Abstract
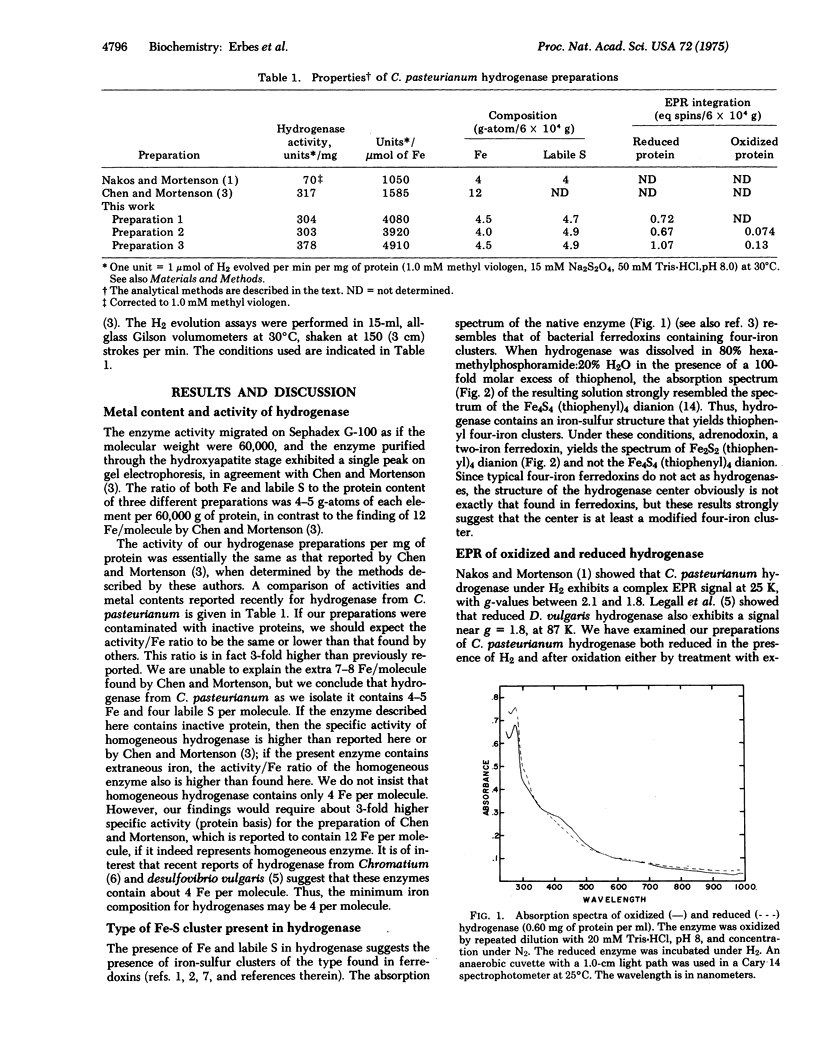
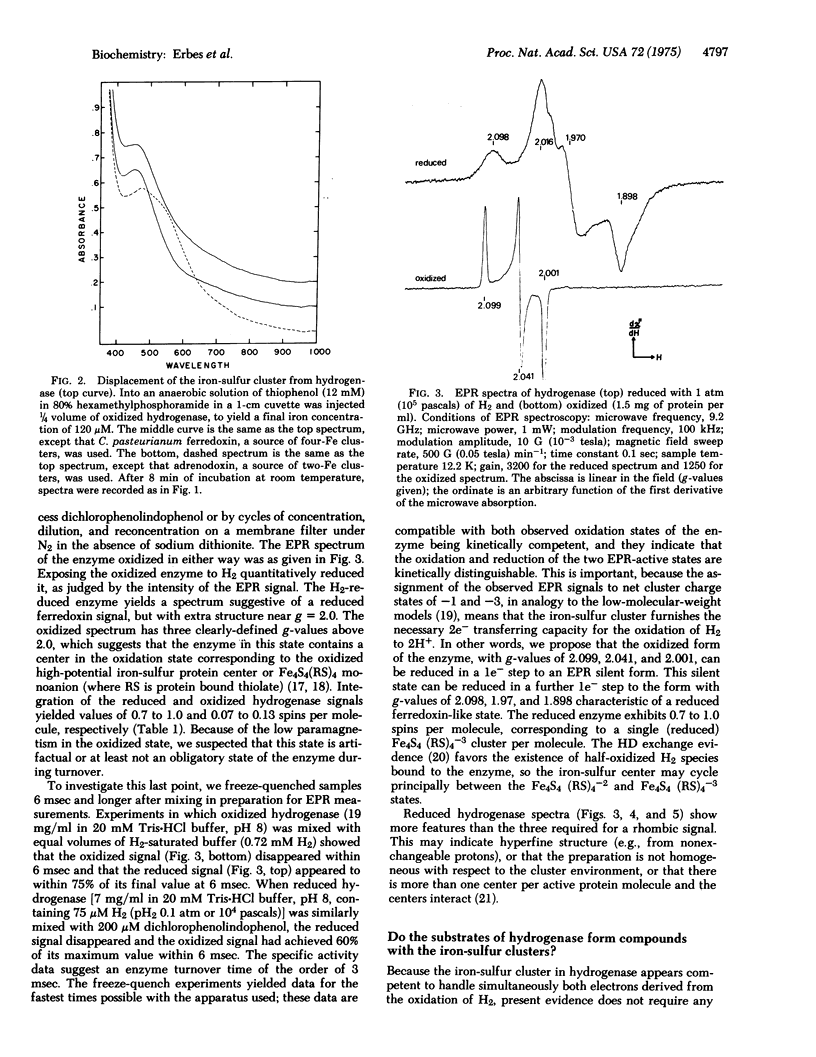
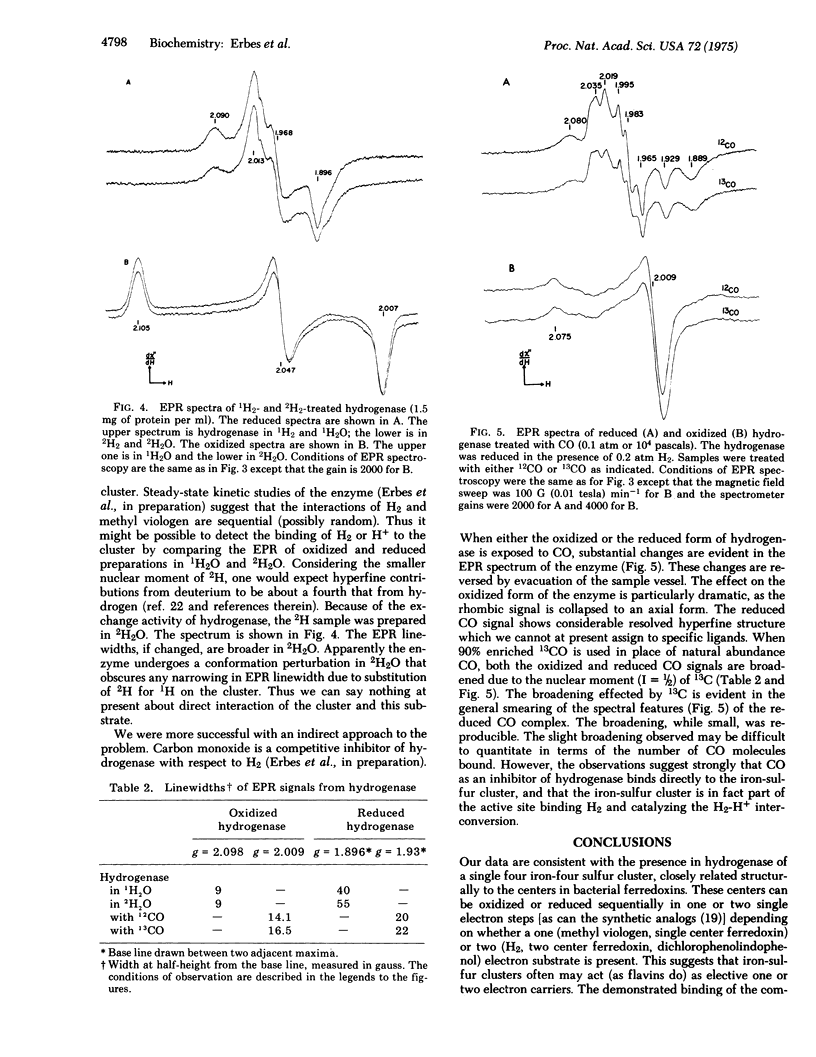
Hydrogenase, purified to an average specific activity of 328 mumol of H2 evolved/(min X mg of protein) from Clostridium pasteurianum W5, was found to have 4-5 Fe and 4-5 labile sulfur atoms per molecule of 60,000 molecular weight, in contrast with earlier reports of 12 Fe per molecule. Displacement of the iron-sulfur cluster from hydrogenase by thiophenol in 80% hexamethyl phosphoramide:20% H2O yielded the Fe4S4 (thiophenyl)4 dianion according to absorption spectroscopy. Electron paramagnetic resonance spectroscopy at 12 K showed that the iron-sulfur cluster in the enzyme could be reduced by the H2 to a state (g-values of 2.098, 1.970, and 1.898) similar to that in reduced ferredoxin and could be oxidized by dichlorophenolindophenol or H+ to a state (g-values at 2.099, 2.041, and 2.001) similar to that in high potential iron-sulfur proteins. These oxidations and reductions appeared to occur within the turnover time of the enzyme. Deuterium failed to narrow the electron paramagnetic resonance signal in either state, but the competitive inhibitor carbon monoxide reversibly formed a compound with either state and substantially altered the electron paramagnetic resonance. 13CO produced a broadening of these signals, suggesting the formation of a direct CO complex with the iron-sulfur cluster. These data are consistent with a model of the active site of the enzyme in which a four-iron four-sulfur cluster is a component that can accept one or two electrons from and donate either one or two electrons to substrates, and in which the iron-sulfur cluster serves as the site of binding of gaseous ligands.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY R. C. Sudden freezing as a technique for the study of rapid reactions. Biochem J. 1961 Oct;81:189–193. doi: 10.1042/bj0810189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUMBY P. E., MILLER R. W., MASSEY V. THE CONTENT AND POSSIBLE CATALYTIC SIGNIFICANCE OF LABILE SULFIDE IN SOME METALLOFLAVOPROTEINS. J Biol Chem. 1965 May;240:2222–2228. [PubMed] [Google Scholar]
- Beinert H., Orme-Johnson W. H. Electron-nuclear and electron-electron spin interactions in the study of enzyme structure and function. Ann N Y Acad Sci. 1969 May 16;158(1):336–360. doi: 10.1111/j.1749-6632.1969.tb56230.x. [DOI] [PubMed] [Google Scholar]
- Cammack R. "Super-reduction" of chromatium high-potential iron-sulphur protein in the presence of dimethyl sulphoxide. Biochem Biophys Res Commun. 1973 Sep 18;54(2):548–554. doi: 10.1016/0006-291x(73)91457-5. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A., Sieker L. C., Adman E., Jensen L. H. A comparison of Fe 4 S 4 clusters in high-potential iron protein and in ferredoxin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3526–3529. doi: 10.1073/pnas.69.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. S., Mortenson L. E. Purification and properties of hydrogenase from Clostridium pasteurianum W5. Biochim Biophys Acta. 1974 Dec 18;371(2):283–298. doi: 10.1016/0005-2795(74)90025-7. [DOI] [PubMed] [Google Scholar]
- Gitlitz P. H., Krasna A. I. Structural and catalytic properties of hydrogenase from Chromatium. Biochemistry. 1975 Jun 17;14(12):2561–2568. doi: 10.1021/bi00683a001. [DOI] [PubMed] [Google Scholar]
- Haschke R. H., Campbell L. L. Purification and properties of a hydrogenase from Desulfovibrio vulgaris. J Bacteriol. 1971 Jan;105(1):249–258. doi: 10.1128/jb.105.1.249-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm R. H. Iron-sulphur clusters in natural and synthetic systems. Endeavour. 1975 Jan;34(121):38–43. doi: 10.1016/0160-9327(75)90067-8. [DOI] [PubMed] [Google Scholar]
- Kleiner D., Burris R. H. The hydrogenase of Clostridium pasteurianum. Kinetic studies and the role of molybdenum. Biochim Biophys Acta. 1970 Sep 16;212(3):417–427. doi: 10.1016/0005-2744(70)90247-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legall J., DerVartanian D. V., Spilker E., Lee J. P., Peck H. D., Jr Evidence for the involvement of non-heme iron in the active site of hydrogenase from Desulfovibrio vulgaris. Biochim Biophys Acta. 1971 Jun 15;234(3):526–530. [PubMed] [Google Scholar]
- Mathews R., Charlton S., Sands R. H., Palmer G. On the nature of the spin coupling between the iron-sulfur clusters in the eight-iron ferredoxins. J Biol Chem. 1974 Jul 10;249(13):4326–4328. [PubMed] [Google Scholar]
- Nakos G., Mortenson L. E. Structural properties of hydrogenase from Clostridium pasteurianum W5. Biochemistry. 1971 Jun 22;10(13):2442–2449. doi: 10.1021/bi00789a003. [DOI] [PubMed] [Google Scholar]
- Nakos G., Mortenson L. Purification and properties of hydrogenase, an iron sulfur protein, from Clostridium pasteurianum W5. Biochim Biophys Acta. 1971 Mar 10;227(3):576–583. doi: 10.1016/0005-2744(71)90008-8. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Hamilton W. D., Jones T. L., Tso M. Y., Burris R. H., Shah V. K., Brill W. J. Electron paramagnetic resonance of nitrogenase and nitrogenase components from Clostridium pasteurianum W5 and Azotobacter vinelandii OP. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3142–3145. doi: 10.1073/pnas.69.11.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Iron-sulfur proteins: structure and function. Annu Rev Biochem. 1973;42(0):159–204. doi: 10.1146/annurev.bi.42.070173.001111. [DOI] [PubMed] [Google Scholar]
- Que L., Jr, Holm R. H., Mortenson L. E. Letter: Extrusion of Fe2S2 and Fe4S4 cores from the active sites of ferredoxin proteins. J Am Chem Soc. 1975 Jan 22;97(2):463–464. doi: 10.1021/ja00835a064. [DOI] [PubMed] [Google Scholar]
- Tsai R., Yu C. A., Gunsalus I. C., Peisach J., Blumberg W., Orme-Johnson W. H., Beinert H. Spin-state changes in cytochrome P-450cam on binding of specific substrates. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1157–1163. doi: 10.1073/pnas.66.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Bogart M., Beinert H. Micro methods for the quantitative determination of iron and copper in biological material. Anal Biochem. 1967 Aug;20(2):325–334. doi: 10.1016/0003-2697(67)90038-3. [DOI] [PubMed] [Google Scholar]
- Vandecasteele J. P., Burris R. H. Purification and properties of the constituents of the nitrogenase complex from Clostridium pasteurianum. J Bacteriol. 1970 Mar;101(3):794–801. doi: 10.1128/jb.101.3.794-801.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


