Abstract
Adult prostatic stromal sarcoma is a rare malignant tumor. The main presenting symptom is urinary retention secondary to bladder outlet obstruction. Prostatic Specific Antigen level can be normal. Imaging features show a prostate mass with or without pelvic organ invasion depending on the aggressiveness of the tumor. We present a patient with prostatic stromal sarcoma who debuted with urinary obstruction, leukocytosis and neutrophilia, prostate enlargement, and hypodense prostate areas on CT images, simulating prostatitis with abscess formation.
Keywords: Stromal sarcoma, Prostatic neoplasm, CT imaging
CASE REPORT
A 39-year-old patient presented at the emergency room with bladder outlet obstruction (BOO). He had no history of urinary tract infection, urinary lithiasis, trauma/congenital urethral anomalies, or other symptoms suggestive of genitourinary pathology.
The digital rectal examination (DRE) revealed a palpable, painful, and tumescent prostatic mass. Blood analysis indicated leukocytosis (16.06 × 103 cells/μl; normal range is 3.5–10.8 × 103 cells/μl) and neutrophilia (87.6; normal range is 1.3–8.0 × 103 cells/μl). All hemocultures and urine cultures were negative, and the Prostatic Specific Antigen (PSA) level was 1 ng/ml (normal PSA < 4 ng/ml).
To rule out pelvic abscesses and because we could not perform a Transrectal Ultrasound (TRUS) since it was very painful, a computed tomography (CT) exploration was performed. CT showed an enlarged (8.6 cm × 9.5 cm × 9 cm in the latero-lateral, antero-posterior and sagittal diameters, respectively) and heterogeneous prostate gland, with low and delayed contrast enhancement and central hypodense areas with peripheral rim enhancement (Figures 1a, b, 2, and 3). Prostatic infection was reported.
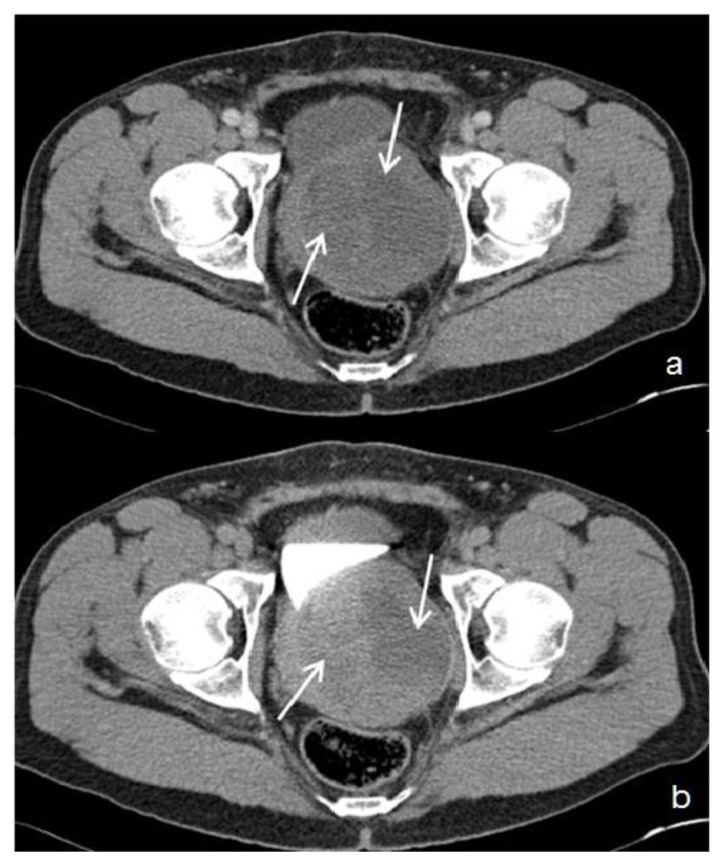
Figure 1.
39 year-old male patient with stromal prostatic sarcoma. CT (16 Multidetector Somatom Sensation 16 - Siemens Medical Systems, Forchheim, Germany). Technique: single-bolus 2-phase protocol (portal and excretory phases at 70 s and 600 s delays, respectively, from the start of contrast material injection (100 ml of 320 mgI/ml Optiray™ 320-Ioversol, Covidien, Spain) with the following acquisition parameters: 150 effective mAs, 120 kV, 2 mm with 1mm reconstruction increment slice thickness.
In the portal phase - Figure 1a: axial image, demonstrates an enlarged (8.6 cm × 9.5 cm in the latero-lateral, antero-posterior diameters, respectively), and heterogeneous prostate gland, with low contrast enhancement and central hypodense areas (white arrows). During the excretory phase (Figure 1b: axial image), the prostatic mass displaces the bladder and the left ureter, and contacts the anterior face of the rectum, displacing it discreetly backwards. No lymphadenopathy or metastases were visible in the rest of the abdomen.
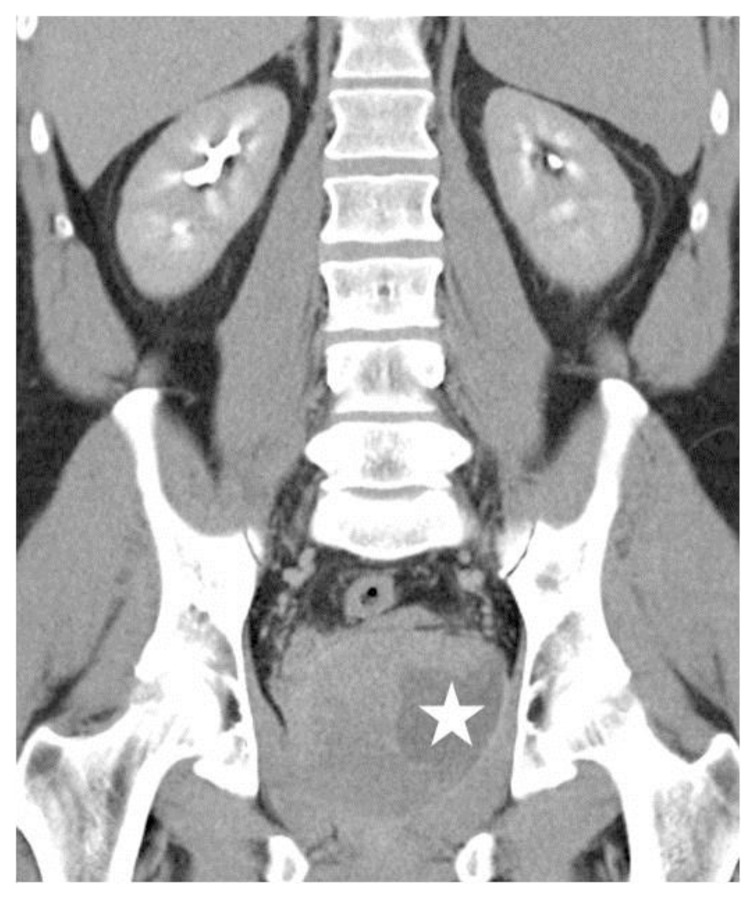
Figure 2.
39 year-old male patient with stromal prostatic sarcoma. CT (16 Multidetector Somatom Sensation 16 - Siemens Medical Systems, Forchheim, Germany). Technique: single- bolus 2-phase protocol (portal and excretory phases at 70 s and 600 s delays, respectively, from the start of contrast material injection, (100 ml of 320 mgI/ml Optiray™ 320-Ioversol, Covidien, Spain) with the following acquisition parameters: 150 effective mAs, 120 kV, 2 mm with 1mm reconstruction increment slice thickness.
Coronal Multiplanar Reformatted Image (MPR) (5 mm thickness) in the excretory phase demonstrates delayed contrast enhancement in the prostate gland and cystic areas (white star) with slight peripheral rim enhancement.
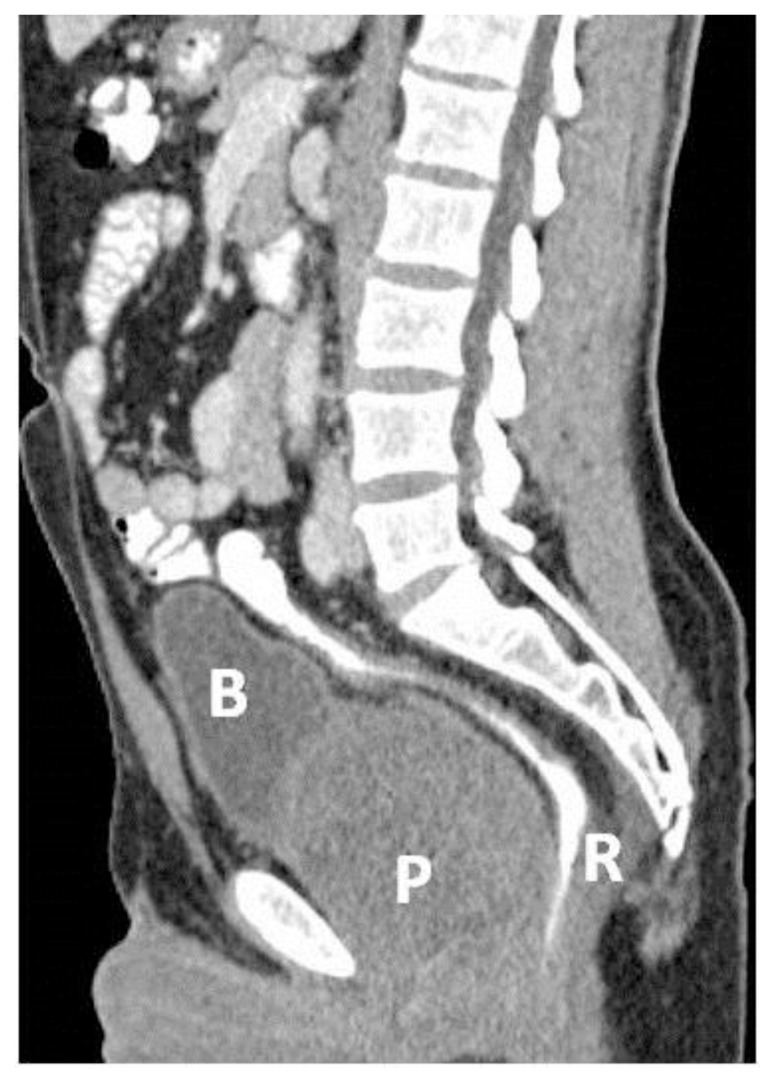
Figure 3.
39 year-old male patient with stromal prostatic sarcoma. CT (16 Multidetector Somatom Sensation 16 - Siemens Medical Systems, Forchheim, Germany), technique: single-bolus 2-phase protocol (portal and excretory phases at 70 s and 600 s delays, respectively, from the start of contrast material injection, (100 ml of 320 mgI/ml Optiray™ 320-Ioversol, Covidien, Spain) with the following acquisition parameters: 150 effective mAs, 120 kV, 2 mm with 1mm reconstruction increment slice thickness.
Sagittal image (MPR) (5 mm thickness) in the portal phase shows an increased (9 cm in the sagittal diameter) prostate gland (P) that displaces the rectum (R) backwards and the bladder (B) upwards.
Analgesic and antibiotic treatment was administered. Despite leukocytosis and neutrophilia, our patient did not show other clinical features of either prostatitis or prostatic abscesses, since blood and urinary cultures were negative. 48 hours after admission and pain relief, a TRUS-guided prostate biopsy was performed and the histological sampling of prostate needle biopsy specimens was prostatic sarcoma.
The patient underwent radical cystoprostatectomy and ileal conduit derivation without pelvic lymphadenectomy (Figure 4). The final histopathological diagnosis was prostatic stromal sarcoma (PSS) with infiltration of the prostate capsule, without infiltration of the bladder and rectum (Figure 5). There were also areas of necrosis and interstitial hemorrhage. The patient continued to show no relapse 30 months after the surgical intervention.
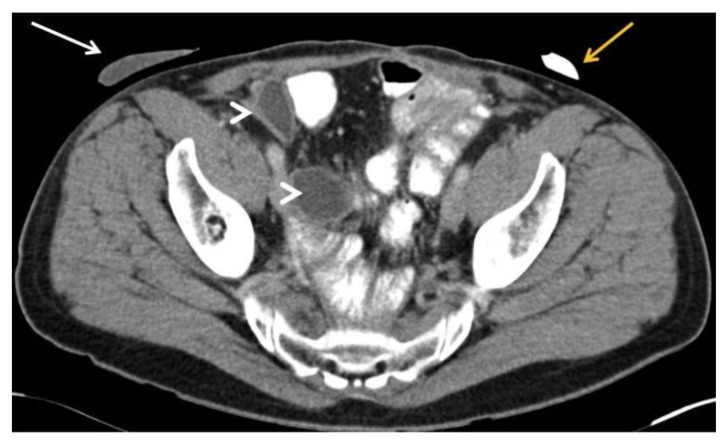
Figure 4.
39 year-old male patient with stromal prostatic sarcoma. CT (16 Multidetector Somatom Sensation 16 - Siemens Medical Systems, Forchheim, Germany). Technique: single-bolus portal phase protocol (70 s delay from the start of contrast material injection, (100 ml of 320 mgI/ml Optiray™ 320-Ioversol, Covidien, Spain) with the following acquisition parameters: 150 effective mAs, 120 kV, 2 mm with 1mm reconstruction increment slice thickness
Axial image after radical cystoprostatectomy, shows bowel loops occupying the pelvic space with left colostomy (yellow arrow), and right ileal conduit (white arrow and arrowheads).
Figure 5.
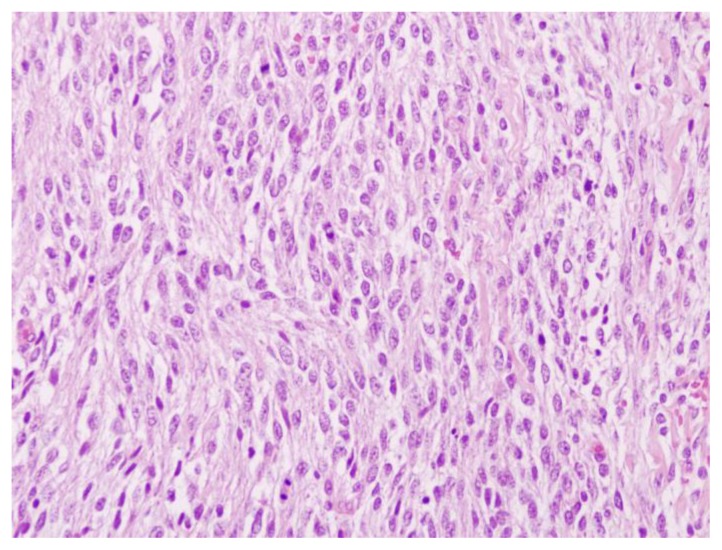
39 year old male patient with stromal prostatic sarcoma. Microscopic pathology. Prostatic stromal sarcoma with high grade prostatic intraepithelial neoplasia (PIN). Nearly all of the prostate is occupied by a tumor composed of large cells with polygonal or spindle morphology. Proliferating cells have clear or slightly eosinophilic cytoplasm and slightly pleomorphic ovoid nuclei equipped with nucleoli. Dominated areas of high cellularity and numerous mitoses are present, some atypical. Immunohistochemical analysis demonstrated expression of Vimentin, with weak and occasional expression of actin and only focal expression of progesterone receptors. The rate of cell proliferation (Ki-67) was 40%.
DISCUSSION
Bladder outlet obstruction (BOO) is a blockage that slows or stops urine flow out of the bladder. Chronic bladder outlet obstruction causes bladder stones, infection and damage to the bladder and kidneys. Benign prostatic hyperplasia (BPH) is the leading cause of BOO in aging men (> 50). Causes of BOO in young men include urethral stricture secondary to urethral injury or surgery, dysfunctional voiding, neurogenic-based detrusor-sphincter dyssynergia (DSD), and primary bladder neck obstruction. In these cases the prostate gland does not show enlargement unlike BPH. Scarring of the urinary channel (urethra) or bladder neck, as a result of injury, surgery, or lower urinary tract infection is the leading cause in young men [1].
Although our patient presented with BOO, the presence of leukocytosis and neutrophilia in a young man, made the diagnosis of an inflammatory/infectious disease more probable. Nevertheless, young men rarely develop urinary tract infections. In male children, infections occur in patients with complicating factors, such as abnormal anatomy, voiding disorders, or urinary tract instrumentation. In contrast, clinical series suggest that few healthy young men presenting with acute urinary tract infections have anatomical or functional urinary tract abnormalities [2]. Previous prostate biopsy and prior fluoroquinolone intake are significant risk factors behind a rising incidence of acute prostatitis [3].
According to the National Institutes of Health (NIH) prostatitis classification, Category I is defined as follows: “Patients with acute bacterial prostatitis present with acute symptoms of a urinary tract infection characteristically including urinary frequency and dysuria. Some patients have symptoms suggestive of systemic infection, such as malaise, fever, and myalgias. Bacteriuria and pyuria are related to infection of the prostate and bladder”. Category II is defined like chronic bacterial prostatitis with recurrent infection of the prostate. Category III is defined as chronic nonbacterial prostatitis or chronic pelvic pain syndrome, where there is no demonstrable infection by conventional microbiologic techniques. Category IV is an asymptomatic inflammatory prostatitis, where there are no subjective symptoms, but white blood cells are found in prostate secretions or in prostate tissue during an evaluation for other disorders [4].
Prostatic abscesses are uncommon in clinical practice because early antibiotic therapy has reduced complications of prostatitis. Prostatic abscess mainly affects diabetic, immunosuppressed patients, and old patients with pre-existent chronic obstructive troubles or urinary episodic inflammation [5]. Escherichia coli (E. Coli) is the causative organism in the majority of cases of acute prostatitis, post-biopsy prostatitis (E. Coli with a high rate of fluoroquinolone resistance), and prostatic abscesses [2–5].
Our patient, since blood and urinary cultures were negative, a TRUS-guided prostate biopsy was necessary to establish the correct diagnosis.
Regarding prostate tumors, the most frequent tumor is prostate adenocarcinoma. It accounts for 95 percent of all prostate cancers. The risk of prostatic adenocarcinoma rises steeply with age, and about three-quarters of all cases occur in men aged 65 or more. With the introduction of PSA screening, increased detection of preclinical disease was allowed. PSA is elevated beyond the arbitrary cut-off point of 4.0 ng/ml in the majority of patients with prostate cancer [6, 7]. Most prostate adenocarcinomas are asymptomatic and detected on PSA screening and prostatic biopsy, by (TRUS)-guided biopsy and more recently, by magnetic resonance imaging (MRI)-guided biopsy [8, 9].
TRUS is primarily used to direct the biopsy needle to desired anatomic locations to estimate the volume of the prostate and assist in sampling prostate tissue in a spatially systematic way, but it is unreliable in differentiating normal prostate gland from cancer tissue, and up to 40% of cancers are missed by TRUS. Prostate adenocarcinomas are located in the peripheral and posterior zone but it can extend or arise in the transitional zone. Contrast-enhanced color Doppler imaging and contrast-enhanced ultrasound, is a promising tool since it takes advantage of the difference in the microvasculature between areas of prostate cancer and benign prostate tissue [10, 11].
CT has low sensitivity to detect and stage prostate cancer because it does not provide sufficient soft-tissue contrast beyond size assessment of the prostate. Although CT is valuable in the evaluation of pelvic lymphadenopathy and bone metastases, MR imaging and bone scanning have been found superior in their assessment [11–13]. MRI and MRI-spectroscopy is a valuable method for detecting and staging prostate cancer. An increase in the choline-to-citrate ratio or the (choline + creatine)/citrate ratio is often used as a marker of malignancy in prostate cancer and increases the specificity of diagnosis; however, it is most reliable in the peripheral zone [11, 14, 15]. Diffusion-weighted MRI (DWI), and dynamic contrast-enhanced MRI (DCE-MRI), have been investigated for potential to complement T2-weighted MRI in improving prostate cancer localization. DWI is based on the diffusion properties of water within tissue. Regions of prostate cancer show increased cell density and reduced apparent diffusion coefficient (ADC) relative to normal prostate. DCE-MRI measures tumor vascularity. After injecting a gadolinium chelate contrast agent, areas of hypervasculature such as prostate cancer show rapid enhancement and early washout of signal intensity. However, some prostate cancers are not detectable by this method because of low vascularity. Combining DCE-MRI with T2-weighted MRI improved prostate cancer detection and staging accuracy [11,14, 16].
Nuclear medicine (Fluorine-18-labeled FDG-PET and 11C and 18F choline and acetate PET/CT) methods are increasingly being used for imaging of prostate cancer. PET/CT, in comparison to FDG-PET, improves especially the lesion localization as well as characterization. On the other hand, FDG-PET has a limited value when the lesion is close enough to a hot source such as urinary bladder due to isotope shine through [17]. At the present time, [(11) C]-choline PET/CT is not recommended in the primary setting but may be utilized in clinically suspected prostate cancer with repeatedly negative prostate biopsies, in preparation of a focused re-biopsy [11,18].
Prostatic urothelial carcinoma represents 0.7–2.8% of prostatic tumors in adults. Most patients have the same age range as bladder urothelial carcinoma (45–90 years). In patients with invasive bladder carcinoma, there is involvement of the prostate in up to 45% of cases. The prostate is a rare site of extranodal lymphoma and only a few cases are reported. Of patients with chronic lymphocytic leukemia, 20% are reported to have prostate involvement at autopsy. The most frequent symptoms are those related to lower urinary obstruction. Metastatic tumors arise outside of the prostate and spread to the gland by vascular channels, since contiguous spread from other pelvic tumors into the prostate does not constitute a metastasis. Metastases from lung, skin (melanoma), gastrointestinal tract, kidney, testis and endocrine glands have been reported. Lung is the most common primary site of metastases to the prostate. Direct spread of bladder carcinoma is the commonest secondary prostatic tumor [6].
Primary prostate sarcomas are rare. Rhabdomyosarcoma is the most common tumor of the lower genitourinary tract in the first two decades of life and it represents 5–10% of malignant solid tumors of childhood. PSS are rare mesenchymal tumors, representing less than 0.1% of primary prostate malignancies in adults [6]. The patient age ranges from 25 to 86 years, and one half of these patients are younger than 50 years [18]. The common clinical presentation of adult prostate sarcoma is urinary retention, hematuria or hematospermia, and a palpable rectal mass. PSA levels are usually normal [6, 19–21]. See table 1.
Table 1.
Summary table of stromal prostatic sarcoma
| Etiology | Mesenchymal tumor arising in the cells of the stromal prostate |
| Incidence | Represent less than 0,1% of primary prostate malignancy in adults |
| Gender ratio | Essentially is a male neoplasm |
| Age predilection | Ranges from 25 to 86 years, and one half of these patients are younger than 50 years |
| Risk factor | No specific risk factor |
| Treatment | Radical prostatectomy or cystoprostatectomy |
| Prognosis | The prognosis is generally still bad for low-grade tumors |
| Imaging findings | Large, round or lobulated, solid or cystic mass with rapid, hypervascular, heterogeneous soft-tissue enhancement. Tumors can invade adjacent structures and organs, with the bladder and rectum the most commonly invaded organs. |
PSS was formally described by Gaudin et al. in 1998 [19]. This tumor originates in the cells of the stromal prostate. The current World Health Organization classification characterizes this tumor as a distinctive spindle cell neoplasm [6]. The term “stromal tumors of uncertain malignant potential” (STUMP) was coined in recent years to describe a proliferation of stromal cells that is behaviorally and histologically distinct from benign hyperplasia and whose behavior cannot be predicted by histological appearance. Gaudin et al. identified four distinct STUMP types based on their histological pattern and degree of atypia. Stromal sarcoma may arise de novo or coexist with preexistent or concurrent STUMP, suggesting a potential for STUMP to dedifferentiate into stromal sarcoma. A literature review of patients younger than 40 years identified only 21 stromal prostatic tumors from 1977 until 2010 [19, 21].
Macroscopically, PSS can be solid or mixed with cystic areas. Necrosis and hemorrhage may also occur, especially in high-grade tumors. Size varies (2–18 cm) in the reported cases; interestingly, size does not correlate with the grade or clinical behavior of the tumor. The tumor tends to occupy the majority of or the entire prostate. Microscopically, PSS is characterized by proliferation of spindle and ovoid stromal cells, some of which poses atypical nuclei, scattered mitotic figures, and necrotic foci. PSS is divided into low-grade and high-grade tumors based on moderate to high cellular atypia and hypercellularity in high-grade tumors. Immunochemically, this neoplasm expresses a positive progesterone receptor, and occasional cases have exhibited estrogen-receptor positivity. CD34 and Vimentin are both expressed diffusely, and in most cases a marked increase in the Ki-67 labeling index is observed if a high grade of intraepithelial neoplasia (PIN) is associated with the tumor [6, 21].
Until now, most reports have been based on histological characteristics, and limited published data is available regarding the CT or MRI characteristics of PSS. The published cases describe a large, round or lobulated, solid or cystic mass with rapid, hypervascular, heterogeneous soft-tissue enhancement [22]. CT in our patient showed an enlarged prostate with little enhancement and hypodense areas with peripheral rim enhancement on the excretory phase, simulating abscess formation (figures 1, 2, and 3). Pathological analysis revealed that these hypodense areas were areas of necrosis, a probable cause of the observed low contrast material enhancement, leukocytosis and neutrophilia. We have not found published data regarding this unusual clinical manifestation of prostate sarcoma. For detailed differential diagnosis between PSS and other prostatic entities, see table 2.
Table 2.
Differential diagnosis table of stromal prostatic sarcoma
| TRUS | CT | MRI | PET | |
|---|---|---|---|---|
| Stromal prostatic sarcoma | Prostate is markedly enlarged and heterogeneous | Homogeneous/heterogen eous mass with unclear delimitation. Slight enhancement after intravenous contrast material injection | Isointense or slightly hypointense mass on the axial, unenhanced, T1-weighted MRI image and heterogeneous signal intensity on T2-weighted MRI image | No experience reported in this area |
| Prostate Abscess | Focal hypoechoic or anechoic lesions with a thickened wall with/without septations | Multiple fluid collections within the prostate gland and/or periprostatic tissues. Peripheral rim enhancement after intravenous contrast material injection | Multiloculated cystic lesion. Slightly increased signal intensity in T2-weighted sequences, relatively isointense in T1-weighted sequences | No experience reported in this area |
| Prostate Carcinoma |
|
CT scanning in arterial-phase the can help differentiate between peripheral and central prostate regions, but it cannot demonstrate intraprostatic pathology; however, it may be helpful in detecting nodal involvement and metastasis |
|
FDG-PET and PET CT improve the lesion localization as well as characterization and have a role in the detection of lymph node metastases from prostate cancer, particularly in patients with relapsed disease after primary treatment. |
| BPH | Hypoechoic/hyperechoic nodules in an enlarged heterogeneous gland | Irregular shapes and various sizes and may contain hemorrhage or calculi | No experience reported in this area |
TRUS: Transrectal Ultrasound, CT: Computed Tomography, MRI: Magnetic Resonance Imaging, PET: Positron Emission Tomography, BPH: Benign prostatic hyperplasia, PC: Prostate carcinoma, ADC: apparent diffusion coefficient, DCE-MRI Dynamic-contrast enhanced MRI, FDG-PET: Fluorine-18-labeled PET, PET/CT: 11C and 18F choline and acetate PET/CT.
Tumors can invade adjacent structures and organs, with the bladder and rectum the most commonly invaded. No published case included pelvic lymphadenopathy on CT and MRI as demonstrated by operation and pathology, and thus pelvic lymphadenectomy is not routinely performed [21, 22].
A poor prognosis still accompanies low-grade prostate sarcoma tumors, which have a high prevalence of spreading to the seminal vesicles, bladder, and rectum. Surgical margins are sometimes difficult to obtain due to involvement of adjacent structures. Vascular invasion and metastases to the liver and lung have also been reported in high-grade sarcomas. Treatment is based on radical prostatectomy or cystoprostatectomy, and there is no current data regarding the effectiveness of chemotherapy or radiotherapy [6].
TEACHING POINT
Prostate sarcoma should be included in the differential diagnosis of a prostatic mass with heterogeneous or low enhancement of the contrast material, and clinical symptoms of inflammatory prostatic disease, in a young patient with normal PSA levels.
ABBREVIATIONS
- BOO
Bladder outlet obstruction
- BPH
Benign prostatic hyperplasia
- CT
Computed Tomography
- DCE-MRI
Dynamic Contrast-Enhanced MRI
- DRE
Digital rectal examination
- DSD
Detrusor-sphincter dyssynergia
- DWI
Diffusion-Weighted MRI
- E. Coli
Escherichia Coli
- FDG-PET
Fluorine-18-labeled PET
- MPR
Multiplanar Reformatted Images
- MRI
Magnetic resonance imaging
- NIH
National Institutes of Health
- PET/CT
11C and 18F choline and acetate PET/CT
- PET
Positron emission tomography
- PIN
Prostatic intraepithelial neoplasia
- PSA
Prostatic Specific Antigen
- PSS
Prostatic stromal sarcoma
- STUMP
Stromal tumors of uncertain malignant potential
- TRUS
Transrectal Ultrasound
REFERENCES
- 1.Dmochowski RR. Bladder Outlet Obstruction: Etiology and Evaluation. Rev Urol. 2005;7(Suppl 6):S3–S13. [PMC free article] [PubMed] [Google Scholar]
- 2.Krieger JN, Dobrindt U, Riley DE, Oswald E. Acute Escherichia coli Prostatitis in Previously Health Young Men: Bacterial Virulence Factors, Antimicrobial Resistance, and Clinical Outcomes. Urology. 2011;77:1420–1425. doi: 10.1016/j.urology.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 3.Mosharafa AA, Torky MH, El Said WM, Meshref A. Rising Incidence of Acute Prostatitis Following Prostate Biopsy: Fluoroquinolone Resistance and Exposure Is a Significant Risk Factor. Urology. 2011;78:511–515. doi: 10.1016/j.urology.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 4.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282(3):236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 5.Barozzil L, Pavlica P, De Matteis M, Canepari M. AJR. Vol. 170. 1998. Prostatic Abscess: Diagnosis and treatment; pp. 753–757. [DOI] [PubMed] [Google Scholar]
- 6.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Tumors of the urinary system and male genital organs. Lyon: IARC Press; 2004. Pathology and genetics; pp. 218–249. (World Health Organization Classification of tumors) [Google Scholar]
- 7.DeAntoni EP, Crawford ED, Oesterling JE, Ross CA, Berger ER, McLeod DG, Staggers F, Stone NN. Age- and race-specific reference ranges for prostate-specific antigen from a large community-based study. Urology. 1996;48:234–239. doi: 10.1016/s0090-4295(96)00091-x. [DOI] [PubMed] [Google Scholar]
- 8.Ravery V, Goldblatt L, Royer B, Blanc E, Toublanc M, Boccon-Gibod L. Extensive biopsy protocol improves the detection rate of prostate cancer. J Urol. 2000;164:393–396. [PubMed] [Google Scholar]
- 9.Yacoub JH, Verma S, Moulton JS, Eggener S, Oto A. Imaging-guided Prostate Biopsy: Conventional and Emerging Techniques. RadioGraphics. 2012;32:819–837. doi: 10.1148/rg.323115053. [DOI] [PubMed] [Google Scholar]
- 10.Hamper UM, Sheth S, Walsh PC, Holtz PM, Epstein JI. Carcinoma of the prostate: value of transrectal sonography in detecting extension into the neurovascular bundle. AJR. 1990;155:1015–1019. doi: 10.2214/ajr.155.5.2120928. [DOI] [PubMed] [Google Scholar]
- 11.Kelloff GJ, Choyke P, Coffey DS for The Prostate Cancer Imaging Working Group. Challenges in Clinical Prostate Cancer: Role of Imaging. AJR. 2009;192:1455–1470. doi: 10.2214/AJR.09.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasch C, Barillot I, Remeijer P, Touw A, van Herk M, Lebesque JV. Definition of the prostate in CT and MRI: a multi-observer study. Int J Radiat Oncol Biol Phys. 1999;1999;43:57–66. doi: 10.1016/s0360-3016(98)00351-4. [DOI] [PubMed] [Google Scholar]
- 13.Dotan ZA. Bone imaging in prostate cancer. Nat Clin Pract Urol. 2008;5(8):434–444. doi: 10.1038/ncpuro1190. [DOI] [PubMed] [Google Scholar]
- 14.Choi YJ, Kim JK, Kim M, Kim KW, Choi EK, Cho K-S. Functional MR Imaging of Prostate Cancer. RadioGraphics. 2007;27:63–77. doi: 10.1148/rg.271065078. [DOI] [PubMed] [Google Scholar]
- 15.Claus FG, Hricak H, Hattery RR. Pretreatment Evaluation of Prostate Cancer: Role of MR Imaging and 1H MR Spectroscopy. RadioGraphics. 2004;24:S167–S180. doi: 10.1148/24si045516. [DOI] [PubMed] [Google Scholar]
- 16.Oto A, Yan CH, Kayhan A, Tretiakova M, Antic T, Schmid-Tannwald C, Eggener S, Karczmar GS, Stadler WM. Diffusion-Weighted and Dynamic Contrast-Enhanced MRI of Prostate Cancer: Correlation of Quantitative MR Parameters With Gleason Score and Tumor Angiogenesis. AJR. 2011;197:1382–1390. doi: 10.2214/AJR.11.6861. [DOI] [PubMed] [Google Scholar]
- 17.Liu Yiyan. Invalidity of SUV measurements of lesions in close proximity to hot sources due to “shine-through” effect on FDG PET-CT interpretation. Radiology research and practice. 2012;2012:1–5. doi: 10.1155/2012/867218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy RC, Kawashima A, Peller PJ. The Utility of 11C-Choline PET/CT for Imaging Prostate Cancer: A Pictorial Guide. AJR. 2011;196:1390–1398. doi: 10.2214/AJR.10.5491. [DOI] [PubMed] [Google Scholar]
- 19.Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol. 1998;22(2):148–62. doi: 10.1097/00000478-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Morikawa T, Goto A, Tomita K, Tsurumaki Y, Ota S, Kitamura T, et al. Recurrent prostatic stromal sarcoma with massive high-grade prostatic intraepithelial neoplasia. J Clin Pathol. 2007;60:330–332. doi: 10.1136/jcp.2006.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombo P, Ceresolli GL, Boiocchi L, et al. Prostatic stromal tumor with fatal outcome in a young man: histopathological an immunohistochemical case presentation. Rare tumors. 2010:2e57. doi: 10.4081/rt.2010.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Fy, Lu JP, Wang J, Ye Jj, Shao Cw, Wan Mj. Adult prostate sarcoma: radiological-clinical correlation. Clinical Radiology. 2009;64:171e177. doi: 10.1016/j.crad.2008.07.013. [DOI] [PubMed] [Google Scholar]