This commentary focuses on critical challenges in the epidemiology, pathogenesis, host defense, laboratory detection, diagnosis, and treatment of the ongoing tragedy of iatrogenic meningitis caused by Exserohilum rostratum. Advances in understanding of this infection may reduce suffering and save lives.
Keywords: Exserohilum rostratum, meningoencephalitis, antifungals, risk factors, pathogenesis
Abstract
The tragedy of the ongoing epidemic of meningitis caused by Exserohilum rostratum brings into focus the epidemiology, risk factors, pathogenesis, diagnosis, and treatment of a multitude of opportunistic mold infections of the central nervous system. Herein we provide our perspective regarding the translational research objectives of this infection that are needed to make an impact on this important healthcare crisis.
Contamination of a steroid injection preparation has recently resulted in a multistate (19 states) epidemic of Exserohilum rostratum meningitis (ERM) or arthritis [1, 2]. More than 700 cases of spinal and paraspinal infections have been encountered, and 46 patients have died (www.cdc.gov/HAI/outbreaks, last updated 8 April 2013). New cases are continuing to be discovered each day with no apparent end in sight. More than 13 000 patients from 20 states are estimated to have received the contaminated product. ERM has been associated with hemorrhagic and ischemic infarcts, mycotic aneurysms, painful arachnoiditis, epidural abscess, phlegmon, discitis, and vertebral osteomyelitis at or near the site of injection. This is a historically unprecedented public health threat of unmeasured dimensions as the natural history of this serious infection and its immunopathogenesis, treatment, and prognosis are unknown. The purpose of this commentary is to discuss critical questions that may guide the science and management of these devastating infections.
VIRULENCE FACTORS AND CENTRAL NERVOUS SYSTEM INFECTION
Exserohilum rostratum is a thermophilic dematiaceous (melanized) mold that is an uncommon cause of human disease [3]. Little is known about immune predisposition of the host, if any, for ERM. Local impairment of immune defenses by concentrated corticosteroids [4] is likely a key determinant of disease in a closed space such as the cerebrospinal fluid (CSF).
In understanding host–pathogen interactions, the effector-to-target-ratio, measured as the number of phagocytic cells to number of fungal elements, is a key factor. Because we do not know the concentration of organisms within the contaminated solution of methylprednisolone, we are not able to accurately quantify the virulence of this organism in humans. Given that multiple vials were injected with no prior indication that the vials displayed visible growth, the inoculum may have been relatively low, thus possibly accounting for the relatively low infection/exposure rate of >600 patients among 13 000 exposed patients. Quantification of organisms in existing vials by quantitative polymerase chain reaction (PCR) and culture would be valuable in understanding virulence in humans.
The predominance of a single fungus infection in the setting of fungal outbreaks raises important questions in fungal pathogenesis. For example, trauma victims following the devastating tornado in Joplin, Missouri, in 2011 had necrotizing mucormycosis due to the uncommon Mucorales Apophysomyces trapeziformis [5]. Although tornado victims were exposed to soil and dirt containing myriad bacteria and fungi, this unusual pathogen emerged. Perhaps unique geoclimatic conditions of a tornado (eg, turbulence), locally traumatized tissue, and a previously dormant organism conferred an advantage in virulence. Similarly, vials of methylprednisolone were contaminated by uncommon species of molds. Among those organisms, only E. rostratum grew at ≥37°C and only this organism caused central nervous system (CNS) infection. Incubation of E. rostratum in a starvation mode (older lots in the presence of sparse nutrient medium with concentrated methylprednisolone) could result in a growth advantage and/or improved immune evasion strategies.
Binding by fungi of human sterols is well described. Corticosteroids in an apparent receptor/ligand system in Aspergillus fumigatus can accelerate growth [6] and metabolism. Ng and colleagues [6] observed an approximately 35% increased growth rate in A. fumigatus and Aspergillus flavus exposed to pharmacological doses of hydrocortisone. If this corticosteroid-induced enhancement of growth and metabolism occurs in E. rostratum, it may coincide with enhanced melanin biogenesis [7, 8], secreted hydrolytic enzymes, toxin production [9], and more neutrophil-associated inflammatory injury (Figure 1). If E. rostratum possesses corticosterone-binding protein, as observed in Candida species, this may also support corticosteroid-enhanced accelerated growth. In Coccidioides species, where progesterone and 17-β-estradiol promote growth and endospore release [10], this response correlated with the presence of a high-affinity cytosol binding protein receptor for estradiol and a low-affinity receptor for testosterone in Coccidioides species [11]. These receptor/ligand interactions are of course consistent with the increased risk of accelerated coccidioidomycosis during pregnancy. The converse applies in Paracoccidioides brasiliensis, where paracoccidioidomycosis predominates in males in association with 17-β-estradiol-induced inhibition of the mycelial-to-yeast transformation [12–14].
Figure 1.
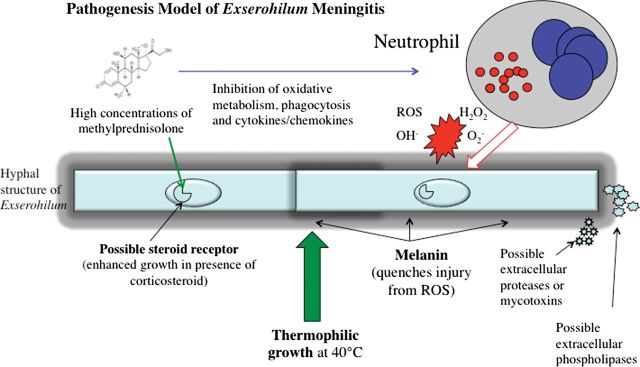
Pathogenesis model of Exserohilum rostratum meningitis. Abbreviations: H2O2, hydrogen peroxide; O2, oxygen; OH, hydroxyl radical; ROS, reactive oxygen species.
Melanin content in E. rostratum's cell wall is a prime candidate for fitness advantage [6, 7]. Fungal melanin serves as a type of “reactive armor” that quenches neutrophil-derived reactive oxygen species, such as superoxide, hydrogen peroxide, and hydroxyl radicals. Melanin also inhibits phagocytosis of conidia and protects filamentous fungi, such as Exophiala (Wangiella) dermatitidis and Aspergillus nidulans, from hydrolytic enzymes. Unlike the melanin of Cryptococcus neoformans, which is synthesized through a phenolic pathway, the melanin of dematiaceous molds, such as E. rostratum, is elaborated through an indole-dependent pentaketide pathway.
The fundamental molecular studies by Paolo et al [15] and Feng et al [16] of the dematiaceous mold E. dermatitidis provide insight into possible contributions of melanin to the pathogenesis and antifungal resistance of E. rostratum. Cell wall pigmentation in E. dermatitidis depends on WdPKS1, which encodes a polyketide synthase required for biosynthesis of the precursor for dihydroxynaphthalene melanin. Organisms with disrupted WdPKS1 or absent gene are more vulnerable to neutrophil killing and are less virulent in mice. They also have thinner cell walls, lack an electron-opaque layer, and are more susceptible to killing by voriconazole, amphotericin B, defensin NP-1, heat, and lysing enzymes than the wild-type melanized strain. Small molecules targeting fungal polyketide synthase would provide potentially novel therapeutic advances against E. rostratum and other dematiaceous molds.
That E. rostratum is able to grow at 40°C is a potential virulence factor that is shared with other neurotropic dematiaceous molds, including Ochroconis gallopava and Cladophialophora bantiana. Both organisms are well known to cause cerebral phaeohyphomycosis [17–19]. What accounts for this propensity to infect the CNS is not well understood. Whether there is “tropism” of specific strains within a genus or a general characteristic within a fungal order for the brain and for their proclivity to create specific clinical manifestations (eg, meningitis vs meningoencephalitis) remains to be understood. An alternative hypothesis is that E. rostratum may be “neurotrophic”; that is, when the organism reaches the CNS, the tissue microenvironment affords a favorable nutritive environment.
Could polymorphisms in genes encoding innate host defense molecules have created a heightened risk in a subpopulation of the >13 000 patients who received the contaminated steroidal product? A haplotype of several polymorphisms may have heightened the risk for infection. Although neutropenia is known to be associated with greater risk of mortality in phaeohyphomycosis [20], the role of neutrophils and other immune cells in host defenses against E. rostratum is not known. The answers to these and other questions will likely reveal a more intricate interaction of fungal pathogenesis and innate host defenses (Figure 1).
DIAGNOSIS AND MONITORING OF THERAPEUTIC RESPONSE
Patients who present with a history of corticosteroid injection and new onset of neurological symptoms and who have a leukocytosis of ≥5 white blood cells per high-powered field are assumed to have ERM warranting antifungal therapy. Culture of E. rostratum in CSF may be delayed or absent.
Development of assays for rapid diagnosis and monitoring of therapeutic response is critically needed. The Centers for Disease Control and Prevention has developed a PCR system using panfungal primers; the amplicon is then sequenced for identification at the species level. However, this assay is used currently for epidemiologic and forensic documentation rather than for immediate bedside care.
In addressing this critical need, Zhao et al [21] from the New Jersey Public Health Research Institute, in collaboration with Weill Cornell Medical Center, developed a quantitative PCR system using beacon probes specific for E. rostratum for rapid diagnosis, monitoring of therapeutic response, and environmental microbiologic surveillance. A key feature of the assay is sensitive detection (50–100 fg) of target nucleic acid even in the presence of excess human DNA. The primers and probes for this assay have been placed in public domain for availability to clinics, hospitals, and reference laboratories.
The cell wall carbohydrate polymer (1→3)-β-D-glucan also has potential utility as a biomarker for diagnosis and monitoring of therapeutic response of ERM. Aspergillus species and other molds, with exception of the Mucorales, broadly express this molecule. Candida species, but not Cryptococcus neoformans, also express (1→3)-β-D-glucan. Although serum (1→3)-β-D-glucan is used successfully in diagnosis of invasive fungal infections in high-risk patients [22], little has been known about its role in fungal meningitis. Our laboratory demonstrated proof of concept of the expression of (1→3)-β-D-glucan in CSF during fungal meningitis in the diagnosis and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis (HCME) [23]. As an extension of these laboratory studies, we have since documented expression of CSF (1→3)-β-D-glucan in pediatric patients with HCME and CNS aspergillosis [24]. Consistent with these findings, 3 of 5 patients with presumed Exserohilum CNS infection had CSF samples with (1→3)-β-D-glucan levels >31 pg/mL, suggesting its utility for diagnosis and monitoring of ERM [25].
As patients are being treated for ERM, there is understandable reluctance in discontinuation of therapy. Discontinuation of therapy based solely upon improvement of symptoms and CSF pleocytosis may not be reliable. Use of CSF PCR and (1→3)-β-D-glucan may provide more objective microbiologic evidence of eradication of ERM.
PHARMACOLOGY, PHARMACODYNAMICS, AND THERAPEUTIC INTERVENTIONS
Currently, voriconazole, a lipophilic triazole that highly penetrates the CNS, is recommended as the initial antifungal agent on the basis of published minimum inhibitory concentrations (MICs) of a collection of Exserohilum isolates [26–28]. However, the correlations between in vitro activity defined by MICs and clinical outcome are difficult to evaluate for this dematiaceous mold. The MICs of voriconazole against these fungal species vary widely, ranging from 0.03 µg/mL to 1.0 µg/mL. Other isolates may have an MIC of 2 μg/mL. For organisms with MICs of 1–2 μg/mL, sustained CNS concentrations may be difficult to achieve without reaching toxic plasma concentrations. Because reduction of voriconazole dosage may lead to clinical progression, alternative therapeutic approaches are needed.
The currently recommended dosage of voriconazole 6 mg/kg intravenously every 12 hours is intended to achieve plasma and CSF exposures that would exceed the MICs of 1–2 μg/mL of infecting strains. Although this dosage is routinely used as a loading dose in adults, little is known about maintenance administration of voriconazole at 6 mg/kg intravenously. Because 6 mg/kg intravenously every 12 hours exceeds the Michaelis-Menten saturation of voriconazole's metabolism, the plasma concentrations will predictably display nonlinear plasma pharmacokinetics, resulting in precipitously high circulating concentrations. Thus, some patients receiving this regimen have shown serum trough levels exceeding 10 µg/mL in association with dose-limiting visual hallucinations, photosensitization, and incapacitating systemic symptoms.
Liposomal amphotericin B (LAmB) is recommended as an alternative to voriconazole. Studies of plasma pharmacokinetics of LAmB at 7.5 mg/kg/day demonstrate an area under the concentration–time curve that would exceed the MIC of 1 µg/mL for this organism [29]. Although the MICs of amphotericin B are within safely achievable concentrations, this higher dose may not be predictive of CNS response [30]. Moreover, dose-limiting nephrotoxicity, especially in older patients and those with diabetes mellitus or other underlying renal impairment, are requiring a lower dosage of LAmB or alternative antifungal options. Whether a lower and less nephrotoxic dosage of LAmB at 5 mg/kg/day may also be effective against ERM in such patients is not known.
Other antifungal triazoles, such as posaconazole and itraconazole, may provide alternative options for single-agent therapy. Posaconazole has in vitro activity within achievable plasma concentrations that exceed the MICs of several dematiaceous molds, including Bipolaris species, C. bantiana, Curvularia species, E. dermatitidis, and Rhinocladiella mackenziei [31]. Studies in clinical CNS cryptococcosis, experimental phaeohyphomycosis, and clinical chromoblastomycosis have shown activity of posaconazole [32–34]. Posaconazole also has favorable in vitro activity against E. rostratum with median MICs of 0.003 μg/mL. However, little is known about the in vivo antifungal activity and CNS pharmacology of posaconazole against this organism or other mold pathogens.
Itraconazole also has in vitro activity against dematiaceous molds, including E. rostratum with MICs that are similar to those of posaconazole. The activity of itraconazole against coccidioidal meningitis [35] provides a foundation for understanding its potential utility in treatment of ERM.
Isavuconazole is an investigational triazole that is structurally similar to voriconazole [36]. Isavuconazole has in vitro activity against E. rostratum; however, the MICs are approximately 4 μg/mL. Although these MIC values are higher than those of the hydrophobic triazoles (posaconazole and itraconazole), the CSF penetration of isavuconazole is approximately 50% in comparison to being almost undetectable for itraconazole. Although isavuconazole is active in vitro against E. rostratum, there is a paucity of data on CNS pharmacology of isavuconazole. Flucytosine (5-fluorocytosine) has a high level of CNS penetration but has minimal in vitro activity against E. rostratum.
The pharmacokinetic and pharmacodynamic exposures needed for successful treatment of ERM using voriconazole, liposomal amphotericin B, posaconazole, itraconazole, or isavuconazole are not known. One should underscore that CSF levels do not necessarily correlate with therapeutic response. Concentrations of antifungal agents needed to treat infected parenchymal brain tissue are not necessarily reflected by CSF levels. For example, amphotericin B and itraconazole, which are active in treatment of CNS mycoses, have minimal CSF levels [37, 38].
Combination antifungal therapy against ERM also may be beneficial but has not been defined either in vitro or in vivo. The rationale for the original recommendation of the combination of voriconazole and LAmB was based on providing a broad spectrum against possible polymicrobial infection. However, for patients who are not able to tolerate the higher dosages of voriconazole, the combination of a triazole with an echinocandin warrants further exploration. While the echinocandins are active in vitro against E. rostratum, they optimally would be used in combination with an antifungal triazole.
The commonly held belief that echinocandins have no activity in the CNS is not accurate. Although echinocandins do not penetrate an intact blood–brain barrier, they do have efficacy in treatment of CNS infections where the blood–brain barrier is disrupted. Laboratory studies demonstrate successful treatment of experimental HCME with echinocandins in association with detectable drug levels in CNS tissue [39]. These findings are compatible with that of an earlier case of HCME successfully treated with caspofungin and were predictive of the results of a randomized trial of caspofungin in treatment of neonatal candidemia and HCME [40, 41].
Optimal duration of antifungal therapy of ERM also is unknown. Early indications suggest that the time to eradication of E. rostratum may be measured in months rather than weeks. As little is known about the outcome of ERM following discontinuation of antifungal therapy, consideration should be given to the possibility that relapse may occur and that some patients may require life-long suppression of ERM. That life-long antifungal therapy may be needed for a CNS mycosis is exemplified in the management of meningeal coccidioidomycosis, where chronic suppression with fluconazole or itraconazole is considered standard of care [35]. If voriconazole is used for chronic suppression, the potential development of cutaneous melanomas and squamous cell carcinomas need to be considered.
Chronic administration of voriconazole of ERM is challenging. Therapeutic drug monitoring of serum voriconazole is warranted to ascertain stability and attainment of target serum concentrations. Because voriconazole achieves an approximately 50% CSF/plasma ratio, CSF concentrations are not necessary. Assuming a voriconazole MIC of 1.0 µg/mL, serum protein binding of 50%, and 50% CSF penetration, one would need a minimum serum trough concentration of 4.0 µg/mL. Some patients clearly do not tolerate this sustained concentration of voriconazole. Other patients have been found to have unremitting decline of their serum concentrations over time on the same dosage. This decline of voriconazole serum concentrations over time also has been observed in oncology patients [42]. Moriyama et al demonstrated that this process is likely related to autoinduction [43]. The approaches to such patients include increase of dosage and addition of a CYP2C19 inhibitor, such as omeprazole. However, when such measures do not overcome the autoinduction effect, alternative antifungal agents are required.
Intrathecal or intraventricular administration has been suggested as adjunctive therapy but has little data to support its usage. The potential for introduction of bacterial pathogens through an Ommaya reservoir is high and the possibility of inducing chemical arachnoiditis via the intrathecal route is prohibitive in patients who already have arachnoidal pathology. Moreover, penetration of intrathecally administered antimicrobial agents beyond the subarachnoid space into CNS parenchymal lesions is relatively unpredictable. By comparison, delivery of antifungal agents via the circulation provides more predictable CSF and parenchymal levels without the risks of bacterial infection, CSF dural leaks, and chemical arachnoiditis.
PROGNOSIS AND OUTCOME
As ERM is treated, patterns of persistence, recurrence, and development of new symptoms and signs may emerge. The new onset of lumbosacral arachnoiditis, epidural abscesses, and paraspinal infections are being reported in patients receiving voriconazole. Development of predictive population-based risk models for assessment of prognosis and outcome is needed. In the absence of such models, quantitative serial molecular diagnostic assays, such as quantitative PCR, nucleic acid sequence-based amplification, and (1→3)-β-D-glucan in CSF, may indicate patients who are at risk for persistent or refractory infection.
Why would ERM persist despite antifungal therapy? Melanization of E. rostratum may contribute to evasion of host defense and increase resistance. In addition to subtherapeutic concentrations of voriconazole in CSF, we do not know if voriconazole truly kills E. rostratum in the CNS. Fungistatic activity of voriconazole coupled with melanin-driven evasion of innate host defenses may be critical factors contributing to persistence or relapse of ERM.
FUTURE DIRECTIONS AND RESEARCH NEEDS
Improved understanding of the pathogenesis and host defenses may permit development of new approaches for immune augmentation in patients with ERM (Table 1). Critically needed in this public health crisis are definitive animal models for characterization of the antifungal pharmacokinetics, pharmacodynamics, molecular detection, therapeutic monitoring, and host defense that will provide a scientific foundation for treating these patients. During the bioterrorist anthrax attacks in the United States in 2001, the availability of data from the primate model of inhalational anthrax was critical in guiding the use of ciprofloxacin over penicillin. Such model systems are urgently needed to meet this public health threat.
Table 1.
Scientific Objectives Needed to Understand and Advance the Care of Patients With Exserohilum rostratum Meningitis
| Pathogenesis |
| • Whole genome sequencing |
| • Effect of glucocorticosteroids on growth and virulence, including toxin secretion and regulation of melanization |
| • Thermotolerance |
| • Development of physiologically and medically relevant models of acute and subacute infections in and innovative mini–host models to study CNS tropism of Exserohilum rostratum |
| • Quantification of organisms in vials and relation to outcome in patients |
| Host defense |
| • Analysis for host immunogenetics for increased susceptibility to ERM through whole genome or candidate gene strategies |
| • Innate host responses to E. rostratum and cognate receptors of relevant effector immune cells (TLR, dectin-1) |
| • Innate host response to E. rostratum and role of melanin |
| • Effect of glucocorticosteroids on CNS host response, including microglial cells through functional genomic, transcriptomic, and proteomic responses |
| • Role of phagocytic and immunoregulatory cells |
| • Functional genomic and transcriptional messenger RNA and proteomic responses of peripheral blood mononuclear cells, neutrophils, and lymphocytes |
| • Effects of corticosteroids on innate host response |
| • Augmentation and immunoregulation of innate host response |
| Molecular detection and monitoring of therapeutic response |
| • Application and study of developed qPCR system |
| • Application and study of (1→3)-β-D-glucan |
| • Development of sensitive neuroimaging strategies for screening and therapeutic monitoring |
| • Predictive models for discontinuation of therapy of ERM based on composite end points that use radiologic and non-culture-based diagnostics |
| CNS pharmacology, pharmacokinetics, and pharmacodynamics of antifungal agents |
| • Development of relevant animal models of ERM to study CNS pharmacology, pharmacokinetics, and pharmacodynamics of available antifungal agents |
| • Combination antifungal therapy to improve therapeutic index and efficacy |
| • Bridging studies to patients for optimization of dose-exposure-response relations and explore combination antifungal therapy to improve therapeutic index and efficacy |
| • Study of the clinical impact of voriconazole and therapeutic drug monitoring on outcome of ERM and long-term drug-related toxicity |
| • Study of the clinical impact of liposomal amphotericin B on outcome of ERM and long-term drug-related toxicity |
| Clinical epidemiology |
| • Prospective registries to determine the burden of ERM and impact of type/timing antifungal use on clinical presentation, diagnosis, and long-term outcome |
| • Predictive models for development and complications of ERM |
Abbreviations: CNS, central nervous system; ERM, Exserohilum rostratum meningitis; qPCR, quantitative polymerase chain reaction; TLR, Toll-like receptor.
Providing a rapid emergency regulatory process with expedited scientific review for approval of new diagnostic systems or protocols for Investigational New Drug applications could accelerate availability for new strategies in the battle against ERM. Whole genome sequencing of the organism may reveal new understanding of its virulence, pathogenesis, and targets for therapy. Understanding host genetic risk factors for ERM may help to define the subpopulation among all patients exposed to the contaminated methylprednisolone solution. More work also is needed to delineate the nature of potential exposure risk, populations at highest risk of disease and adverse outcomes, and prophylaxis or preemptive treatment strategies. Development of a Bayesian predictive model may allow identification of the patients at highest risk for early intervention. This public health crisis also should be a catalyst for better regulation and oversight of the manufacturing process of such products and enforcement of safe practices by compounding pharmacies so that such tragic epidemics may be prevented [44].
Notes
Financial support. T. J. W. is a Scholar of the Henry Schueler Foundation and a Scholar of Pediatric Infectious Diseases of the Sharpe Family Foundation, and also receives support from the Save Our Sick Kids Foundation (R34HL117352 and 1$01AI103315-01A1). D. P. K. acknowledges the Frances King Black Endowed Professorship for Cancer Research.
Potential conflicts of interest. T. J. W. has received research grants for experimental and clinical antimicrobial pharmacotherapeutics from Astellas, Novartis, Merck, ContraFect, and Pfizer and has served as a consultant to Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. D. P. K. has received research grants from Merck, Pfizer, Astellas, and Gilead and serves on the advisory board of Merck and on the speakers’ bureau of Gilead. D. S. P. has received research support from Astellas, Merck, bioMérieux, Myconostica, and Pfizer. E. R. reports no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention (CDC) Multistate fungal meningitis outbreak—interim guidance for treatment. MMWR Morb Mortal Wkly Rep. 2012;61:842.. [PubMed] [Google Scholar]
- 2.Kainer MA, Reagan DR, Nguyen DB, et al. the Tennessee Fungal Meningitis Investigation Team. Fungal infections associated with contaminated methylprednisolone in Tennessee. N Engl J Med. 2012;367:2194–2203. doi: 10.1056/NEJMoa1212972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon DM, Polak-Wyss A. The medically important dematiaceous fungi and their identification. Mycoses. 1991;34:1–18. doi: 10.1111/j.1439-0507.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 4.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–38. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 5.Green JP, Karras DJ. Update on emerging infections: news from the Centers for Disease Control and Prevention. Notes from the field: fatal fungal soft-tissue infections after a tornado—Joplin, Missouri, 2011. Ann Emerg Med. 2012;59:53–5. doi: 10.1016/j.annemergmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Ng TT, Robson GD, Denning DW. Hydrocortisone-enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology. 1994;140:2475–9. doi: 10.1099/13500872-140-9-2475. [DOI] [PubMed] [Google Scholar]
- 7.Liu GY, Nizet V. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 2009;17:406–13. doi: 10.1016/j.tim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nosanchuk J, Casadevall A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother. 2006;50:3519–28. doi: 10.1128/AAC.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orciuolo E, Stanzani M, Canestraro M, et al. Effects of Aspergillus fumigatus gliotoxin and methylprednisolone on human neutrophils: implications for the pathogenesis of invasive aspergillosis. J Leukoc Biol. 2007;82:839–48. doi: 10.1189/jlb.0207090. [DOI] [PubMed] [Google Scholar]
- 10.Drutz DJ, Huppert M. Coccidioidomycosis: factors affecting the host-parasite interaction. J Infect Dis. 1983;147:372–90. doi: 10.1093/infdis/147.3.372. [DOI] [PubMed] [Google Scholar]
- 11.Powell BL, Drutz DJ. Identification of a high-affinity binder for estradiol and a low-affinity binder for testosterone in Coccidioides immitis. Infect Immun. 1984;45:784–6. doi: 10.1128/iai.45.3.784-786.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Restrepo A, Salazar ME, Cano LE, Stover EP, Feldman D, Stevens DA. Estrogens inhibit mycelium-to-yeast transformation in the fungus Paracoccidioides brasiliensis: implications for resistance of females to paracoccidioidomycosis. Infect Immun. 1984;46:346–53. doi: 10.1128/iai.46.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aristizábal BH, Clemons KV, Cock AM, Restrepo A, Stevens DA. Experimental Paracoccidioides brasiliensis infection in mice: influence of the hormonal status of the host on tissue responses. Med Mycol. 2002;40:169–78. doi: 10.1080/mmy.40.2.169.178. [DOI] [PubMed] [Google Scholar]
- 14.Shankar J, Wu TD, Clemons KV, Monteiro JP, Mirels LF, Stevens DA. Influence of 17β-estradiol on gene expression of Paracoccidioides during mycelia-to-yeast transition. PLoS One. 2011;6:e28402. doi: 10.1371/journal.pone.0028402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paolo WF, Jr, Dadachova E, Mandal P, Casadevall A, Szaniszlo PJ, Nosanchuk JD. Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella [Exophiala] dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiol. 2006;6:55. doi: 10.1186/1471-2180-6-55. (1–16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng B, Wang X, Hauser M, et al. Molecular cloning and characterization of WdPKS1, a gene involved in dihydroxynaphthalene melanin biosynthesis and virulence in Wangiella (Exophiala) dermatitidis. Infect Immun. 2001;69:1781–94. doi: 10.1128/IAI.69.3.1781-1794.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh TJ, Dixon DM, Polak A, Salkin IF. Comparative histopathology of Dactylaria constricta, Fonsecaea pedrosoi, Wangiella dermatitidis, and Xylohypha bantiana in experimental phaeohyphomycosis of the central nervous system. Mykosen. 1987;30:215–25. doi: 10.1111/j.1439-0507.1987.tb03970.x. [DOI] [PubMed] [Google Scholar]
- 18.Dixon DM, Walsh TJ, Merz WG, McGinnis MR. Human central nervous system infections due to Xylohypha bantiana (Cladosporium trichoides) Rev Infect. Dis. 1989;11:515–25. doi: 10.1093/clinids/11.4.515. [DOI] [PubMed] [Google Scholar]
- 19.Shoham S, Pic-Aluas L, Taylor J, et al. Pulmonary Ochroconis gallopavum infections. Transplant Infect Dis. 2008;10:442–8. doi: 10.1111/j.1399-3062.2008.00327.x. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Ami R, Lewis RE, Raad II, Kontoyiannis DP. Phaeohyphomycosis in a tertiary care cancer center. Clin Infect Dis. 2009;48:1033–41. doi: 10.1086/597400. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Petraitiene R, Walsh TJ, Perlin DS. A real-time PCR assay for rapid detection and quantification of Exserohilum rostratum, a causative pathogen of fungal meningitis from injection of contaminated methylprednisolone. J Clin Microbiol. 2013;51:1034–6. doi: 10.1128/JCM.03369-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odabasi Z, Mattiuzzi G, Estey E, et al. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004;39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- 23.Petraitiene R, Petraitis V, Hope WW, et al. CSF and plasma (1→3)-β-D-glucan as surrogate markers for detection and therapeutic response of experimental hematogenous Candida meningoencephalitis. Antimicrob Agents Chemother. 2008;52:4121–9. doi: 10.1128/AAC.00674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvatore CM, Chen T, Toussi SS, et al. (1→3)-β-D-glucan in cerebrospinal fluid as a biomarker for Candida and Aspergillus infections of the central nervous system in infants, children, and adolescents. Accepted for presentation at the 2014 Interscience Conference on Antimicrobial Agents and Chemotherapy (abstract in press) [Google Scholar]
- 25.Lyons JL, Roos KL, Marr KA, et al. Cerebrospinal fluid (1,3) β-D-glucan detection as an aid to diagnose iatrogenic fungal meningitis. J Clin Microbiol. 2013;51:1285–7. doi: 10.1128/JCM.00061-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Cunha KC, Sutton DA, Gené J, Capilla J, Cano J, Guarro J. Molecular identification and in vitro response to antifungal drugs of clinical isolates of Exserohilum. Antimicrob Agents Chemother. 2012;56:4951–4. doi: 10.1128/AAC.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoham S, Marr KA. Treatment of iatrogenic fungal infections: a black mold defines a new gray zone in medicine. Ann Intern Med. 2012;158:208–10. doi: 10.7326/0003-4819-158-3-201302050-00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauffman CA, Pappas PG, Patterson TF. Fungal infections associated with contaminated methylprednisolone injections—preliminary report [Epub ahead of print 19 October 2012] N Engl J Med. 2012 doi: 10.1056/NEJMra1212617. doi:10.1056/NEJMoa1213978. [DOI] [PubMed] [Google Scholar]
- 29.Walsh TJ, Goodman JL, Pappas P, et al. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob Agents Chemother. 2001;45:3487–96. doi: 10.1128/AAC.45.12.3487-3496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clemons KV, Schwartz JA, Stevens DA. Experimental central nervous system aspergillosis therapy: efficacy, drug levels and localization, immunohistopathology, and toxicity. Antimicrob Agents Chemother. 2012;56:4439–49. doi: 10.1128/AAC.06015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graybill JR, Najvar LK, Johnson E, Bocanegra R, Loebenberg D. Posaconazole therapy of disseminated phaeohyphomycosis in a murine model. Antimicrob Agents Chemother. 2004;48:2288–91. doi: 10.1128/AAC.48.6.2288-2291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negroni R, Tobón A, Bustamante B, Shikanai-Yasuda MA, Patino H, Restrepo A. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2005;47:339–46. doi: 10.1590/s0036-46652005000600006. [DOI] [PubMed] [Google Scholar]
- 34.Flores VG, Tovar RM, Zaldivar PG, Martinez EA. Meningitis due to Cryptococcus neoformans: treatment with posaconazole. Curr HIV Res. 2012;10:620–3. doi: 10.2174/157016212803305970. [DOI] [PubMed] [Google Scholar]
- 35.Tucker RM, Denning DW, Dupont B, Stevens DA. Itraconazole therapy for chronic coccidioidal meningitis. Ann Intern Med. 1990;112:108–12. doi: 10.7326/0003-4819-112-2-108. [DOI] [PubMed] [Google Scholar]
- 36.Livermore J, Hope W. Evaluation of the pharmacokinetics and clinical utility of isavuconazole for treatment of invasive fungal infections. Expert Opin Drug Metab Toxicol. 2012;8:759–65. doi: 10.1517/17425255.2012.683859. [DOI] [PubMed] [Google Scholar]
- 37.Sorensen KN, Sobel RA, Clemons KV, Pappagianis D, Stevens DA, Williams PL. Comparison of fluconazole and itraconazole in a rabbit model of coccidioidal meningitis. Antimicrob Agents Chemother. 2000;44:1512–7. doi: 10.1128/aac.44.6.1512-1517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groll AH, Giri N, Petraitis V, et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis. 2000;182:274–82. doi: 10.1086/315643. [DOI] [PubMed] [Google Scholar]
- 39.Hope WW, Mickiene D, Petraitis V, et al. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis. 2008;197:163–71. doi: 10.1086/524063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odio CM, Araya R, Pinto LE, et al. Caspofungin therapy of neonates with invasive candidiasis. Pediatr Infect Dis J. 2004;23:1093–7. [PubMed] [Google Scholar]
- 41.Mohamed WA, Ismail M. A randomized, double-blind, prospective study of caspofungin vs. amphotericin B for the treatment of invasive candidiasis in newborn infants. J Trop Pediatr. 2012;58:25–30. doi: 10.1093/tropej/fmr025. [DOI] [PubMed] [Google Scholar]
- 42.Moriyama B, Elinoff J, Danner RL, et al. Accelerated metabolism of voriconazole and its partial reversal by cimetidine. Antimicrob Agents Chemother. 2009;53:1712–4. doi: 10.1128/AAC.01221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulanovich V, Lewis RE, Raad II, Kontoyiannis DP. Random plasma concentrations of voriconazole decline over time. J Infect. 2007;55:e129–30. doi: 10.1016/j.jinf.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Perfect JR. Iatrogenic fungal meningitis: tragedy repeated. Ann Intern Med. 2012;157:825–6. doi: 10.7326/0003-4819-157-11-201212040-00558. [DOI] [PubMed] [Google Scholar]


