fluorescent antibody studies on the immune response in sporozoite-induced and blood-induced vivax malaria and the relationship of antibody production to parasitemia. Am J Trop Med Hyg. 1966;15:676–83. doi: 10.4269/ajtmh.1966.15.676. [DOI] [PubMed] [Google Scholar]
- 10.Trenholme CM, Williams RL, Desjardins RE, et al. Mefloquine (WR 142,490) in the treatment of human malaria. Science. 1975;190:792–4. doi: 10.1126/science.1105787. [DOI] [PubMed] [Google Scholar]
- 11.Herrera S, Fernandez O, Manzano MR, et al. Successful sporozoite challenge model in human volunteers with Plasmodium vivax strain derived from human donors. Am J Trop Med Hyg. 2009;81:740–6. doi: 10.4269/ajtmh.2009.09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett J, Pybus B, Yadava A, et al. Primaquine failure for radical cure of P. vivax hypnozoites in humans is associated with an absence or deficiency in the cytochrome P450 isoenzyme 2D6. 2013 N Engl J Med. [Google Scholar]
- 13.Antibiotic_Expert_Group. Therapeutic guidelines: antibiotic. 14th ed. Melbourne, Australia: Therapeutic Guidelines; 2010. [Google Scholar]
- 14.The Australian Red Cross Blood Service. Donation testing. http://www.transfusion.com.au/blood_products/collection_testing . Accessed 3 June 2013.
- 15.Australaian Government Department of Health and Ageing, Therapeutic Goods Administration. Australian regulatory guidelines for biologicals. www.tga.gov.au/pdf/biologicals-argb-app04.pdf. Accessed 3 June 2013.
- 16.US Food and Drug Information. Code of Federal Regulations. Title 21, chapter I, subchapter F, part 610. General biological products standards. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=610. Accessed 3 June 2012 .
- 17.US Food and Drug Information. Code of Federal Regulations. Title 21, chapter I, subchapter F, part 640. Additional standards for human blood and blood products. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=640. Accessed 3 June 2012.
- 18.Borlon C, Russell B, Sriprawat K, et al. Cryopreserved Plasmodium vivax and cord blood reticulocytes can be used for invasion and short term culture. Int J Parasitol. 2012;42:155–60. doi: 10.1016/j.ijpara.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Australia New Zealand Clinical Trials Registry. Trial ID: ACTRN12612001096842. http://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363064. Accessed 3 June 2013. [Google Scholar]
- 20.Safeukui I, Millet P, Boucher S, et al. Evaluation of FRET real-time PCR assay for rapid detection and differentiation of Plasmodium species in returning travellers and migrants. Malar J. 2008;7:70. doi: 10.1186/1475-2875-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockett RJ, Tozer SJ, Peatey C, et al. A real-time, quantitative PCR method using hydrolysis probes for the monitoring of Plasmodium falciparum load in experimentally infected human volunteers. Malar J. 2011;10:48. doi: 10.1186/1475-2875-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuboi T, Kaslow DC, Gozar MM, Tachibana M, Cao YM, Torii M. Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol Med. 1998;4:772–82. [PMC free article] [PubMed] [Google Scholar]
- 23.Dharia NV, Bright AT, Westenberger SJ, et al. Whole-genome sequencing and microarray analysis of ex vivo Plasmodium vivax reveal selective pressure on putative drug resistance genes. Proc Natl Acad Sci U S A. 2010;107:20045–50. doi: 10.1073/pnas.1003776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neafsey DE, Galinsky K, Jiang RH, et al. The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nat Genet. 2012;44:1046–50. doi: 10.1038/ng.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan ER, Menard D, David PH, et al. Whole genome sequencing of field isolates provides robust characterization of genetic diversity in Plasmodium vivax. PLoS Negl Trop Dis. 2012;6:e1811. doi: 10.1371/journal.pntd.0001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manske M, Miotto O, Campino S, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–9. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auburn S, Campino S, Miotto O, et al. Characterization of within-host Plasmodium falciparum diversity using next-generation sequence data. PLoS ONE. 2012;7:e32891. doi: 10.1371/journal.pone.0032891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kublin JG, Dzinjalamala FK, Kamwendo DD, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–8. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- 29.Mu J, Ferdig MT, Feng X, et al. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol Microbiol. 2003;49:977–89. doi: 10.1046/j.1365-2958.2003.03627.x. [DOI] [PubMed] [Google Scholar]
- 30.Raj DK, Mu J, Jiang H, et al. Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J Biol Chem. 2009;284:7687–96. doi: 10.1074/jbc.M806944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–9. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 32.Imwong M, Pukrittayakamee S, Cheng Q, et al. Limited polymorphism in the dihydropteroate synthetase gene (dhps) of Plasmodium vivax isolates from Thailand. Antimicrob Agents Chemother. 2005;49:4393–5. doi: 10.1128/AAC.49.10.4393-4395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menegon M, Majori G, Severini C. Genetic variations of the Plasmodium vivax dihydropteroate synthase gene. Acta Trop. 2006;98:196–9. doi: 10.1016/j.actatropica.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Hastings MD, Maguire JD, Bangs MJ, et al. Novel Plasmodium vivax dhfr alleles from the Indonesian Archipelago and Papua New Guinea: association with pyrimethamine resistance determined by a Saccharomyces cerevisiae expression system. Antimicrob Agents Chemother. 2005;49:733–40. doi: 10.1128/AAC.49.2.733-740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hastings MD, Porter KM, Maguire JD, et al. Dihydrofolate reductase mutations in Plasmodium vivax from Indonesia and therapeutic response to sulfadoxine plus pyrimethamine. J Infect Dis. 2004;189:744–50. doi: 10.1086/381397. [DOI] [PubMed] [Google Scholar]
- 36.Korsinczky M, Fischer K, Chen N, Baker J, Rieckmann K, Cheng Q. Sulfadoxine resistance in Plasmodium vivax is associated with a specific amino acid in dihydropteroate synthase at the putative sulfadoxine-binding site. Antimicrob Agents Chemother. 2004;48:2214–22. doi: 10.1128/AAC.48.6.2214-2222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–77. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 38.Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol. 2011;11:57–64. doi: 10.1038/nri2902. [DOI] [PubMed] [Google Scholar]
- 39.Engwerda CR, Minigo G, Amante FH, McCarthy JS. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol. 2012;28:515–21. doi: 10.1016/j.pt.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Miller FG, Grady C. The ethical challenge of infection-inducing challenge experiments. Clin Infect Dis. 2001;33:1028–33. doi: 10.1086/322664. [DOI] [PubMed] [Google Scholar]
- 41.Kotloff KL. Human challenge studies with infectious agents. J Investig Med. 2003;51(Suppl 1):S6–11. [PubMed] [Google Scholar]
- 42.Church LW, Le TP, Bryan JP, et al. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J Infect Dis. 1997;175:915–20. doi: 10.1086/513990. [DOI] [PubMed] [Google Scholar]
- 43.Epstein JE, Rao S, Williams F, et al. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis. 2007;196:145–54. doi: 10.1086/518510. [DOI] [PubMed] [Google Scholar]
- 44.Roestenberg M, Bijker EM, Sim BK, et al. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2013;88:5–13. doi: 10.4269/ajtmh.2012.12-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieman AE, de Mast Q, Roestenberg M, et al. Cardiac complication after experimental human malaria infection: a case report. Malar J. 2009;8:277. doi: 10.1186/1475-2875-8-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrera S, Solarte Y, Jordan-Villegas A, et al. Consistent safety and infectivity in sporozoite challenge model of Plasmodium vivax in malaria-naive human volunteers. Am J Trop Med Hyg. 2011;84:4–11. doi: 10.4269/ajtmh.2011.09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyd M, Kitchen S. On attempts to hyperimmunize convalescents from vivax malaria. Am J Trop Med Hyg. 1943;s1–23:209–25. [Google Scholar]
- 48.Roestenberg M, O'Hara GA, Duncan CJ, et al. Comparison of clinical and parasitological data from controlled human malaria infection trials. PLoS ONE. 2012;7:e38434. doi: 10.1371/journal.pone.0038434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collins WE, Jeffery GM, Roberts JM. A retrospective examination of the effect of fever and microgametocyte count on mosquito infection on humans infected with Plasmodium vivax. Am J Trop Med Hyg. 2004;70:638–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.