Abstract
Objective:
To test the hypothesis that higher levels of red blood cell (RBC) docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) have a protective association with domain-specific cognitive function in women aged 65 years and older.
Methods:
A total of 2,157 women with normal cognition enrolled in a clinical trial of postmenopausal hormone therapy were followed with annual cognitive testing for a median of 5.9 years. In this retrospective cohort study, we assessed the relationship between prerandomization RBC DHA + EPA levels and a) cognitive measures at baseline, and b) cognitive change over time. Endpoints were composite cognitive function and performance in 7 cognitive domains: fine motor speed, verbal memory, visual memory, spatial ability, verbal knowledge, verbal fluency, and working memory.
Results:
After adjustment for demographic, clinical, and behavioral characteristics, no significant (p < 0.01) cross-sectional cognitive differences were found between women in the high and low DHA + EPA tertiles at the time of the first annual cognitive battery. In addition, no significant (p < 0.01) differences were found between the high and low DHA + EPA tertiles in the rate of cognitive change over time.
Conclusions:
We did not find an association between RBC DHA + EPA levels and age-associated cognitive decline in a cohort of older, dementia-free women.
Cognitive aging can be defined as gradual, subclinical deterioration in neurocognitive function associated with normal adult aging.1 This decline has been attributed to age-related changes in cerebral volume, vasculature, and functional integration.2 The rate of age-associated cognitive decline can vary considerably between individuals,2 prompting efforts to identify modifiable factors such as exercise, nutrition, and social support that may contribute to optimal cognitive aging.3,4
Increasing omega-3 polyunsaturated fatty acid (PUFA) intake has been proposed as a possible intervention for preventing or delaying age-associated cognitive decline.5 Researchers have described several mechanisms by which omega-3s, particularly docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), may slow cognitive decline. First, DHA is a major structural component of brain tissue, comprising 30% of mammalian gray matter neuronal membranes.6 Animal and cell models suggest that DHA contributes to synaptic function, neurotransmission, and neuronal membrane fluidity.7,8 Second, DHA has antioxidant, anti-inflammatory, and anti-apoptotic effects, which may slow brain deterioration.7,9 Third, DHA and EPA may reduce the risk of vascular dementia by reducing triglycerides, blood pressure, and inflammation, and improving endothelial function.10 Based on these physiologic mechanisms and results from previous observational studies,10 we hypothesized that higher levels of red blood cell (RBC) DHA and EPA would have a protective effect on performance in one or more cognitive domains in a study population of older postmenopausal women.
METHODS
This study was a secondary analysis of data collected for the Women's Health Initiative Study of Cognitive Aging (WHISCA), an ancillary study within the Women's Health Initiative (WHI) hormone therapy (HT) trials.11 In the HT trials, women aged 65 to 80 years with prior hysterectomy were randomized to estrogen or placebo, and women without hysterectomy to estrogen plus progestin or placebo. Starting in 1999, 2,302 dementia-free WHI HT trial participants were enrolled in WHISCA. WHISCA subjects' first cognitive assessment occurred a median of 3.0 years (range = 1.1–5.6 years) after HT randomization, and they completed a battery of cognitive tests on a yearly basis. Although the WHI estrogen-plus-progestin and estrogen-only HT trials ended in 2002 and 2004, respectively, follow-up of the WHISCA study cohort continued until 2007. Results from WHISCA and the larger Women's Health Initiative Memory Study showed that both forms of HT were harmful to cognitive function.12 Additional information about WHISCA may be found in prior publications.12–14
Standard protocol approvals, registrations, and patient consents.
The NIH and Institutional Review Boards for all participating institutions approved the study procedures and documents. Written informed consent was obtained from all participants as part of the original WHISCA study.
Fatty acid measurement.
Blood samples were obtained from 2,208 WHISCA participants before WHI randomization; RBCs were isolated, frozen, and stored at −80°C. RBCs were later thawed, aliquoted, and analyzed by gas chromatography as previously described.15 RBC omega-3 PUFA content correlates with the omega-3 content of other tissues,16 and serves as a stable biological marker of average omega-3 PUFA intake over the 1 to 2 months before measurement.17 In our analyses, the exposure of interest was the proportion of subjects' RBC membranes composed of DHA and EPA (DHA + EPA).
During aliquoting, the RBC samples were stored improperly at −20°C for a period of approximately 2 weeks, causing oxidative degradation of the membrane PUFAs before measurement. Before our study, Pottala et al.15 corrected the RBC PUFA measurements as described previously. Briefly, they performed experiments to determine the rates at which RBC PUFAs degrade at −20°C. Results from these experiments were used to develop regression calibration equations and estimate subjects' predegradation RBC PUFA levels. Because the correction process is imperfect, a random error term was added to the best estimate of each subject's predegradation DHA and EPA levels to produce 10 imputed DHA and EPA values for each subject. Multiple imputation allowed the imprecision in subjects' corrected DHA + EPA levels to be quantified and properly accounted for in our statistical analyses. Fifty-one subjects were excluded because their RBC PUFAs had degraded significantly and did not meet the quality-control criterion recommended by Pottala et al.15 This reduced the number of eligible WHISCA subjects to 2,157.
The validity of the corrected RBC DHA + EPA measurements can be evaluated by comparison with participants' reported dietary habits. In both the WHI and the Nurses' Health Study,18 RBC DHA and EPA contents were measured and dietary DHA and EPA intakes estimated from food frequency questionnaires. Correlations between the RBC biomarkers and dietary intake estimates for DHA and EPA were similar in the 2 studies (see appendix e-1 on the Neurology® Web site at www.neurology.org). Additionally, test/retest reliability statistics were available for a subset of 796 participants whose RBC DHA + EPA levels were measured both at WHI screening and at their first annual visit. The correlation between these 2 measurements was reasonably strong (Pearson r = 0.70; intraclass correlation coefficient = 0.69).
Cognitive assessments.
The annual WHISCA test battery included 1) the Finger Tapping Test to assess fine motor speed, 2) the Card Rotations Test to assess spatial ability, 3) the Benton Visual Retention Test to assess short-term visual memory, 4) the California Verbal Learning Test to assess verbal memory, 5) the Primary Mental Abilities Vocabulary test to assess verbal knowledge, 6) letter and category fluency tests to assess verbal fluency, and 7) the Digit Span Forward and Backward Test to assess working memory. For each domain, subjects' raw test scores were standardized to z scores as described previously19; we also calculated a composite cognitive function z score for each participant. Details can be found in appendix e-1, and participants' raw scores in table e-1. For all cognitive outcomes, a higher standardized score indicates better performance.
Covariates.
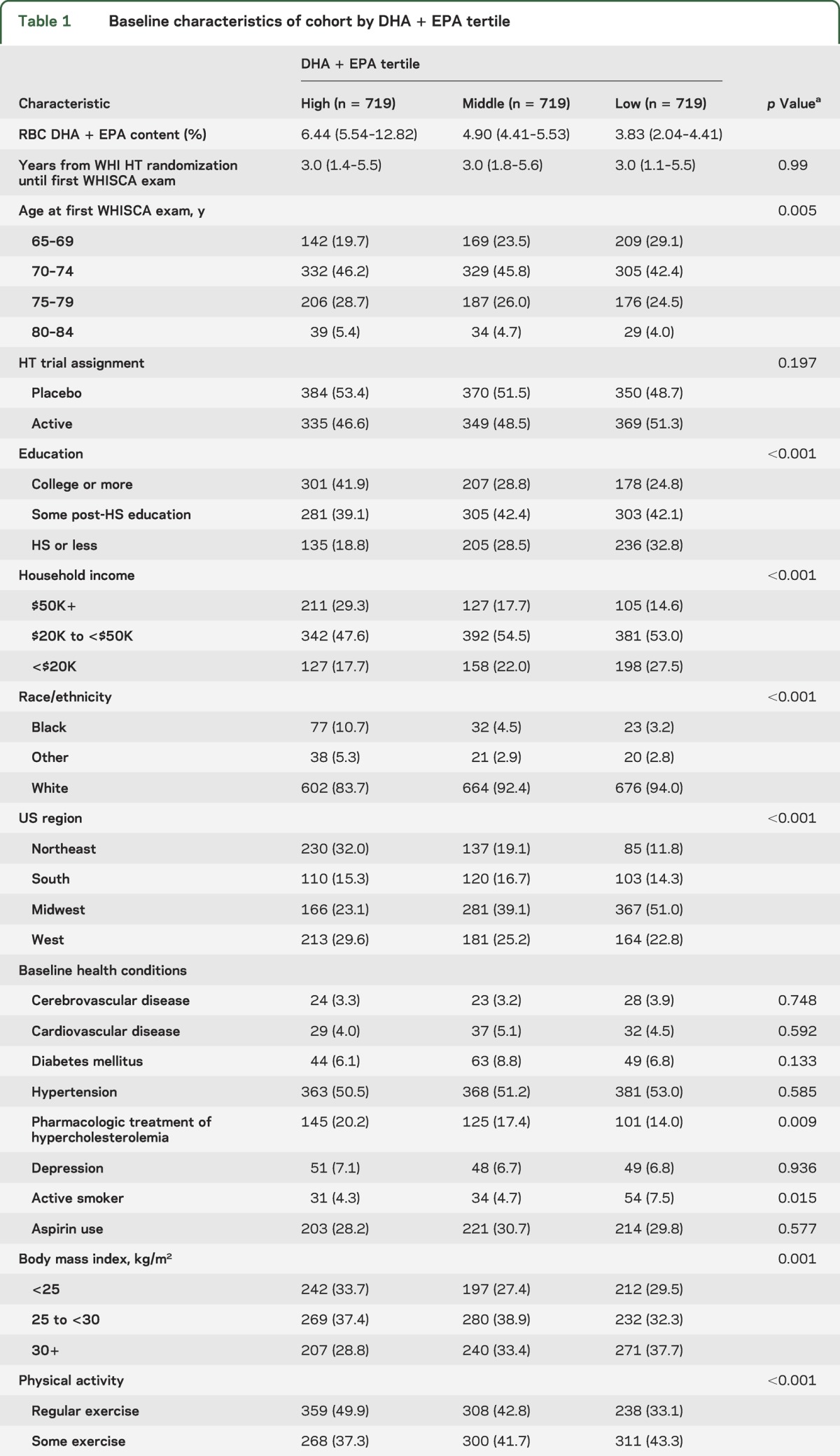
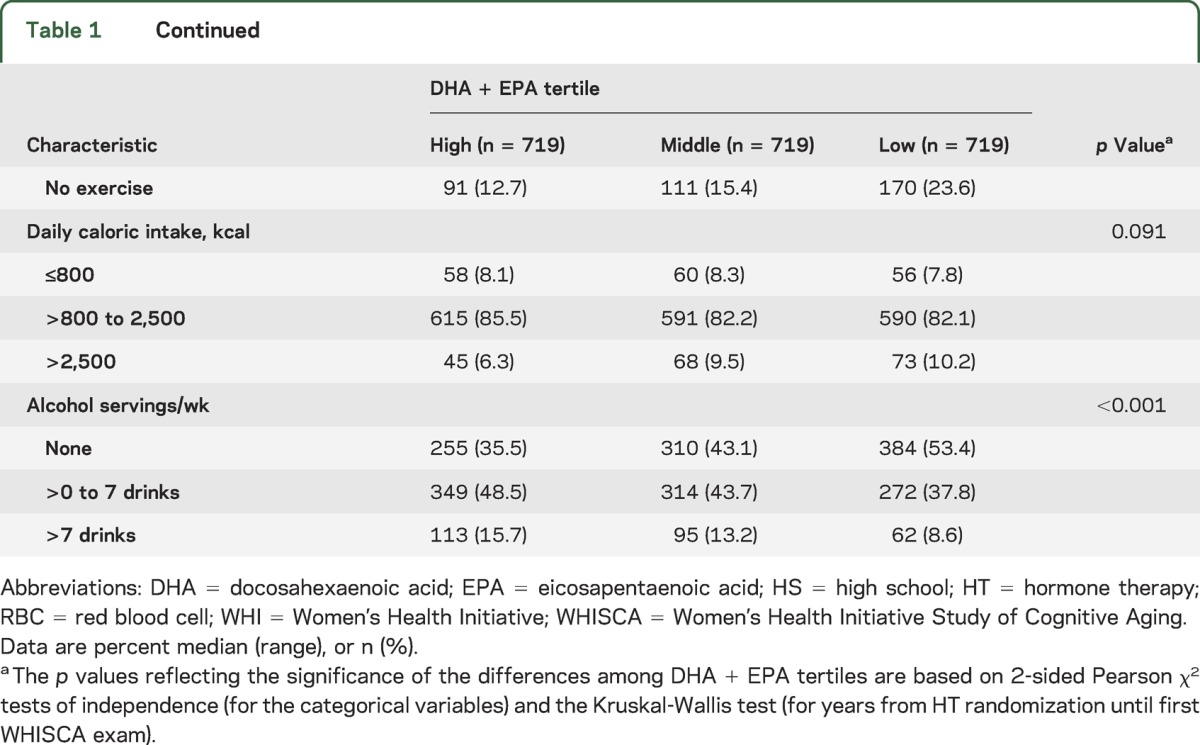
Variables representing participant demographics, WHI HT treatment assignment, health history, health behavior, and anthropometric measures were considered for inclusion in multivariable analyses based on their possible association with DHA + EPA levels and the longitudinal WHISCA outcomes. All covariates were measured during screening for the WHI trials before randomization. See table 1 for a complete list of these covariates and appendix e-1 for information on how they were defined.
Table 1.
Baseline characteristics of cohort by DHA + EPA tertile
Statistical methods.
Multivariable linear mixed models were used to estimate the independent effect of DHA + EPA on cognitive function at baseline and cognitive change over time. Analyses were performed separately for each of the 10 RBC DHA + EPA imputations, and the parameter estimates were pooled using Rubin's method.20 Random intercept and time slope effects were included to account for within-subject test score correlations. Baseline DHA + EPA was categorized by tertile because a) initial analyses were suggestive of nonlinear relationships between DHA + EPA and cognitive function, and b) participants in the lowest tertile had RBC DHA + EPA levels (2.04%–4.41%) within or near the <4% range that previous research has identified as a risk factor for cardiovascular disease.21
For each cognitive domain, 2 multivariable models were developed. Model 1 has minimal covariate adjustment for age, HT trial assignment, and response trends over time. Model 2 has additional adjustment for important demographic, medical, and behavioral factors that may confound the relationship between DHA + EPA and cognitive function. Covariates included in models 1 and 2 are listed in figures 1 and 2. Rationales for their inclusion and a detailed description of the model selection process are provided in appendix e-1. Cardiovascular disease, cerebrovascular disease, diabetes, hypertension, and depression were predictive of cognitive function, but were not included as covariates in models 1 or 2 because they may mediate the causal relationship between DHA + EPA and cognition. In sensitivity analyses, these variables were added to model 2 to determine whether their addition affected the DHA + EPA effect estimates. Because it is conceivable that HT and DHA + EPA may have interactive effects on inflammatory pathways and cholesterol levels,22 or that assignment to HT could affect participants' dietary habits, we tested for interactive effects of DHA + EPA and HT on cognitive function.
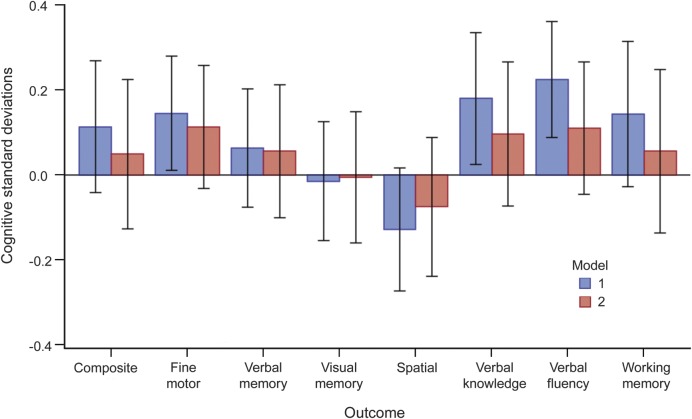
Figure 1. Mean baseline cognitive differences between high and low DHA + EPA tertiles with 99% confidence intervals, adjusted for model 1 and model 2 covariates.
Model 1: DHA + EPA tertile, DHA + EPA tertile*time interaction, time (linear and quadratic), first test indicator variable, age at first WHISCA examination (linear and quadratic), age*time interaction, HT trial arm, HT trial arm*time interaction, verbal memory test form (for composite cognition and verbal memory outcomes). Model 2: model 1 + education, education*time interaction, race, region, income, body mass index, physical activity, alcohol consumption, smoking, daily caloric intake. DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; HT = hormone therapy; WHISCA = Women's Health Initiative Study of Cognitive Aging.
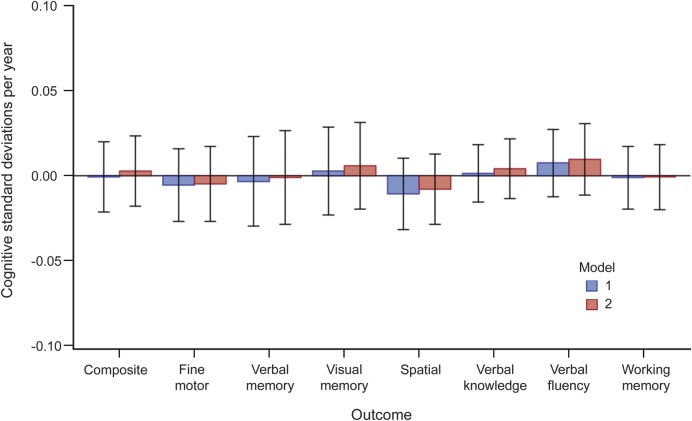
Figure 2. Mean differences in cognitive change over time between high and low DHA + EPA tertiles with 99% confidence intervals, adjusted for model 1 and model 2 covariates.
Model 1: DHA + EPA tertile, DHA + EPA tertile*time interaction, time (linear and quadratic), first test indicator variable, age at first WHISCA examination (linear and quadratic), age*time interaction, HT trial arm, HT trial arm*time interaction, verbal memory test form (for composite cognition and verbal memory outcomes). Model 2: model 1 + education, education*time interaction, race, region, income, body mass index, physical activity, alcohol consumption, smoking, daily caloric intake. DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; HT = hormone therapy; WHISCA = Women's Health Initiative Study of Cognitive Aging.
Our primary endpoints were the mean differences between the high and low DHA + EPA tertiles in a) cognitive performance at baseline, and b) change over time in composite cognitive function and each of the 7 cognitive domains. We designated 0.01 as the critical value for statistical significance because of the number of cognitive domains included as endpoints. Because of correlations among the WHISCA cognitive outcomes, we chose not to apply the more conservative Bonferroni correction for multiple comparisons.13
Several secondary analyses were performed. Using the modeling approach described above, DHA and EPA were examined individually for associations with cognitive performance. We also explored treating DHA + EPA as a continuous variable and tested for linear and polynomial effects on cognitive function. The SAS software package (version 9.3 for Windows; SAS Institute, Cary, NC) was used to conduct the statistical analyses and generate the figures presented in this report.
RESULTS
Participants' RBC DHA + EPA contents (calculated as the mean of the 10 DHA + EPA imputations for descriptive statistics) followed an approximately normal distribution (mean = 5.16%, SD = 1.47%, range = 2.04%–12.82%), as did participants' cognitive domain scores at baseline. RBC DHA + EPA ranges were 2.04%–4.41% for the first tertile, 4.41%–5.53% for the second, and 5.54%–12.82% for the third.
Descriptive baseline statistics for the study cohort by DHA + EPA tertile are shown in table 1. Bivariate analyses indicated that lower levels of DHA + EPA were associated with a number of risk factors for cognitive impairment and vascular disease. Compared with subjects in the highest DHA + EPA tertile, subjects in the lowest tertile were less likely to have a college degree (24.8% vs 41.9%), a household income ≥$50,000/year (14.6% vs 29.3%), a body mass index <25 kg/m2, or to exercise regularly. Significant differences among DHA + EPA tertiles in age, region, race, alcohol consumption, smoking, and hypercholesterolemia treatment rates were also observed.
Median length of follow-up for all subjects was 5.9 years (range = 0.0–7.9). We estimated cognitive response trends with multivariable models using all available observations. No subjects were excluded because of missing covariates in the model 1 analyses; approximately 7% of subjects were excluded for this reason in the model 2 analyses. We found no significant interaction between HT assignment and DHA + EPA, so we report DHA + EPA effect estimates for the study cohort as a whole. Cross-sectional differences in cognitive SDs between the high and low DHA + EPA tertiles, evaluated at the time of the first WHISCA examination in the multivariable linear models, are shown in figure 1 and table e-2. For all outcomes, a positive coefficient favors the high tertile. Based on the model 1 effect estimates, participants in the high DHA + EPA tertile exhibited better fine motor speed, verbal knowledge, and verbal fluency. However, after full covariate adjustment (model 2), these associations were attenuated and no longer statistically significant (fine motor ability: +0.11 SD, 99% confidence interval [CI]: −0.03 to 0.26, p = 0.04; verbal knowledge: +0.10 SD, 99% CI: −0.08 to 0.27, p = 0.14; verbal fluency: +0.11 SD, 99% CI: −0.05 to 0.27, p = 0.07). No other significant (p < 0.01) effects were found. When cardiovascular disease, cerebrovascular disease, diabetes, hypertension, and depression were added to model 2, the DHA + EPA effect estimates were virtually unchanged.
Differences in the rate of cognitive change (in SD per year) between the high and low DHA + EPA tertiles are shown in figure 2 and table e-3. In both the minimally and fully adjusted models, no significant (p < 0.01) difference was found between the high and low DHA + EPA tertiles in the rate of change over follow-up in any cognitive domain.
Results from our secondary analyses were consistent with the lack of association found between DHA + EPA and cognitive function in the primary analyses. After covariate adjustment, no significant (p < 0.01) associations were found when we tested for a) trends across tertiles, b) linear and polynomial effects of RBC DHA + EPA when treated as a continuous variable, and c) individual effects of DHA and EPA.
DISCUSSION
In our study, after adjustment for confounding factors, RBC DHA + EPA levels were not significantly (p < 0.01) associated with baseline cognitive function or cognitive change over time in any of the tested cognitive domains. Marginally significant cross-sectional differences favoring the high DHA + EPA tertile over the low DHA + EPA tertile were found for fine motor speed and verbal fluency. But overall, our results were consistent with the null hypothesis. Strengths of our study include its large sample size (N = 2,157), yearly cognitive examinations, and high retention of subjects over an extended 7.9-year follow-up period, which yielded effect estimates with precise CIs.
As with other observational studies of omega-3s, a potential source of bias is misclassification of exposure. Some misclassification is likely attributable to intraindividual biological variation, year-to-year variation in participants' DHA + EPA intake, and sample degradation caused by the improper storage of the blood samples. Despite the sample degradation issue, the validity statistics for our corrected RBC DHA and EPA measurements were comparable to those reported in a previous study18 (see methods section and appendix e-1 for details). On the assumption that measurement error resulted in nondifferential misclassification of DHA + EPA status, we would expect modest bias in our effect estimates toward the null.
A second limitation of our study is that APOE genotyping was unavailable for WHISCA participants. Some previous studies have reported that the protective association between omega-3s and cognitive function varies by APOE genotype, although these findings have been inconsistent.23–28 Our results represent estimates of the average cognitive effects of DHA + EPA across APOE ε4 carriers and noncarriers, who account for approximately 30% and 70% of the US population, respectively.29
Another potential limitation is the fact that the first battery of WHISCA cognitive tests was administered an average of 3 years (range = 1–5 years) after DHA + EPA exposure and baseline covariates were measured during screening for the WHI HT trials. Because dietary patterns in older adults are generally stable and the hypothesized effect of DHA + EPA on cognition is likely to be gradual and cumulative, the impact of the lag would likely be small in our study. Additionally, to the extent that DHA + EPA was associated with cognitive changes that occurred between screening for the WHI HT trials and the first WHISCA cognitive examination, these effects would appear in our baseline cross-sectional results.
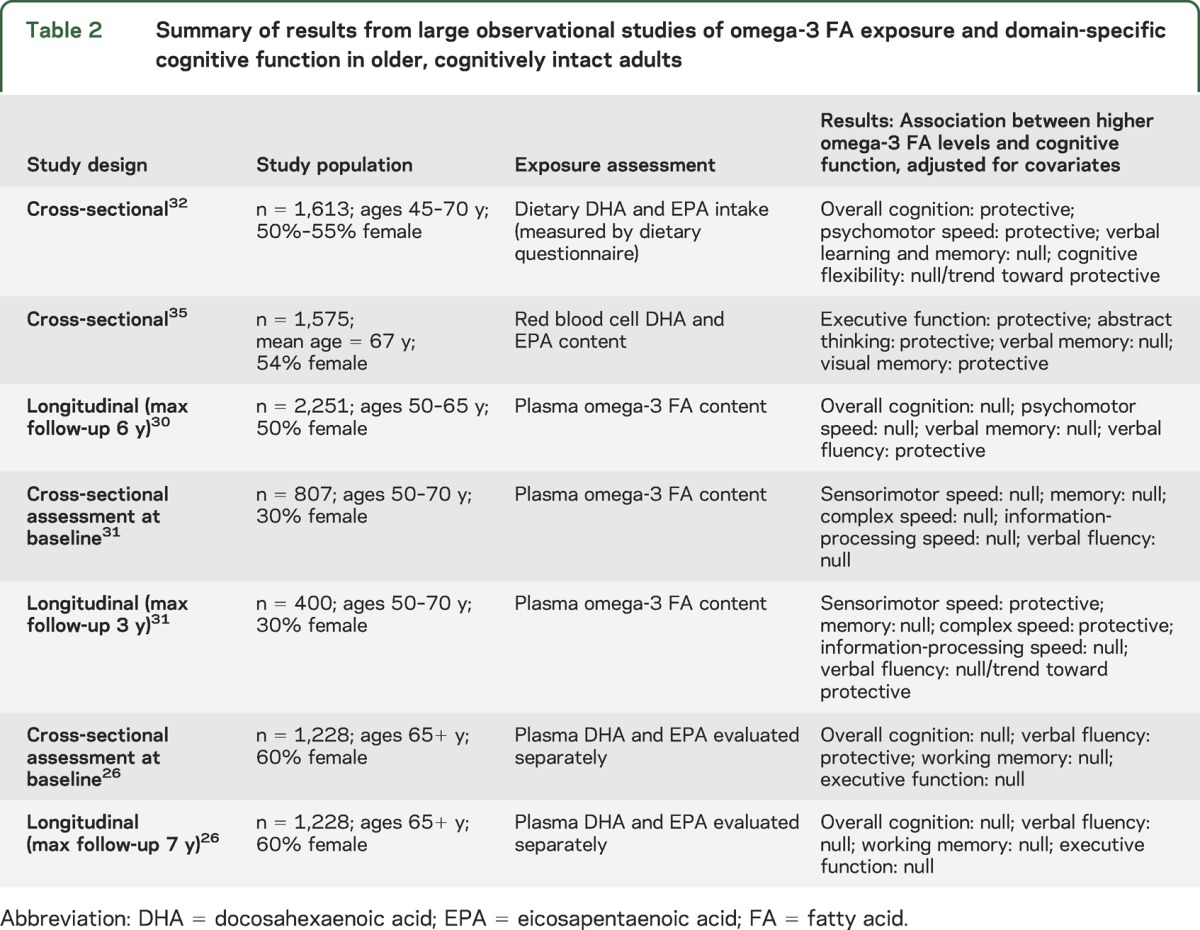
Our study adds to a growing body of cross-sectional and longitudinal observational studies on the relationship between omega-3s and domain-specific cognitive aging in dementia-free older adults26,30–32,35 (see table 2). In contrast to previous study populations, our sample was a) entirely female, and b) comprised of HT trial participants who were healthier and more educated than the general population of older dementia-free women in the United States. The low rates of cerebrovascular disease, diabetes, and other cardiovascular risk factors in our study population may limit the generalizability of our results to other populations. Nevertheless, some areas of agreement can be found with previous studies. First, none found a relationship between omega-3s and verbal or semantic memory, which is consistent with our results. Second, some evidence exists for a protective association between omega-3s and a) sensorimotor/psychomotor speed,31,32 and b) verbal fluency.26,30 This is consistent with the marginally significant cross-sectional protective associations we observed for fine motor speed and verbal fluency.
Table 2.
Summary of results from large observational studies of omega-3 FA exposure and domain-specific cognitive function in older, cognitively intact adults
Despite suggestive findings from some observational studies of a protective association between omega-3s and improved cognitive aging, results from randomized controlled trials of omega-3 supplements have generally not been supportive of a protective effect in cognitively intact older adults over short treatment periods.10,33,34 This is consistent with our finding of no association between RBC DHA + EPA content and cognitive change over a median follow-up of 5.9 years. If the marginally significant cross-sectional differences that we found for fine motor speed and verbal fluency do represent genuine causal effects, these effects are likely to be of small magnitude (on the order of 0.1 SD based on our DHA + EPA high vs low tertile model 2 effect estimates) and may be the result of years or decades of cumulative exposure to higher levels of omega-3s.
Supplementary Material
GLOSSARY
- CI
confidence interval
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- HT
hormone therapy
- PUFA
polyunsaturated fatty acid
- RBC
red blood cell
- WHI
Women's Health Initiative
- WHISCA
Women's Health Initiative Study of Cognitive Aging
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Mr. Ammann collaborated in data analysis and interpretation, and manuscript drafting and revision. Dr. Pottala collaborated on the statistical analyses and interpretation, and manuscript revision. Dr. Harris collaborated in data interpretation, manuscript revision, and obtaining funding. Dr. Espeland collaborated on data analysis, interpretation, and manuscript revision. Dr. Wallace collaborated in data collection and critical review of the manuscript. Dr. Denburg collaborated in data interpretation and manuscript revision. Dr. Carnahan collaborated in data analysis and interpretation, and manuscript revision. Dr. Robinson collaborated in the study design and conceptualization, data analysis and interpretation, drafting and revising of the manuscript, acquisition of the data, statistical analysis, study supervision, and obtaining funding.
STUDY FUNDING
Supported in part under a contract with the National Heart, Lung, and Blood Institute (BAA 19). The WHI program is funded by the National Heart, Lung, and Blood Institute, NIH, and US Department of Health and Human Services through contracts HSN268201100046C, HHSN268201100001C, HHSN268201100002C, HSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
DISCLOSURE
E. Ammann reports no disclosures. J. Pottala is an employee of OmegaQuant Analytics, LLC, a company that offers RBC fatty acid testing. W. Harris is an employee of Health Diagnostic Laboratory, Inc., and the owner of OmegaQuant Analytics, LLC. The former is a clinical laboratory and the latter a research laboratory; both offer RBC fatty acid testing. M. Espeland, R. Wallace, N. Denburg, and R. Carnahan report no disclosures. J. Robinson has served as principal investigator for grants received by the University of Iowa from Amarin, Amgen, Daiichi-Sankyo, Genentech/Hoffman-La Roche, and GlaxoSmithKline (>$10,000 each). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Daffner KR. Promoting successful cognitive aging: a comprehensive review. J Alzheimers Dis 2010;19:1101–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. J Geriatr Psychiatry Neurol 2010;23:75–93 [DOI] [PubMed] [Google Scholar]
- 3.Depp CA, Harmell A, Vahia IV. Successful cognitive aging. Curr Top Behav Neurosci 2012;10:35–50 [DOI] [PubMed] [Google Scholar]
- 4.Bielak AA. How can we not “lose it” if we still don't understand how to “use it”? Unanswered questions about the influence of activity participation on cognitive performance in older age: a mini-review. Gerontology 2010;56:507–519 [DOI] [PubMed] [Google Scholar]
- 5.Joseph J, Cole G, Head E, Ingram D. Nutrition, brain aging, and neurodegeneration. J Neurosci 2009;29:12795–12801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001;36:945–959 [DOI] [PubMed] [Google Scholar]
- 7.Carrié I, Abellan Van Kan G, Rolland Y, Gillette-Guyonnet S, Vellas B. PUFA for prevention and treatment of dementia? Curr Pharm Des 2009;15:4173–4185 [DOI] [PubMed] [Google Scholar]
- 8.Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging 2002;23:843–853 [DOI] [PubMed] [Google Scholar]
- 9.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu Rev Nutr 2011;31:321–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson JG, Ijioma N, Harris W. Omega-3 fatty acids and cognitive function in women. Womens Health 2010;6:119–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- 12.Coker LH, Espeland MA, Rapp SR, et al. Postmenopausal hormone therapy and cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS). J Steroid Biochem Mol Biol 2010;118:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials 2004;1:440–450 [DOI] [PubMed] [Google Scholar]
- 14.Maki PM, Henderson VW. Hormone therapy, dementia, and cognition: the Women's Health Initiative 10 years on. Climacteric 2012;15:256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pottala JV, Espeland MA, Polreis J, Robinson J, Harris WS. Correcting the effects of −20 degrees C storage and aliquot size on erythrocyte fatty acid content in the Women's Health Initiative. Lipids 2012;47:835–846 [DOI] [PubMed] [Google Scholar]
- 16.Harris WS, Sands SA, Windsor SL, et al. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation 2004;110:1645–1649 [DOI] [PubMed] [Google Scholar]
- 17.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 1997;38:2012–2022 [PubMed] [Google Scholar]
- 18.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007;86:74–81 [DOI] [PubMed] [Google Scholar]
- 19.Espeland MA, Miller ME, Goveas JS, et al. Cognitive function and fine motor speed in older women with diabetes mellitus: results from the Women's Health Initiative Study of Cognitive Aging. J Womens Health 2011;20:1435–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc 1996;91:473–489 [Google Scholar]
- 21.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004;39:212–220 [DOI] [PubMed] [Google Scholar]
- 22.Saldeen P, Saldeen T. Women and omega-3 fatty acids. Obstet Gynecol Surv 2004;59:722–730 [DOI] [PubMed] [Google Scholar]
- 23.Whalley LJ, Deary IJ, Starr JM, et al. n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. Am J Clin Nutr 2008;87:449–454 [DOI] [PubMed] [Google Scholar]
- 24.Barberger-Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology 2007;69:1921–1930 [DOI] [PubMed] [Google Scholar]
- 25.Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology 2005;65:1409–1414 [DOI] [PubMed] [Google Scholar]
- 26.Samieri C, Feart C, Proust-Lima C, et al. Omega-3 fatty acids and cognitive decline: modulation by ApoEepsilon4 allele and depression. Neurobiol Aging 2011;32:2317.e13–2317.e22 [DOI] [PubMed] [Google Scholar]
- 27.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 2008;71:430–438 [DOI] [PubMed] [Google Scholar]
- 28.Kroger E, Verreault R, Carmichael PH, et al. Omega-3 fatty acids and risk of dementia: the Canadian Study of Health and Aging. Am J Clin Nutr 2009;90:184–192 [DOI] [PubMed] [Google Scholar]
- 29.Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer's disease. Annu Rev Med 1996;47:387–400 [DOI] [PubMed] [Google Scholar]
- 30.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr 2007;85:1103–1111 [DOI] [PubMed] [Google Scholar]
- 31.Dullemeijer C, Durga J, Brouwer IA, et al. n-3 Fatty acid proportions in plasma and cognitive performance in older adults. Am J Clin Nutr 2007;86:1479–1485 [DOI] [PubMed] [Google Scholar]
- 32.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004;62:275–280 [DOI] [PubMed] [Google Scholar]
- 33.Mazereeuw G, Lanctot KL, Chau SA, Swardfager W, Herrmann N. Effects of omega-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol Aging 2012;33:1482.e17–1482.e29 [DOI] [PubMed] [Google Scholar]
- 34.Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane database Syst Rev 2012;6:CD005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan ZS, Harris WS, Beiser AS, et al. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology 2012;78:658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.