Abstract
Objective:
To report the clinical features of 20 newly diagnosed patients with GABAB receptor (GABABR) antibodies and determine the frequency of associated tumors and concurrent neuronal autoantibodies.
Methods:
Clinical data were retrospectively obtained and evaluated. Serum and CSF samples were examined for additional antibodies using methods previously reported.
Results:
Seventeen patients presented with seizures, memory loss, and confusion, compatible with limbic encephalitis (LE), one patient presented with ataxia, one patient presented with status epilepticus, and one patient presented with opsoclonus-myoclonus syndrome (OMS). Nineteen (95%) patients eventually developed LE during the course of the disease. Small-cell lung cancer (SCLC) was identified in 10 (50%) patients, all with LE. Treatment and outcome was available from 19 patients: 15 showed complete (n = 7) or partial (n = 8) neurologic improvement after steroids, IV immunoglobulins, or plasma exchange and oncologic treatment when indicated; 1 patient died of tumor progression shortly after the first cycle of immunotherapy, and 3 were not treated. Five patients with SCLC had additional onconeuronal antibodies (Ri, amphiphysin, or SOX1), and 2 without tumor had GAD65 and NMDAR antibodies, respectively. GABABR antibodies were not detected in serum of 116 patients with SCLC without neurologic symptoms.
Conclusion:
Our study confirms GABABR as an autoantigen of paraneoplastic and nonparaneoplastic LE and expands the phenotype of GABABR antibodies to ataxia, OMS, and status epilepticus. The long-term prognosis is dictated by the presence of a tumor. Recognition of syndromes associated with GABABR antibodies is important because they usually respond to treatment.
Autoimmune encephalitides are caused by humoral or cellular responses against specific neuronal antigens. Recently, neuronal surface receptors and synaptic proteins have been identified as autoantigens of some of these disorders. The clinical picture of these patients may include limbic encephalitis (LE), Morvan syndrome, psychosis, or abnormal movements and can occur preferentially as paraneoplastic or nonparaneoplastic syndromes depending on the type of autoantibody.1,2 Surface receptor antibodies are highly specific and sensitive diagnostic markers, and the associated syndromes often respond to immunotherapy.3 One of these autoantibodies was found to be directed against the GABAB receptor (GABABR).4 In the series where these antibodies were first reported, all 15 patients had LE and prominent seizures and some had additional antibodies against GAD65 or SOX1, with or without the presence of an underlying small-cell lung cancer (SCLC).4 These immunologic and oncologic associations gained further attention since similar patients were previously considered to have antibody-negative LE, or the neurologic syndrome had been attributed to the concurrent antibodies.4,5 However, the limited number of patients reported since the original series, which was focused on LE, indicates a need for additional studies to define the spectrum of GABABR antibody–associated symptoms, and to assess the impact of oncologic and immunologic therapies. It is also unclear whether GABABR antibodies may occur in patients with SCLC without neurologic symptoms. To address these questions, we report 20 new patients with GABABR antibodies who were identified by examining sera and CSF from patients with symptoms suspected to be autoimmune but without selection for a specific neurologic syndrome. We provide a systematic investigation on their clinical picture, additional antibodies, the presence of an underlying tumor, and the response to treatment. In addition, we determined whether the target epitopes of GABABR antibodies are conformational, and whether the antibodies occur in patients with SCLC but without paraneoplastic neurologic symptoms (PNS).
METHODS
Patients.
We investigated 9,076 sera or CSF of patients with suspected autoimmune encephalitis or PNS (including patients with LE, nonfocal encephalitis, encephalomyelitis, Morvan syndrome, and cerebellar dysfunction) that were received for antibody studies between May 2009 and September 2012 in the Department of Neurology, University of Pennsylvania, and the Service of Neurology, University of Barcelona. In addition, we investigated 346 CSF samples of patients with rapidly progressive neurologic symptoms, suspected to be prion disorders, received for evaluation of 14-3-3 protein between January and December 2012 at the Service of Neurology, University of Barcelona. Clinical information was obtained from questionnaires filled out by the referring neurologists and telephone interviews. One of the patients was previously described as a case report,6 another patient was included in a series of patients with LE and antibodies against neuronal cell-surface antigens antedating the discovery of GABABR as autoantigen.7
Standard protocol approvals, registrations, and patient consents.
Samples are deposited in the collection of biological samples named “neuroinmunologia” registered in the biobank of IDIBAPS, Barcelona, Spain. Samples of patients with SCLC without PNS were obtained at the Department of TB, National Koranyi Institute of TB and Pulmonology, Hungary, and at the Division of Thoracic Surgery, Medical University of Vienna, Austria. Informed consent for research for antineuronal antibodies was obtained in all patients. The study was approved by the institutional review boards of the Hospital of the University of Pennsylvania, University of Barcelona, the Koranyi Institute of TB and Pulmonology, Hungary, and the Medical University of Vienna.
Screening for antineuronal antibodies.
Serum and CSF samples were tested for antibodies to intracellular and cell-surface antigens on pre-fixed and postfixed rat brain, as described previously (figure 1, A and B).8 Samples showing specific tissue staining were further examined with a commercial immunoblot assay (Ravo Diagnostika, GmbH, Freiburg, Germany) for antibodies against classic paraneoplastic antigens (Hu, Yo, Ri, CV2, amphiphysin, Ma1/2, SOX1, and GAD65). The identity of cell-surface antigens was established using live hippocampal neurons (figure 1C) and a cell-based assay with HEK293 cells expressing LGI1, CASPR2, NMDAR, AMPAR, GABABR, GlycineR, mGluR1, mGluR5, or DPPX, as reported (figure 1D).9
Figure 1. Screening for GABABR antibodies in serum and CSF of patients.
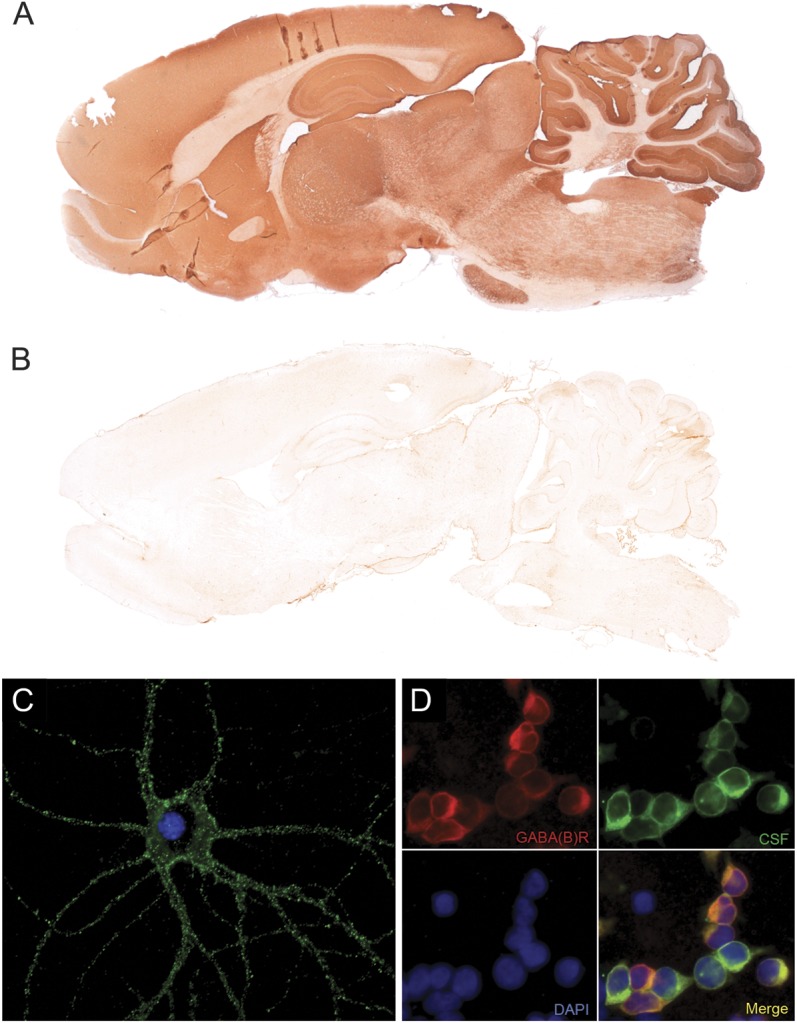
Indirect immunohistochemistry on rat brain with a patient's serum shows widespread labeling of GABAB receptor (GABABR) in the cortex and subcortical gray matter, with strong positivity of hippocampus and molecular layer of the cerebellum (A), whereas the serum from a healthy individual is negative (B). GABABR antibodies of a patient strongly label the surface of a live embryonic rat hippocampal neuron (C). Antibodies of patients are identified on HEK293 cells transfected with the R1 and R2 subunit of the GABABR (D; red: commercial antibody against the R1 subunit of the GABABR, green: CSF from a patient with GABABR antibodies, blue: nuclear staining with DAPI). C, D: ×400.
Immunoblot.
To confirm that human serum or CSF antibodies recognize conformation-dependent epitopes of the GABABR subunit, immunoblots with lysates of GABABR-transfected cells were performed. Nontransfected cells were used as control. HEK cell homogenates were resolved in an 8% polyacrylamide gel electrophoresis and blotted onto a polyvinylidene difluoride membrane. Strips were incubated overnight with patient's or normal human sera diluted 1:1,000 and a commercial GABABR antibody (R1 subunit, p.c.; Santa Cruz Biotechnology Inc. Santa Cruz, CA) diluted 1:5,000. The reactivity of patients and control sera was visualized with an avidin-biotin peroxidase assay (Vector Labs, Burlingame, CA).
Statistical analysis.
Age and time until diagnosis were analyzed by a Mann-Whitney U test. The difference in antibody positivity between serum and CSF was calculated with McNemar paired test. For survival, Kaplan-Meier curves were created, using log-rank tests. Statistical analysis was performed with GraphPad Prism 6.01 (La Jolla, CA).
RESULTS
Patients.
We identified 20 patients with GABABR antibodies. A summary of these patients is shown in table 1. The male: female ratio was 12: 8. The 10 patients without SCLC were younger (median age 39 years, range 16–67 years) than those with SCLC (median 67.5 years, range 60–77 years) (p = 0.0006). The median time from symptom onset until diagnosis was 4 weeks (range 2–104 weeks). In 9 of 10 patients with SCLC, the neurologic syndrome preceded the diagnosis of SCLC (median 0.75 months, range 0.75–22 months), and in 1 patient the SCLC was identified 1 week before onset of LE.
Table 1.
Clinical presentation and immunologic findings in patients with GABABR autoimmune encephalitis

Clinical presentation.
At the time of hospital admission, the clinical picture of the patients was categorized into 4 types.
Limbic encephalitis and seizures.
Seventeen patients presented with LE, including memory loss, confusion, hallucinations, personality change, and seizures, which in 5 patients were difficult to control. Additionally, one patient developed autonomic dysfunction and hypoventilation, one had mild limb spasticity, and another prominent psychiatric symptoms. One patient who additionally had amphiphysin antibodies (see below) showed a mixed clinical picture of LE and diffuse encephalomyelitis with gait ataxia.7 In 9 patients, the MRI showed unilateral or bilateral increased fluid-attenuated inversion recovery (FLAIR)/T2 signal in the hippocampus and amygdala, and 1 patient showed pial enhancement; the other 7 patients had normal MRI. EEG was available from 12 patients: 7 had temporal lobe epileptic activity with or without general slowing; the others were unremarkable, although they did not have continuous monitoring. CSF analysis was available from 16 patients: 11 showed lymphocytic pleocytosis (median 37 white blood cells, range 12–159), elevated protein concentration (median 68.5 mg/dL, range 51–176), or both (table 1).
Status epilepticus.
A 63-year-old woman presented with prominent seizures, preceded 2 weeks earlier by prodromal fever and nonspecific respiratory symptoms. She then developed abdominal myoclonic jerks and dystonia of the right leg that was interpreted as focal seizures and the patient was treated with anticonvulsants. Brain MRI was normal, and the EEG demonstrated epileptic activity in the left temporal lobe; CSF showed 11 white blood cells/mm3 and protein 50 mg/dL. Four weeks after symptom onset, the patient developed status epilepticus that was controlled with multiple anticonvulsants, but she died of respiratory failure and aspiration. No underlying tumor was found. Autopsy was not available.
Ataxia.
A 52-year-old man presented with gait ataxia, spasticity, and dysarthria. Additionally, he developed depressive mood and mild cognitive deficits but did not have seizures. Brain MRI showed leukoencephalopathy in frontal and temporal lobes. EEG was unremarkable; CSF showed 21 white blood cells/mm3 and protein 106.2 mg/dL. The patient responded partially to immunotherapy. He is alive and no tumor has been identified.
Opsoclonus-myoclonus.
A 33-year-old woman presented with opsoclonus-myoclonus syndrome (OMS) that partially responded to steroids, IV immunoglobulin (IVIg), plasmapheresis, and rituximab. Three months later, she developed hallucinations, disorientation, and symptoms of LE. At this time, GABABR antibodies were identified in serum; serum from 3 months earlier was no longer available for antibody determination. Brain MRI showed increased signal in T2/FLAIR and contrast enhancement in cortex and white matter of both frontal and temporal lobes and cingulum, the EEG demonstrated diffuse slow activity, and the CSF revealed 30 white blood cells/mm3 and 76 mg/dL protein. Symptoms of LE responded partially to steroids.
Treatment and follow-up.
Fifteen patients received immunotherapy (steroids, IVIg, plasma exchange, rituximab, cyclophosphamide, or mycophenolate mofetil), 3 of them along with oncologic treatment (chemotherapy, 2 additionally radiation therapy), 1 patient received chemotherapy alone, and 3 did not receive any of these treatments; information was not available from 1 patient (table 2). Overall, 7 patients showed complete neurologic response to immunotherapy or oncologic therapy, and 8 had partial response. Seven of these 8 patients were able to function by themselves, 4 of them requiring minimal assistance, and 1 who only received oncologic treatment died a few weeks after neurologic improvement of complications of chemotherapy. Of the 15 patients who responded to therapy, 9 did not have SCLC and 6 had SCLC.
Table 2.
Treatment and outcome of patients with GABABR autoimmune encephalitis

Among the 4 patients who did not have neurologic improvement, only one received immunotherapy. This patient had a very poor general condition and died shortly after the first cycle of IVIg and steroids and therefore the effects of immunotherapy were not assessable. The other 3 patients were in such poor general condition that they only had palliative treatment before they died.
At the last follow-up, 12 patients are alive and 8 have died; 7 of these 8 patients died as a result of tumor progression or complications of chemotherapy. The only patient without tumor who died developed seizures in association with concurrent antibodies to GAD65; the cause of death was refractory status epilepticus. Patients with SCLC had a shorter survival compared with patients without tumor (p = 0.029).
Immunologic studies.
A systematic screening of all GABABR antibody–positive sera or CSF for the presence of other antibodies showed that 5 patients with SCLC had additional antibodies: 3 SOX1, 1 amphiphysin, and 1 Ri (ANNA2). The patient with additional amphiphysin antibodies was diagnosed with paraneoplastic encephalomyelitis. He received one cycle of IVIg and steroids for 3 days but did not improve and died of pneumonia. At autopsy, a SCLC was found, and the brain showed lymphocytic inflammatory infiltrates throughout the whole brain including cerebellum and brainstem, but with a more prominent involvement of limbic areas. The inflammatory infiltrates were mainly composed of perivascular and to a minor extent parenchymal CD3-, CD4-, and CD8-positive T cells.7 The 3 patients with SOX1 antibodies and the patient with Ri antibodies presented with sudden onset of epilepsy and psychiatric symptoms. All 4 were in the group of poor general condition and thus 2 of them only received palliative care support, the other 3 received immunotherapy or chemotherapy that improved neurologic symptoms, but all died soon due to tumor progression. The median survival of the 5 patients with SCLC and additional antibodies was 1.5 months compared to 11 months for patients with SCLC and GABABR antibodies alone (p = 0.07).
Among patients without SCLC, one had antibodies against GAD65 (discussed earlier) and another against the NR1 subunit of the NMDAR, which associated with prominent psychiatric symptoms in addition to LE.
None of the sera from 116 patients with SCLC without PNS showed reactivity with HEK293 cells expressing GABABR. The survival of patients with SCLC and GABABR antibodies (median survival 3 months, interquartile range [IQR] 1.5–14 months) did not significantly differ from survival of those without PNS (median survival 7 months, IQR 2–13 months, p = 0.68) (figure 2).
Figure 2. Survival of patients with SCLC with GABABR antibodies does not differ from that of patients with SCLC without PNS.
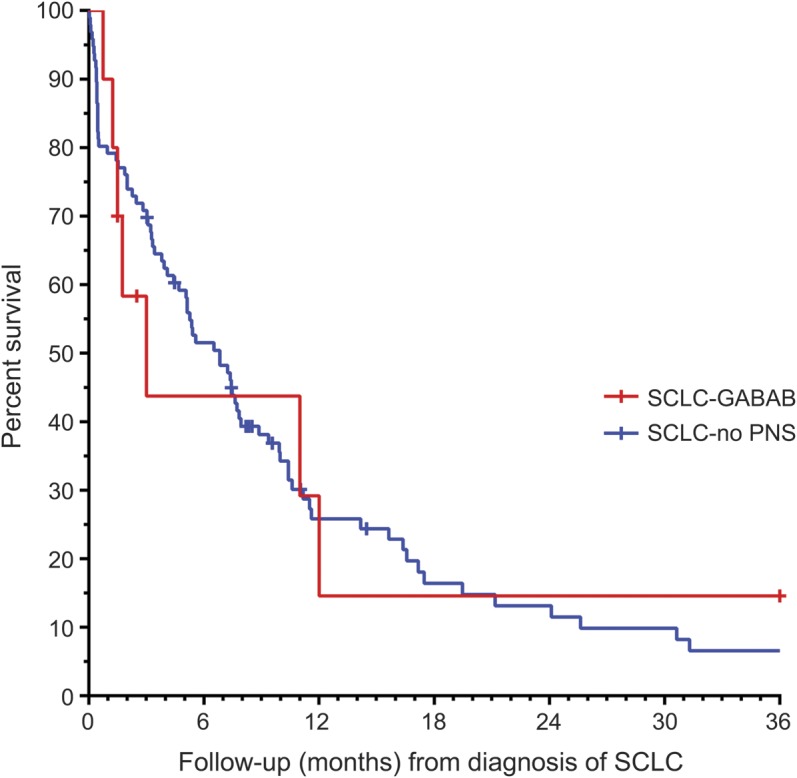
Kaplan-Meier survival curves for 96 patients with small-cell lung cancer (SCLC) without paraneoplastic neurologic symptoms (PNS) (blue) and 10 patients with SCLC and PNS associated with GABAB receptor (GABABR) antibodies (red). Censored cases of surviving patients or patients lost to follow-up are marked by a cross.
Immunoblot of protein extracts from HEK cells expressing GABABR were tested with a commercial GABABR antibody and sera of 6 patients from whom enough sample was available. While the commercial antibody reacted with a major band of approximately 106 kDa corresponding to the R1 subunit of the receptor, none of the 6 patients' sera showed immunoblot reactivity despite being strongly positive with HEK cells expressing GABABR (data not shown). These findings suggest that patient antibodies are directed against conformal epitopes of the GABABR.
DISCUSSION
We report 20 patients with GABABR antibodies, representing the largest case series of patients with this disorder reported to date. Four relevant findings are provided: 1) the study confirms that most patients with these antibodies develop LE, 2) 2 patients had novel forms of symptom presentation, ataxia, and opsoclonus, 3) GABABR antibodies occurred in patients with neurologic dysfunction, with or without SCLC, but were not detected in cancer patients without neurologic symptoms, and 4) analysis of the GABABR antibodies and the repertoire of concurrent autoimmunities showed that the GABABR epitopes are conformational, and the type of concurrent antibodies varies according to the presence or absence of cancer, and may suggest outcome.
A previous series of patients from the University of Pennsylvania—a referral center for autoimmune and paraneoplastic encephalitis—showed a predominant association of GABABR antibodies with LE and seizures.4 To determine whether this association holds in a broader referral setting, the current study was conducted at the University of Pennsylvania and at the Service of Neurology, University of Barcelona, which has a referral pattern that includes autoimmune disorders of the gray and white matter, paraneoplastic syndromes, and prion disorders. Despite this wider spectrum of disorders, including also sera of 116 patients with SCLC but without PNS, 19 of the 20 patients with GABABR antibodies developed LE, 85% of them in association with prominent seizures. Interestingly, the initial symptom presentation of 2 patients did not suggest LE, one had cerebellar symptoms, similar to a case recently described,10 and the other had opsoclonus. This patient developed the opsoclonus several months before the development of LE. We cannot rule out that he had 2 unrelated paraneoplastic syndromes with opsoclonus preceding the development of GABABR antibody–associated symptoms. To support this possibility, a previously reported patient presented with opsoclonus without onconeuronal antibodies, and several months later developed anti-Hu-associated encephalomyelitis.11
The occurrence of cerebellar symptoms, including opsoclonus, is not surprising given the high density of expression of GABABR in the cerebellum.12 Moreover, considering the wide anatomical distribution of GABABR,13 it is reasonable to assume that with future systematic screening more clinically “atypical” cases may be found.
Fifty percent of the patients had an underlying tumor, which in all cases was an SCLC that was usually identified after the development of neurologic symptoms. Taking these 20 patients together with all previously reported cases, 27 of all 47 (58%) patients showed a paraneoplastic etiology.4,5,10 The median age of patients in the paraneoplastic group was older compared with that of the nonparaneoplastic group (66 years, range 47–77, vs 43.5 years, range 16–69). In all but 2 cases, where histologic characterization was available, the underlying tumor was an SCLC; the 2 exceptions included a patient with a carcinoid of the thymus5 and another patient with melanoma.10 Like SCLC, these 2 types of tumors derive from the neuroectoderm and may thus be able to express GABABR; further studies with additional patients are needed to confirm these tumor associations.
Consistent with the extracellular location of the epitopes of the GABABR, 14/15 patients treated with immunotherapy (3 combined with oncologic therapy) had full or substantial neurologic responses. Even 4 patients (one with tumor) with treatment delays of 3 to 4 months improved with immunotherapy. In patients with cancer, the outcome was dictated by the successful treatment of the tumor and the presence of onconeuronal antibodies against intracellular antigens, which usually indicate cytotoxic T-cell mechanisms and less response to immunotherapy.7 Interestingly, one patient without additional onconeuronal antibodies, who was treated for SCLC without immunotherapy, also showed neurologic improvement, emphasizing the role of antigen presentation by the tumor. Only one of the 8 patients who died did not have SCLC, but this patient had additional antibodies to GAD65 and died of refractory status epilepticus, consistent with the poor prognosis that these antibodies confer to LE and seizures.14
In patients with anti-GABABR-associated encephalitis, the occurrence of additional antibodies has been previously noted,5 but several novel implications can be obtained from our findings. The type of additional antibodies varied depending on the presence or absence of a SCLC, so that detection of these antibodies may lead to identify the cancer and suggest a clinical prognosis. For example, the 5 patients with onconeuronal antibodies (amphiphysin, SOX1, or Ri/ANNA2) had an underlying SCLC. In these 5 patients, the presence of the tumor and probably cytotoxic T-cell mechanisms might have influenced the outcome. Indeed, all had a rapid clinical progression to death, and the patient with amphiphysin antibodies showed autopsy findings consistent with a cytotoxic T-cell–mediated disorder.7 On the other hand, in the 2 patients without SCLC but with additional antibodies, these were directed against GAD65 and NMDAR, respectively. In both patients, the presence of these antibodies appeared to modify the neurologic phenotype; indeed, the patient with NMDAR antibodies showed prominent psychiatric symptoms, and the patient with GAD65 antibodies developed refractory seizures as indicated above. Interestingly, the serum and CSF of the patient with OMS did not show any additional antibody, as occurs in most cases of autoimmune opsoclonus.15
The mechanisms involved in the effects of patients' GABABR antibodies are under study. The strong reactivity of these antibodies with cultured live hippocampal neurons, and the response of patients' symptoms to immunotherapy, suggest a direct pathogenic role as it occurs with NMDAR antibodies.16 However, in contrast to NMDAR antibodies, preliminary studies suggest that GABABR antibodies do not decrease the synaptic levels of receptors, but alter the synaptic function, blocking the inhibitory effects of baclofen on the spontaneous firing of cultured neurons.17 In the current study, we show that antibodies are directed against conformal epitopes, visible in HEK293 cells transfected with GABABR or in live neurons, but not visible when the receptor protein is denaturated in immunoblot.
Overall, findings from this and previous studies have several practical implications. Testing for GABABR antibodies should be considered in all patients with LE with or without SCLC, and rare cases of cerebellar dysfunction and opsoclonus in which no other antibodies are identified. The presence of a tumor, mainly SCLC, should be suspected in older patients as well as those who have additional onconeuronal antibodies. In patients with known SCLC, the development of LE without Hu antibodies (ANNA1) is strongly associated with GABABR antibodies. The diagnosis of this disorder is important, because in most patients neurologic symptoms respond to immunotherapy regardless of being paraneoplastic or not.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Mercè Alba, Eva Caballero, and Esther Aguilar for technical support.
GLOSSARY
- FLAIR
fluid-attenuated inversion recovery
- GABABR
GABAB receptor
- IQR
interquartile range
- IVIg
IV immunoglobulin
- LE
limbic encephalitis
- OMS
opsoclonus-myoclonus syndrome
- PNS
paraneoplastic neurologic symptoms
- SCLC
small-cell lung cancer
Footnotes
Editorial, p. 1482
AUTHOR CONTRIBUTIONS
Design/conceptualization of the study: R.H., M.T., B.D., F.G., J.D.; analysis/interpretation of the data: R.H., M.T., L.S., B.D., A.R., B.H., M.A.H., V.L., H.J.A., L.H., S.B., A.d.F., A.S., F.G., J.D.; statistical analysis: M.T.; drafting/revising the manuscript: R.H., M.T., B.D., L.H., F.G., J.D. All authors give final approval of the version to be published.
STUDY FUNDING
Supported in part by grant PI12/00611 from the Fondo de Investigaciones Sanitarias, Madrid, Spain (F.G.), NIH RO1NS077851 (J.D.), RO1CA89054 (J.D.), a McKnight Neuroscience of Brain Disorders award (J.D.), Fondo de Investigaciones Sanitarias (FIS, Spain, 11/01780, J.D.), and Fundació la Marató de TV3 (J.D.). J.D. receives royalties from Athena Diagnostics for a patent for the use of Ma2 as autoantibody test, and licensing fees from Euroimmun for a patent for the use of NMDAR as autoantibody test. R.H. was funded by the Fonds zur Förderung der wissenschaftlichen Forschung, Austria, Project J3230. M.T. was supported by a ErasmusMC fellowship B.D. was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4 A/-11-1-2012-0001 “National Excellence Program.” Further support: OTKA MOB 80325 (BH), KTIA-AIK 12-1-2013-0041 (B.D., V.L., A.R.), OTKA K109626 (B.D.), OTKA K108465 (B.D.), and the Hans und Blanca Moser Foundation (M.A.H.).
DISCLOSURE
R. Höftberger, M. Titulaer, L. Sabater, B. Dome, A. Rózsás, B. Hegedus, M. Hoda, V. Laszlo, H. Ankersmit, L. Harms, S. Boyero, A. de Felipe, and A. Saiz report no disclosures. J. Dalmau has a research grant from Euroimmun and receives royalties from patents for the use of Ma2 and NMDAR as autoantibody tests. F. Graus reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Graus F, Dalmau J. Paraneoplastic neurological syndromes. Curr Opin Neurol 2012;25:795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lancaster E, Dalmau J. Neuronal autoantigens: pathogenesis, associated disorders and antibody testing. Nat Rev Neurol 2012;8:380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010;9:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology 2011;76:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenholz DM, Wong VS, Bateman LM, et al. Treatment of gamma-aminobutyric acid B receptor-antibody autoimmune encephalitis with oral corticosteroids. Arch Neurol 2012;69:1061–1063 [DOI] [PubMed] [Google Scholar]
- 7.Graus F, Saiz A, Lai M, et al. Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology 2008;71:930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoftberger R, Dalmau J, Graus F. Clinical neuropathology practice guide 5-2012: updated guideline for the diagnosis of antineuronal antibodies. Clin Neuropathol 2012;31:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarius S, Steinmeyer F, Knobel A, et al. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J Neuroimmunol 2013;256:94–96 [DOI] [PubMed] [Google Scholar]
- 11.Ducray F, Graus F, Vigliani MC, et al. Delayed onset of a second paraneoplastic neurological syndrome in eight patients. J Neurol Neurosurg Psychiatry 2010;81:937–939 [DOI] [PubMed] [Google Scholar]
- 12.Lujan R, Shigemoto R. Localization of metabotropic GABA receptor subunits GABAB1 and GABAB2 relative to synaptic sites in the rat developing cerebellum. Eur J Neurosci 2006;23:1479–1490 [DOI] [PubMed] [Google Scholar]
- 13.Billinton A, Ige AO, Wise A, et al. GABA(B) receptor heterodimer-component localisation in human brain. Brain Res Mol Brain Res 2000;77:111–124 [DOI] [PubMed] [Google Scholar]
- 14.Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol 2010;67:470–478 [DOI] [PubMed] [Google Scholar]
- 15.Bataller L, Rosenfeld MR, Graus F, Vilchez JJ, Cheung NK, Dalmau J. Autoantigen diversity in the opsoclonus-myoclonus syndrome. Ann Neurol 2003;53:347–353 [DOI] [PubMed] [Google Scholar]
- 16.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010;30:5866–5875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancaster E, Lai MZ, Hughes E, et al. Autoantibodies to the GABA(B) receptor associate with limbic encephalitis and seizures, and alter GABA(B) receptor function. Neurology 2010;74(suppl 2):A402. Abstract [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


