Abstract
While drug-related periostitis has been known of for many years, the specific association of diffuse periostitis with voriconazole (most frequently in transplant patients) has only been recently explicitly addressed in the literature. Recognition of the radiologic and clinical manifestations of voriconazole-related periostitis is important for helping to narrow an otherwise broad differential diagnosis. We present two cases that illustrate different radiologic presentations of this painful cause of diffuse periostitis. Case 1 features a 60 year-old woman with a history of orthotopic heart transplant who was hospitalized for “full body pain” with progressively worsening bone tenderness involving the humeri, knees, femurs, hips, and hands. Case 2 describes a 48 year-old man with a history of acute lymphoblastic leukemia status post stem cell transplant who presented with diffuse arthralgias involving bilateral ankles, knees, wrists, and elbows.
Keywords: Periostitis deformans, hypertrophic osteoarthropathy, voriconazole, lung transplant
CASE REPORT
Case 1
A 60 year-old woman with previous history of orthotopic heart transplant (eight months prior to admission) with mild acute cellular rejection was admitted with a two week history of refractory polyarticular pain with associated fever and malaise. There was no associated history of morning stiffness. Physical exam demonstrated normal muscular strength and tone and all joints with full range of motion. Both knees demonstrated minimal effusions however the right greater than left knees were exquisitely tender to palpation with the remainder of the joints similarly sensitive. Of note the patient had started on prophylactic antifungal therapy with voriconazole five months prior to the most recent presentation. Additionally, the patient had been hospitalized six weeks prior to the most recent presentation and treated for presumptive pulmonary aspergillosis with a therapeutic course of voriconazole. The patient remained on voriconazole until the time of the case presentation. Laboratory values were as follows: alkaline phosphatase was markedly elevated at 304 (reference range 29–111 U/L) and had been steadily increasing over the four months prior to hospitalization. However, other serologies were within normal limits. Creatine kinase was 29 (normal reference range 39–189 U/L). Antinuclear antibody (ANA) was < 40, double-stranded DNA was < 30 IU/mL, and anti-histone antibody was <1.0, all within normal limits. White blood cell count was normal.
Noncontrast computed tomography (CT) of the chest was performed shortly prior to admission which demonstrated exuberant periosteal bone formation involving several left-sided ribs (Figure 1). Plain radiographs obtained at the same time demonstrated extensive periosteal bone formation along the proximal medial cortex of the left humerus (Figure 2). Additional plain radiographs at admission demonstrated periostitis of the proximal femoral shafts and humeral shafts (Figure 2) which correlated with evidence of diffuse abnormal uptake involving the clavicles, left sixth and seventh ribs, bilateral proximal humeri, bilateral proximal femurs, proximal tibial diaphyses, right third proximal phalanx, and right fifth metacarpal on Tc-99m MDP whole body bone scan (Figure 3). On the basis of the laboratory and radiological imaging findings, voriconazole-related periostitis was suspected and this medication was discontinued. Secondary hypertrophic osteoarthropathy was considered as an alternative diagnosis, however, given the specific history of voriconazole use, the presentation was highly suspicious for voriconazole-related periostitis. The patient reported improvement in symptoms soon after cessation of voriconazole. Follow-up noncontrast chest CT (Figure 1) and bone scan (Figure 3), three and fourth months respectively following the admission, demonstrated marked interval resolution of previously seen abnormal periostitis.
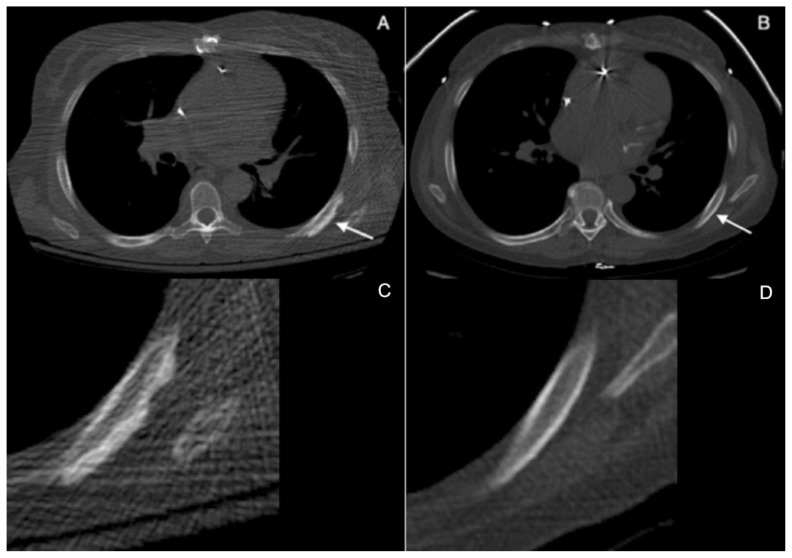
Figure 1.
60 year-old woman with voriconazole-induced periostitis following heart transplant. (A) Axial noncontrast CT image at presentation demonstrates fluffy, irregular periosteal new bone formation along the posterolateral aspect of the left seventh rib (solid arrow) with coned down image below (C) (GE LightSpeed16 Slice CT Scanner, 120 kVp, slice thickness = 2.5 mm. CTDIvol (mGy) = 1.75, DLP (mGy-cm) = 48.63). (B) Axial noncontrast CT image four months following discontinuation of voriconazole the periostitis has completely resolved, with coned down image below (D) (GE LightSpeed VCT Scanner, 120 kVp = 120, mAs = 39, slice thickness = 2.5 mm. CTDIvol (mGy) = 1.29, DLP (mGy-cm) = 43.30, 30% adaptive statistical iterative reconstruction (ASIR)).
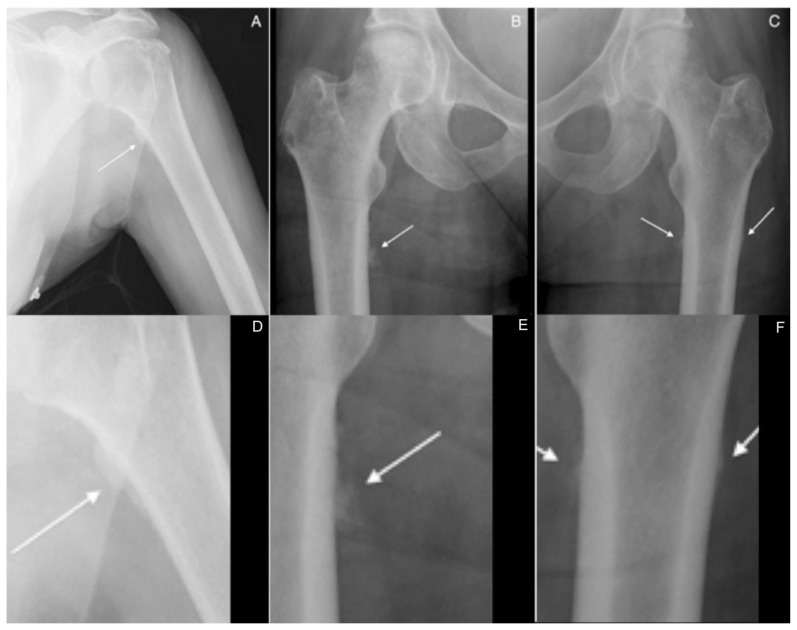
Figure 2.
60 year-old woman with voriconazole-induced periostitis following heart transplant. Anteroposterior views of the left humerus (A) and bilateral hips (B, C) demonstrate multifocal, dense, irregular periosteal new bone formation along the medial aspect of the left humeral neck; the medial proximal right femur, inferior to the lesser trochanter; and along the medial and lateral proximal left femur, inferior to the lesser trochanter (solid arrows). The respective coned down images are shown in the second row of the figure (D, E, F).
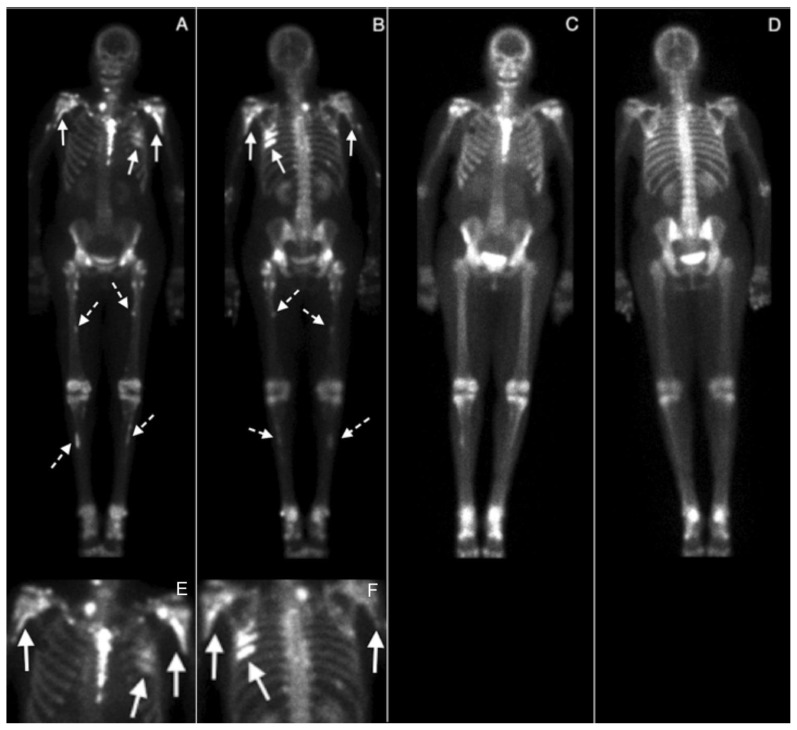
Figure 3.
60 year-old woman with voriconazole-induced periostitis following heart transplant. At presentation, anterior and posterior (A, B) whole body views were obtained 3 hours following the intravenous injection of 19 mCi of Tc-99m MDP (ADAC Vertex Gamma Camera), with coned down views of the thorax from A and B shown in E and F, respectively. There are multiple abnormal scattered foci of uptake, most prominent at the medial left proximal humeral neck and the left sixth and seventh ribs (arrows) but also involving the medial right humeral neck (arrow); the bilateral proximal femoral diaphyses, inferior to the lesser trochanters (dashed arrows); and the proximal tibial diaphyses (dashed arrows). Five months following discontinuation of voriconazole, anterior and posterior whole body views (C and D) were obtained 3 hours following the intravenous injection of 20.1 mCi Tc-99m MDP which demonstrate marked interval resolution of the previously noted abnormal foci of uptake (ADAC Vertex Gamma Camera).
Case 2
A 48 year-old man with history of acute lymphocytic leukemia status post stem cell transplant 10 months prior to onset of symptoms presented with joint pain involving bilateral elbows, ankles, knees, and wrists. He denied any history of erythema or swelling of joints. The pain was constant, severe, and disabling to the point that the patient was bedridden secondary to the pain. The patient began to take dilaudid which provided some temporary relief but the pain did not resolve. A course of corticosteroids provided temporary resolution of pain but the symptoms returned when the dose was tapered. His most recent bone marrow biopsy confirmed remission of leukemia. Physical exam was remarkable for bilateral olecranon hyperostosis, limited rotation of bilateral elbows with otherwise full range of motion. Laboratory values were as follows: alkaline phosphatase was markedly elevated at 245 (reference range 29–111 U/L). Erythrocyte sedimentation rate was elevated at 93 (normal reference range 0–10 mm/h). C-Reactive protein was elevated at 34.6 (normal reference range <6.3 mg/L). Serologies for anti-nuclear antibodies, serum rheumatoid factor, and double-stranded DNA antibody were all negative. Other serologies were within normal limits. Creatine kinase was 29 (normal reference range 39–189 U/L). ANA was < 40, double-stranded DNA was < 30 IU/mL, and anti-histone antibody was <1.0, all within normal limits. White blood cell count was normal.
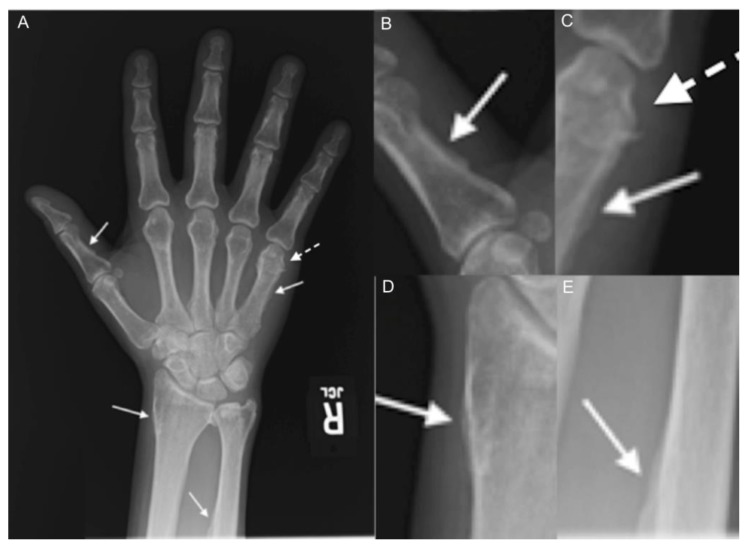
Plain radiographs of the elbow demonstrated extensive periosteal bone formation along the bilateral distal humeri, proximal ulnae, and the proximal radial and ulnar diaphysis (Figure 4). No significant degenerative changes or signs of an inflammatory arthropathy were seen. No juxta-articular osteopenia was noted. Additional radiographs of the hands demonstrated periosteal bone formation along the right fifth metacarpal, the distal right ulna and radius, the left second and fifth metacarpal, and the proximal first phalanges bilaterally (Figure 5). Voriconazole was discontinued. The patient reported dramatic improvement in pain since stopping voriconazole with significantly less pain medication requirements.
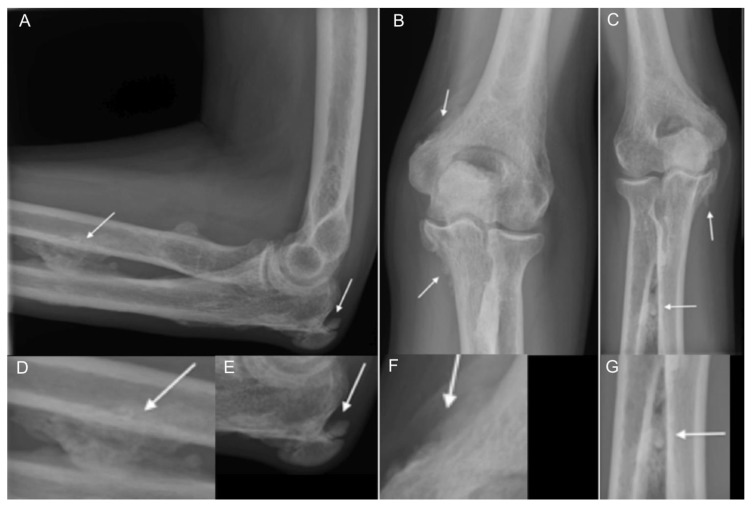
Figure 4.
48 year-old man with voriconazole-induced periostitis following stem cell transplant for acute lymphoblastic leukemia. Lateral (A), anteroposterior (B), and oblique (C) views of the elbow demonstrate dense, irregular, bulky periosteal new bone formation about the elbow joint (arrows). Specifically, there is bulky periosteal new bone along the proximal radial and ulnar diaphyses, at the posterior and medial aspects of the olecranon, and along the distal medial humeral metaphysis in the supracondylar region. Coned down images of each view are shown in the lower row of the figure (D/E, F, and G, respectively).
Figure 5.
48 year-old man with voriconazole-induced periostitis following stem cell transplant for acute lymphoblastic leukemia. Single anteroposterior (A) radiograph of the right hand and coned down images at right demonstrate irregular, bulky periosteal new bone formation involving the first proximal phalanx medially (B), the fifth metacarpal medially (C) (arrows), the distal radial metadiaphysis laterally (D), and the distal ulnar diaphysis laterally (E). Coned down image of the fifth metacarpal (C) also demonstrates sclerosis and abnormal morphology of the fifth metacarpal head consistent with prior fracture (dashed arrow).
DISCUSSION
Diffuse bone pain can be seen in a wide variety of conditions including multiple metabolic/inflammatory processes along with primary and metastatic bone tumors. Infection can also at times be a confounding diagnostic consideration. The specific subset of patients with diffuse periostitis is noteworthy because of the extremely severe pain that these patients can experience owing to the abundant innervation of the periosteum [1].
Voriconazole is a powerful second-generation triazole antifungal agent approved by the Food and Drug Administration in 2002. The drug is similar to fluconazole, however, was created by substituting a fluorinated pyrimidine in lieu of the triazole moiety, leading to expanded antifungal properties [2]. Voriconazole is commonly used in immunocompromised patients to treat both invasive aspergillosis and candidemia with comparable efficacy to regimens of intravenous amphotericin B and/or fluconazole [2,3]. With more than 25,000 lung transplants performed worldwide and an incidence of invasive aspergillus infection from 6–17% (with a 50–100% mortality rate), the powerful voriconazole has become an attractive therapy for both the treatment of and prophylaxis against serious fungal infections [4–6]. The most common side effect of voriconazole is a transient visual disturbance. Less common side effects include rash and elevations in hepatic transaminases [2].
Several case reports have recently documented periostitis in the setting of long-term voriconazole therapy [7]. Wang et al initially described periostitis associated with voriconazole in lung transplant patients [7]. Periostitis on bone scans and plain radiographs was characteristic, in association with severe bone pain, absence of clubbing, and elevated alkaline phosphatase. These findings resolved in all patients following voriconazole discontinuation. A similar series by Chen and Mulligan reported dense and irregular periosteal reaction in lung transplant patients on chronic voriconazole in contrast to the smooth and single layer periostitis described in lung-cancer-associated hypertrophic osteoarthropathy. Additional case reports have recently described this phenomenon of radiographically dramatic periostitis in the setting of chronic voriconazole use which completely resolves upon discontinuation of the medication [8–12].
The precise mechanism of periostitis in association with voriconazole therapy is unclear however recent reports have suggested that fluoride toxicity might be the underlying etiology. Periostitis deformans in the setting of bone fluorosis was first described by Soriano in 1952 with subsequent description of the radiological findings of dramatic “sclero-atrophic osteitis” [13,14]. Fluoride induces bone formation by stimulating osteoblasts [15]. The radiological findings in many of the reported cases of voriconazole-induced periostitis are similar in appearance to the initial descriptions of periostitis deformans in the setting of fluoride excess, first establishing the possible link. More recently Wermers et al studied plasma fluoride levels in 10 adult post-transplant patients who had received voriconazole for at least 6 months and compared to 10 patients who had not received voriconazole. All subjects who received voriconazole had markedly elevated plasma fluoride levels and no subjects in the control group had elevated levels (14.32 μmol/L ± 6.41 compared to 2.54 ± 0.67 μmol/L, p<0.001) [16]. Half of the treated group had evidence of periostitis. Discontinuation of voriconazole in patients with periostitis resulted in improved pain and a reduction in alkaline phosphatase and fluoride levels.
In addition to fluorosis and voriconazole, other causes of diffuse periostitis include primary and secondary hypertrophic osteoarthropathy, thyroid achropachy, venous stasis, leukemia, and other medications (vitamin A, prostaglandin) [17]. The clinical history is helpful in including or excluding most of these diagnoses as part of the differential diagnosis, however, hypertrophic osteoarthropathy can be difficult to exclude. Indeed, in the earliest reports of voriconazole-induced periostitis, hypertrophic osteoarthropathy was the leading alternative consideration and it was initially hypothesized that the two processes had the same pathophysiological mechanism [7]. Of note, however, voriconazole-induced periostitis can often be distinguished from hypertrophic osteoarthropathy by more diffuse involvement (as opposed to predominantly long bone involvement), the absence of clubbing, and universally elevated alkaline phosphatase [7]. Thyroid achropachy predominantly involves the metacarpals/metatarsals with a spiculated, lacy appearance. Venous stasis is usually seen in the lower extremities, and can have associated phleboliths/cellulitis. Bony leukemia in adults is most often seen in the axial skeleton with smooth, lamellated periosteal reaction [17].
TEACHING POINT
Voriconazole-induced periostitis is an important etiology of painful diffuse periostitis in immunocompromised patients characterized by dense, irregular, diffuse periostitis and increased uptake on Tc-99m MDP whole body bone scans. Clinicians and radiologists should carefully correlate with the clinical history in the setting of diffuse bony changes, particularly in immunocompromised patients.
Table 1.
Summary table for voriconazole-induced periostitis
| Etiology | Likely fluoride excess |
| Incidence | Unknown, however a small case control study of 20 patients noted radiographic evidence in 50% |
| Gender Ratio | No specific gender predilection noted |
| Age Predilection | Predominantly middle-aged adults |
| Risk Factors | Chronic voriconazole exposure |
| Treatment | Cessation of voriconazole therapy |
| Prognosis | Resolution of symptoms and radiographic findings following withdrawal of the medication |
| Findings on Imaging | Dense, irregular, bulky periosteal new bone formation on radiograph/CT. Associated increased uptake on Tc-99m MDP whole body bone scan |
Table 2.
Differential diagnosis table for voriconazole-induced periostitis
| Differential Diagnosis | X-Ray/CT | Bone scintigraphy |
|---|---|---|
| Voriconazole-induced periostitis |
|
Associated increased uptake |
| Fluoride excess/Periostitis deformans |
|
Associated increased uptake |
| Hypertrophic Osteoarthropathy |
|
Associated increased uptake |
| Thyroid Achropachy |
|
Associated increased uptake |
| Venous Stasis |
|
Associated increased uptake |
| Leukemia |
|
Increased uptake but may underestimate disease |
| Hypervitaminosis A |
|
Associated increased uptake |
ABBREVIATIONS
- ANA
Antinuclear antibody
- CT
Computed tomography
- DNA
Deoxyribonucleic acid
- MDP
Methylene-diphosphonate
REFERENCES
- 1.Gronblad M, et al. Innervation of human bone periosteum by peptidergic nerves. The Anatomical record. 1984 Jul;209(3):297–299. doi: 10.1002/ar.1092090306. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LB, Kauffman CA. Voriconazole: a new triazole antifungal agent. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2003 Feb;36(5):630–637. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]
- 3.Ally R, et al. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2001 Sep;33(9):1447–1454. doi: 10.1086/322653. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Husain S. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2003 Mar;22(3):258–266. doi: 10.1016/s1053-2498(02)00477-1. [DOI] [PubMed] [Google Scholar]
- 5.Cahill BC, et al. Aspergillus airway colonization and invasive disease after lung transplantation. Chest. 1997 Nov;112(5):1160–1164. doi: 10.1378/chest.112.5.1160. [DOI] [PubMed] [Google Scholar]
- 6.Mehrad B, et al. Spectrum of Aspergillus infection in lung transplant recipients: case series and review of the literature. Chest. 2001 Jan;119(1):169–175. doi: 10.1378/chest.119.1.169. [DOI] [PubMed] [Google Scholar]
- 7.Wang TF, et al. Periostitis secondary to prolonged voriconazole therapy in lung transplant recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009 Dec;9(12):2845–2850. doi: 10.1111/j.1600-6143.2009.02837.x. [DOI] [PubMed] [Google Scholar]
- 8.Ayub A, Kenney CV, McKiernan FE. Multifocal nodular periostitis associated with prolonged voriconazole therapy in a lung transplant recipient. Journal of clinical rheumatology: practical reports on rheumatic & musculoskeletal diseases. 2011 Mar;17(2):73–75. doi: 10.1097/RHU.0b013e31820aff12. [DOI] [PubMed] [Google Scholar]
- 9.Lustenberger DP, Granata JD, Scharschmidt TJ. Periostitis secondary to prolonged voriconazole therapy in a lung transplant recipient. Orthopedics. 2011 Nov;34(11):793–796. doi: 10.3928/01477447-20110922-35. [DOI] [PubMed] [Google Scholar]
- 10.Wise SM, Wilson MA. A case of periostitis secondary to voriconazole therapy in a heart transplant recipient. Clinical nuclear medicine. 2011;36(3):242–4. doi: 10.1097/RLU.0b013e31820902d8. [DOI] [PubMed] [Google Scholar]
- 11.Rossier C, et al. Voriconazole-induced periostitis. European journal of nuclear medicine and molecular imaging. 2012 Feb;39(2):375–376. doi: 10.1007/s00259-011-1922-x. [DOI] [PubMed] [Google Scholar]
- 12.Becce F, et al. Clinical images: Voriconazole-induced periostitis deformans. Arthritis and rheumatism. 2012 Oct;64(10):3490. doi: 10.1002/art.34618. [DOI] [PubMed] [Google Scholar]
- 13.Soriano M. Periostitis deformans. Annals of the rheumatic diseases. 1952 Jun;11(2):154–61. doi: 10.1136/ard.11.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano M, Manchon F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology. 1966 Dec;87(6):1089–1094. doi: 10.1148/87.6.1089. [DOI] [PubMed] [Google Scholar]
- 15.Khokher MA, Dandona P. Fluoride stimulates [3H]thymidine incorporation and alkaline phosphatase production by human osteoblasts. Metabolism: clinical and experimental. 1990 Nov;39(11):1118–1121. doi: 10.1016/0026-0495(90)90081-m. [DOI] [PubMed] [Google Scholar]
- 16.Wermers RA, et al. Fluoride excess and periostitis in transplant patients receiving long-term voriconazole therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011 Mar;52(5):604–611. doi: 10.1093/cid/ciq188. [DOI] [PubMed] [Google Scholar]
- 17.Roberts CC. Periosteum: Solid Periostitis. 2012. [cited 2012 10/4]; Available from: https://my.statdx.com/STATdxMain.jsp?rc=false#edxExpandedContent;solid_periostitis_expert-ddx.