Abstract
Cancer is a major component of health-care expenditures in most developed countries. The costs of cancer care are expected to increase due to rising incidence (as the population ages) and increasing use of targeted anticancer therapies. However, epidemiological analysis of patterns of care may be required prior to empirically well-grounded cost analyses. Additionally, comparisons of care between health-care delivery systems and countries can identify opportunities to improve practice. They can also increase understanding of patient outcomes and economic consequences of differences in policies related to cancer screening, treatment, and programs of care. In this study, we compared patterns of colorectal cancer treatment during the first year following diagnosis in two cohorts of elderly patients from some areas of Italy and the United States using cancer registry linked to administrative data. We evaluated hospital use, initial treatments (surgery, chemotherapy, and radiation), and timeliness of surgery and adjuvant therapy, taking into account patient characteristics and clinical features, such as stage at diagnosis and the cancer subsite. We observed greater use of adjuvant chemotherapy in stage III and IV colon cancer patients and adjuvant therapy in all stages of rectal cancer patients in the US cohort. We found a higher rate of open surgeries in the Italian cohort, a similar rate of hospitalization, but a higher number of hospital days in the Italian cohort. However, in spite of structural differences between the United States and Italy in health-care organization and delivery as well as in data collection, patterns of care and the timing of care in the year after diagnosis are generally similar among patients within stage of disease at diagnosis. Comparative studies of the costs associated with patterns of cancer care will be important for future research.
Cancer care is a major component of health-care expenditures in most developed countries. The costs of cancer care are expected to increase, in large part due to rising incidence as the population ages. Additionally, the costs of anticancer therapies have increased dramatically in recent years (1–4). Health-care systems will face the challenge of providing increasingly expensive cancer care to a growing number of patients. In the current climate of constrained resources that is present in most developed countries, policy makers are striving to identify the ways to provide the most efficient and economical care.
Internationally there is tremendous diversity in health-care systems and patterns of cancer care delivery (5,6). These differences offer an opportunity to compare existing patterns of care, patient outcomes, and costs of care between health-care systems or countries. Such comparisons have the potential to inform evaluation, develop policies related to cancer screening and treatment, and identify the need for programs of care delivery (eg, hospice for patients at the end of life). Findings from these comparisons can also be used to establish benchmarks of cancer outcomes for evaluating the introduction of cancer control interventions prospectively.
Several studies have used data from population-based cancer registries for international comparisons of cancer incidence (7,8,9), survival (10,11), and prevalence (12). The European Cancer Registry (EUROCARE)–based Study on Survival and Care of Cancer Patients and the CONCORD Program for a Global Surveillance of Cancer Survival have conducted more detailed systematic international comparisons of cancer site–specific survival, accounting for underlying population characteristics, such as age, gender, and geographical area. As part of the EUROCARE project, high-resolution (HR) studies collected a sample of registered cases with detailed clinical and pathological information for selected cancer sites. The additional information from HR studies is not usually available in population-based cancer registries and represents a way of assessing the overall performance of health-care services and of improving the interpretation of survival differences across countries and over time (13,14). There have been a limited number of international comparisons of patterns or costs of cancer care, in part because of lack of key data elements collected in a systematic way and differences in how the information is reported for common treatments (eg, surgery, radiation therapy, chemotherapy) and biologically targeted therapies and hormonal treatments. To date, the studies that have compared patterns of care internationally have focused on the United States and Canada (5,6,15,16) or in multiple European countries (13). To our knowledge, there has not been a detailed systematic comparison of specific types of cancer treatment between the United States and a European country. Furthermore, this kind of epidemiological analysis of patterns of health-care delivery will provide useful information for empirically grounded cost analyses, and should be carried out prior to any cost analysis.
In this study, we compared patterns of treatment in colorectal cancer patients during the first year following diagnosis in some areas of Italy and the United States, using cancer registry linked to administrative data available in both countries. We chose colorectal cancer for our comparisons because it is a common cancer in men and women, is treated with multiple modalities of cancer therapy (namely surgery, chemotherapy, and radiation), and can be detected early through routine screening. We also explored the time from diagnosis to initial surgery and the time between surgery and adjuvant therapy in cohorts in both countries.
Data and Methods
Health-Care Delivery Systems
Italy and the United States differ substantially in the structure of their health-care systems. In Italy, the public welfare system guarantees universal health care for hospital, ambulatory, and other health-care services. In the United States, health insurance is employment-based for most working age adults and contracted through one of multiple health insurance companies, resulting in separate and generally discontinuous data for the working-age population. However, the Medicare program in the United States provides comprehensive health-care delivery for the population aged 65 and older and persons with select disabilities. Approximately 97% of the population 65 years and older has Medicare. As a result, there is comprehensive data about services for elderly patients in the Medicare program, which can be compared with the comprehensive services provided for elderly patients in Italy.
Data Sources
Both Italy and the United States maintain population-based cancer registries. These registries collect information about all newly diagnosed cancer patients within defined geographical areas. In both countries, the registry data for individual cancer patients have been linked to their health claims. We used these linked data to obtain information on clinical characteristics, receipt of cancer treatment, including surgery, radiation therapy, and chemotherapy, and timing of cancer treatment. We also obtained information about hospitalizations, both before and after the cancer diagnosis.
In Italy, we combined data from two cancer registries: Firenze-Prato, encompassing two provinces of the Tuscany Cancer Registry (17) in Central Italy and covering 1.2 million residents, and Padova, a local health unit of the Veneto Cancer Registry (18) in Northern Italy, which covers 0.4 million residents. Together these areas cover 2.7% of the Italian population. The combined Veneto–Tuscany Cancer Registry (VTCR) database includes information on date of birth, sex, date of diagnosis, date of last follow-up, tumor site, morphology, diagnostic confirmation, and stage at diagnosis. All patients included in the registries are actively followed up to determine vital status. These registries contain information about cancer diagnoses starting in 1990.
In Italy, health claims come from the hospital discharge card (HDC) administrative database, a data system used for reimbursement for services that occur in the hospital setting. Information on outpatient or ambulatory services and physician visits are not included in the database. However, during the period of this study, hospitals were the locus of all open surgical care and infusion chemotherapy; additionally, data for radiation treatments that are performed in outpatient or ambulatory care were added for this study. Claims for hospital-based services reflect information on the HDC completed by the treating physician for each time that the patient goes to the hospital. HDC includes information about inpatient hospital (IH) care and day hospital (DH) care. IH care occurs when a patient is formally admitted to an institution for treatment and/or care and stays for a minimum of one night; any medical treatment provided during the stay is included. DH care comprises medical and paramedical services delivered to patients seen in the clinic for diagnosis, treatment, or other type of health care, without an overnight hospital stay. DH care may last 1 or more days depending on the cycle of treatments. One HDC refers to a single hospital admission or service (IH or DH). It contains demographic information (date of birth, sex, place of birth, place of residence) and clinical information [type of diagnosis, interventions, and procedures coded by the International Classification of Diseases, Ninth Revision, Clinical Modifications (ICD-9-CM) (19)]. Different HDCs for the same individual can be linked by a unique personal identification code.
Newly diagnosed colon and rectum cancer patients in 2000–2001 in VTCR database were linked with the corresponding regional HDC databases from 1999 to 2002, in order to obtain all hospital admissions and hospital-based care and corresponding procedures received in the year prior to diagnosis and the first year following diagnosis. The deterministic linkage was based on a unique identification code, with 95% of all colorectal cancer patients linked to one or more HDCs. Less than 1% of cancer patients were diagnosed and treated in private hospitals operating outside the National Health System (20). For these patients, although present in the registry, there is no information on HDC.
In the United States, registry data were from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program of cancer registries. The SEER registries are geographically defined and collect detailed clinical information on the site, pathology, and extent of disease at the time of each cancer diagnosis; stage, month, and year of diagnosis; and patient age and sex. For this study, we included cancer patients from 11 registries—five states (Connecticut, Hawaii, Iowa, New Mexico, and Utah) and six metropolitan areas (Atlanta, Detroit, Los Angeles, San Francisco–Oakland, San Jose–Monterey, and Seattle–Puget Sound), altogether representing 14% of the total US population. All patients included in the registries are actively followed to determine vital status. Most of these registries contained information on cancer diagnoses from 1975 onward, except Los Angeles and San Jose–Monterey, which joined the SEER program in 1992.
For US patients with fee-for-service coverage, their Medicare claims are contained in different files, depending on the type of service. These include inpatient hospitalizations, outpatient clinic services, and physician visits. Each file includes ICD-9-CM codes for the patient’s diagnoses and dates of service. Procedures on inpatient files are billed using ICD-9-CM codes. Procedures billed by outpatient clinics and physicians are coded using the Healthcare Common Procedure Coding System (HCPCS) (available at http://www.cms.gov/MedHCPCSGenInfo/).
All patients in the SEER data have been included in a deterministic match against Medicare’s master enrollment file. Approximately 94% of individuals aged 65 or older who have a cancer diagnosis in the SEER data have been linked to Medicare’s master enrollment file (21). For SEER patients who were Medicare-eligible, all available Medicare health claims were obtained. For a more detailed description of SEER–Medicare linked data, refer to http://healthservices.cancer.gov/seermedicare/.
Study Populations—VTCR and SEER–Medicare
We selected patients aged 66 and older newly diagnosed with colon cancer (International Classification of Diseases for Oncology [ICD-O] topography codes C18.0, C18.2–9) or rectal cancer (ICD-O topography codes C19.9, C20.9) in the period January 1, 2000, to December 31, 2001 (VTCR = 1844, SEER–Medicare = 46 571). Although Medicare coverage begins at age 65, we selected patients at age 66 and older in order to obtain information on comorbidities in the year period prior to diagnosis. In the SEER–Medicare data, we excluded patients not covered by both Medicare Parts A and B in the year prior and the year after diagnosis (33.7%) to ensure that we had complete claims for all individuals in this study. In both cohorts, we excluded individuals diagnosed through autopsy or death certificate only (0.8% in both databases), patients with a prior cancer diagnosis (VTCR = 6.5% and SEER–Medicare = 12.9%), patients with another cancer diagnosis in the year following colorectal cancer diagnosis (VTCR = 0% and SEER–Medicare = 1.5%), patients with 1 month or less of survival following diagnosis (VTCR = 2.7% and SEER–Medicare = 5%), and unstaged patients (VTCR = 13.2% and SEER–Medicare = 6.1%). The final analysis cohorts consisted of 1396 Italian and 18 438 US patients with a primary diagnosis of invasive colorectal cancer.
Variables Included in the Analysis
Patient Characteristics.
Patient characteristics for both cohorts were obtained from the time of diagnosis. Patient age was categorized into five groups (66–69, 70–74, 75–79, 80–84, 85+). The American Joint Committee on Cancer’s (AJCC) Cancer Staging Version 3 (22) was used by both registries to classify tumors by their spread and severity of disease. We also used registry data to determine if the tumor was found on the left or right side of the colon or rectum, using ICD-O topography codes (right: C18.0–C18.4; left: C18.5–C20.9). Comorbidity was measured in the year prior to diagnosis using the Charlson Comorbidity Score (23) for inpatient care in both countries. In VTCR, comorbid conditions were identified from the HDC; in SEER–Medicare, from hospital claims. The macro to compute these scores is publicly available at http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
We compared differences in hospital use between colorectal cancer patients in the two countries, both before cancer diagnosis to assess underlying differences in the two populations and after diagnosis to assess patterns of health-care use. Specifically we assessed the number of admissions, defined by any overnight stay in the HDC or any record of a hospital admission in the Medicare data. We also calculated the total number of inpatient days from the length of stay for each hospitalization, summarized over the course of the year by patient. These were categorized into 0, 1, 2+ weeks in the year before diagnosis, and 0, 1, 2, 3, 4+ weeks in the year after diagnosis.
Initial Treatment.
Initial treatment was defined by receipt of open surgery, radiation therapy, or adjuvant chemotherapy during the year following diagnosis. Open surgery for colorectal cancer included colectomy, hemicolectomy, pelvic exenteration, and permanent colostomy. We also assessed the use of chemotherapy, defined as any claim for administration of chemotherapy, and examined use separately for patients who did and did not undergo surgery. We report information for stage I and II colon cancer combined, because guidelines at the time of the study recommend the same therapeutic approach (24): no adjuvant chemotherapy, wide surgical resection, and anastomosis. By contrast, adjuvant chemotherapy was recommended for stage III colon cancer. Rectal cancer surgery is reported separately for all stages, as guidelines for chemotherapy and radiation therapy vary by stage. For rectal cancer patients, we also examined the use of neoadjuvant radiation treatment and chemotherapy, which is intended to allow for sphincter-sparing surgery. These treatments are rarely recommended for colon cancer. See Appendix A for a complete list of ICD-9-CM procedure codes and HCPCS codes used to identify cancer treatments in SEER–Medicare and VTCR–HDC.
Time to Treatment.
Time between diagnosis and initial surgery and time between surgery and start of adjuvant chemotherapy was estimated in days because some patients died during the period of observation. Because the SEER registries only collect month and year of diagnosis, we designated the first day of the month as the date of diagnosis in both cohorts. Actual dates for surgery and chemotherapy were available in both countries. For patients with more than one open surgery, we selected the first surgery after diagnosis for the analysis of time from diagnosis to surgery. We used the last surgery date as the starting date for the analysis of time from surgery to adjuvant chemotherapy.
To account for patients who died during the year after diagnosis, we used a person-day approach. For example, if a patient died 40 days after diagnosis and surgery had not occurred, the patient contributed 10 person-days to the time period 31–60 days and zero to the number of surgeries in that time period. If, instead, one surgery occurred at day 40 after diagnosis with death at day 60, the patient contributed 30 days to the time period 31–60 days and 1 to the number of surgeries in that time period. All patients were followed for a maximum of 365 days post-diagnosis.
Results
Sample Characteristics
In the VTCR and SEER–Medicare colorectal cancer cohorts, the majority of patients had colon cancer (VTCR 71%; SEER–Medicare 76%) (Table 1). The SEER–Medicare cohort was older than the VTCR cohort (aged 80 and older: 37% vs 28%) and had more female patients (55% vs 46%). The stage at diagnosis varied in the two colorectal cohorts, with substantially more SEER–Medicare patients diagnosed with stage I or II than the VTCR patients (61% vs 48%). More patients in the SEER–Medicare cohort were diagnosed with rightsided tumors than in the VTCR cohort (46% vs 34%). Additionally, a larger proportion of the SEER–Medicare cohort had higher comorbidity scores than the VTCR cohort (Charlson index 1+; 14% vs 7%).
Table 1.
Characteristics of patients aged 66+ diagnosed with colon–rectal cancer in 2000–2001; Surveillance, Epidemiology, and End Results (SEER)–Medicare and Veneto–Tuscany Cancer Registry (VTCR)*
| Colorectal cases | Colon cases | Rectum cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEER– Medicare (n = 18 438) | VTCR (n = 1396) | SEER–Medicare (n = 13 906) | VTCR (n = 987) | SEER– Medicare (n = 4532) | VTCR (n = 409) | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age at diagnosis | ||||||||||||
| 66–69 | 2736 | 15 | 247 | 18 | 1950 | 14 | 150 | 15 | 786 | 17 | 97 | 24 |
| 70–74 | 4190 | 23 | 367 | 26 | 3060 | 22 | 274 | 28 | 1130 | 25 | 93 | 23 |
| 75–79 | 4673 | 25 | 389 | 28 | 3524 | 25 | 273 | 28 | 1149 | 25 | 116 | 28 |
| 80–84 | 3640 | 20 | 210 | 15 | 2823 | 20 | 158 | 16 | 817 | 18 | 52 | 13 |
| 85+ | 3199 | 17 | 183 | 13 | 2549 | 18 | 132 | 13 | 650 | 14 | 51 | 12 |
| Sex | ||||||||||||
| Male | 8238 | 45 | 753 | 54 | 5926 | 43 | 513 | 52 | 2312 | 51 | 240 | 59 |
| Female | 10 200 | 55 | 643 | 46 | 7980 | 57 | 474 | 48 | 2220 | 49 | 169 | 41 |
| AJCC stage at diagnosis | ||||||||||||
| I | 5430 | 29 | 213 | 15 | 3672 | 26 | 126 | 13 | 1758 | 39 | 87 | 21 |
| II | 5905 | 32 | 463 | 33 | 4736 | 34 | 349 | 35 | 1169 | 26 | 114 | 28 |
| III | 4565 | 25 | 412 | 30 | 3532 | 25 | 301 | 30 | 1033 | 23 | 111 | 27 |
| IV | 2538 | 14 | 308 | 22 | 1966 | 14 | 211 | 21 | 572 | 13 | 97 | 24 |
| ICD-O topography | ||||||||||||
| Right side (C18.0–C18.4) | 8443 | 46 | 469 | 34 | 8443 | 46 | 469 | 34 | — | — | — | — |
| Left side (C18.5–C20.9) | 9995 | 54 | 927 | 66 | 5463 | 30 | 518 | 37 | 4532 | 24 | 409 | 29 |
| Charlson comorbidity score (hospital claims only) | ||||||||||||
| 0 | 15 819 | 86 | 1291 | 92 | 11 942 | 86 | 909 | 92 | 3877 | 86 | 382 | 93 |
| 1 | 1312 | 7 | 71 | 5 | 990 | 7 | 54 | 5 | 322 | 7 | 17 | 4 |
| 2+ | 1307 | 7 | 34 | 2 | 974 | 7 | 24 | 2 | 333 | 7 | 10 | 2 |
| Number of hospital admissions in the one year prior to diagnosis | ||||||||||||
| 0 | 14 491 | 79 | 1093 | 78 | 10 746 | 77 | 767 | 78 | 3745 | 83 | 326 | 80 |
| 1 | 2634 | 14 | 216 | 15 | 2100 | 15 | 152 | 15 | 534 | 12 | 64 | 16 |
| 2 | 850 | 5 | 65 | 5 | 675 | 5 | 51 | 5 | 175 | 4 | 14 | 3 |
| 3+ | 463 | 3 | 22 | 2 | 385 | 3 | 17 | 2 | 78 | 2 | 5 | 1 |
| Total number of hospital days in the one year prior to diagnosis | ||||||||||||
| 0 | 14 491 | 79 | 1093 | 78 | 10 746 | 77 | 767 | 78 | 3745 | 83 | 326 | 80 |
| 1 week (1–7 days) | 2664 | 14 | 133 | 10 | 2130 | 15 | 96 | 10 | 534 | 12 | 37 | 9 |
| 2+ weeks (8+ days) | 1283 | 7 | 170 | 12 | 1030 | 7 | 124 | 13 | 253 | 6 | 46 | 11 |
| Mean days in hospital in the one year prior to diagnosis | 1.7 | — | 3.4 | — | 1.8 | — | 3.5 | — | 1.3 | — | 3.0 | — |
* AJCC = American Joint Committee on Cancer; ICD-O = International Classification of Diseases for Oncology.
Treatment Patterns for Colon Cancer
Most colon cancer patients underwent open surgery within a year from diagnosis (SEER–Medicare 90%; VTCR 94%), with the highest rates of surgery occurring in patients with stage III cancer (Table 2). Among patients who underwent open surgery, about one-third received adjuvant chemotherapy within a year from diagnosis. However, there was variation between the two cohorts by stage. For patients diagnosed with stage III disease, a group for whom chemotherapy is recommended, 61% of SEER–Medicare cohort received chemotherapy compared with 45% of patients in VTCR. Stage IV patients in the SEER–Medicare data were also more likely to undergo chemotherapy than VTCR patients (57% vs 45%).
Table 2.
Treatment regimen and colostomy information for the first year following colon cancer diagnosis in 2000–2001; Surveillance, Epidemiology, and End Results (SEER)–Medicare and Veneto–Tuscany Cancer Registry (VTCR)
| All cases (stages I–IV) | Stage I and II | Stage III | Stage IV | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEER–Medicare (n = 13 906) | VTCR (n = 987) | SEER– Medicare (n = 8408) | VTCR (n = 475) | SEER– Medicare (n = 3532) | VTCR (n = 301) | SEER– Medicare (n = 1966) | VTCR (n = 211) | |||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Patients receiving surgery within a year from diagnosis | 12 535 | 90 | 925 | 94 | 7607 | 90 | 466 | 98 | 3429 | 97 | 300 | 100 | 1499 | 76 | 159 | 75 |
| Patients receiving adjuvant chemotherapy after surgery (% of patients with surgery) | 4180 | 33 | 292 | 32 | 1229 | 16 | 86 | 18 | 2099 | 61 | 135 | 45 | 852 | 57 | 71 | 45 |
| Patients without surgery (% of total patients) | 1371 | 10 | 62 | 6 | 801 | 10 | 9 | 2 | 103 | 3 | 1 | 0 | 467 | 24 | 52 | 25 |
| Chemotherapy only | 215 | 2 | 9 | 1 | 30 | 1 | 0 | 0 | 17 | 1 | 1 | 0 | 168 | 9 | 8 | 4 |
| Not treated | 1156 | 8 | 53 | 5 | 771 | 9 | 9 | 2 | 86 | 2 | 0 | 0 | 299 | 15 | 44 | 21 |
The time from diagnosis to surgery was similar between SEER–Medicare and VTCR patients (Figure 1). In general, most patients received treatment within the first 3 months after diagnosis; 67% of stage III SEER–Medicare patients had surgery within the month following diagnosis compared with 54% of VTCR patients. The time from surgery to chemotherapy varied according to stage. For both groups, the percentage receiving chemotherapy rose appreciably between the first and second month following surgery, with the majority of patients in both groups receiving chemotherapy within 3 months of surgery.
Figure 1.
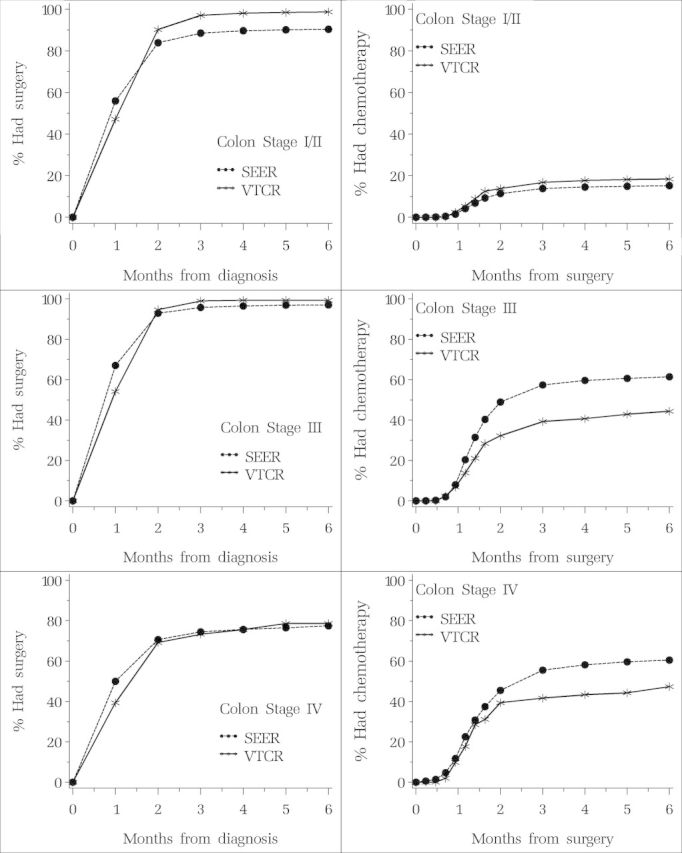
Percent having surgery by time since diagnosis and percent having chemotherapy by time since surgery. Patients diagnosed with colon cancer in Surveillance, Epidemiology, and End Results (SEER)–Medicare and Veneto–Tuscany Cancer Registry (VTCR) by stage.
Treatment Patterns for Rectal Cancer
The percentage of open surgeries among rectal cancer patients in the year after diagnosis was higher in the VTCR cohort than in SEER–Medicare cohort (93% vs 82%). This difference was largest in stage I patients, where 77% of SEER–Medicare patients underwent open surgery contrasted with 95% of the VTCR patients (Table 3). Neoadjuvant therapy in the year prior to surgery was slightly higher in VTCR, more so for patients with stages III and IV disease. However, adjuvant therapies were generally more frequent in SEER–Medicare patients, particularly the use of chemotherapy and radiation. For stages II and III SEER–Medicare patients, the percent who received chemotherapy and radiation therapy was 23% and 36%, respectively, whereas for VTCR patients, the percent who received chemotherapy and radiation was 4% in stage II patients and 12% in stage III patients. Patterns of colostomies were similar, with VTCR patients generally receiving slightly more colostomies compared with SEER–Medicare patients, except for stage III, where SEER–Medicare patients had more colostomies (37% vs 28%).
Table 3.
Treatment regimen and colostomy information for the first year following rectal cancer diagnosis in 2000–2001; Surveillance, Epidemiology, and End Results (SEER)–Medicare and Veneto–Tuscany Cancer Registry (VTCR)*
| All cases (stages I–IV) | Stage I | Stage II | Stage III | Stage IV | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEER– Medicare (n = 4532) | VTCR (n = 409) | SEER– Medicare (n = 1758) | VTCR (n = 87) | SEER– Medicare (n = 1169) | VTCR (n = 114) | SEER– Medicare (n = 1033) | VTCR (n = 111) | SEER– Medicare (n = 572) | VTCR (n = 97) | |||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Patients receiving surgery within a year from diagnosis | 3717 | 82 | 379 | 93 | 1348 | 77 | 83 | 95 | 1055 | 90 | 112 | 98 | 973 | 94 | 110 | 99 | 341 | 60 | 74 | 76 |
| Patients receiving neoadjuvant therapy before surgery (% of patients with surgery) | 486 | 13 | 58 | 15 | 149 | 11 | 11 | 13 | 183 | 17 | 20 | 18 | 116 | 12 | 16 | 15 | 38 | 11 | 11 | 15 |
| Chemotherapy only | 4 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Radiation therapy only | 74 | 2 | 18 | 5 | 26 | 2 | 2 | 2 | 25 | 2 | 9 | 8 | 17 | 2 | 2 | 2 | 6 | 2 | 5 | 7 |
| Chemo + radiation therapy | 389 | 11 | 39 | 10 | 116 | 9 | 8 | 10 | 153 | 15 | 11 | 10 | 93 | 10 | 14 | 13 | 27 | 8 | 6 | 8 |
| Patients receiving adjuvant (after surgery) therapy (% of patients with surgery) | 1596 | 43 | 142 | 37 | 227 | 17 | 7 | 9 | 477 | 45 | 33 | 29 | 670 | 69 | 56 | 51 | 222 | 66 | 46 | 62 |
| Chemotherapy only | 695 | 19 | 98 | 26 | 79 | 6 | 3 | 4 | 165 | 15 | 19 | 17 | 275 | 28 | 40 | 36 | 176 | 52 | 36 | 49 |
| Radiation therapy only | 197 | 5 | 20 | 5 | 66 | 5 | 4 | 5 | 74 | 7 | 9 | 8 | 44 | 5 | 3 | 3 | 13 | 4 | 4 | 5 |
| Chemo + radiation therapy | 704 | 19 | 24 | 6 | 82 | 6 | 0 | 0 | 238 | 23 | 5 | 4 | 351 | 36 | 13 | 12 | 33 | 10 | 6 | 8 |
| Patients without surgery (% of total patients) | 815 | 18 | 33 | 7 | 410 | 23 | 4 | 5 | 114 | 10 | 2 | 2 | 60 | 6 | 1 | 1 | 231 | 40 | 23 | 24 |
| Chemotherapy only | 49 | 1 | 1 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 42 | 7 | 1 | 1 |
| Radiation therapy only | 91 | 2 | 5 | 1 | 28 | 2 | 0 | 0 | 22 | 2 | 0 | 0 | 11 | 1 | 0 | 0 | 30 | 5 | 5 | 5 |
| Chemo + radiation therapy | 167 | 4 | 4 | 1 | 48 | 3 | 0 | 0 | 38 | 3 | 0 | 0 | 17 | 2 | 0 | 0 | 64 | 11 | 4 | 4 |
| None | 508 | 11 | 20 | 5 | 329 | 18 | 4 | 5 | 54 | 5 | 2 | 2 | 30 | 3 | 1 | 1 | 95 | 17 | 13 | 14 |
| Patients receiving a colostomy in the initial surgery (% of patients with surgery) | 1285 | 28 | 124 | 30 | 321 | 18 | 21 | 24 | 417 | 36 | 42 | 37 | 387 | 37 | 31 | 28 | 160 | 28 | 30 | 31 |
The time from diagnosis to surgery was similar in the two cohorts for rectal cancer patients (Figure 2), whereas the time from surgery to chemotherapy (with or without radiotherapy) was generally longer for rectal cancer than for colon cancers, likely a consequence of greater use of neoadjuvant therapies in rectal cancer patients.
Figure 2.
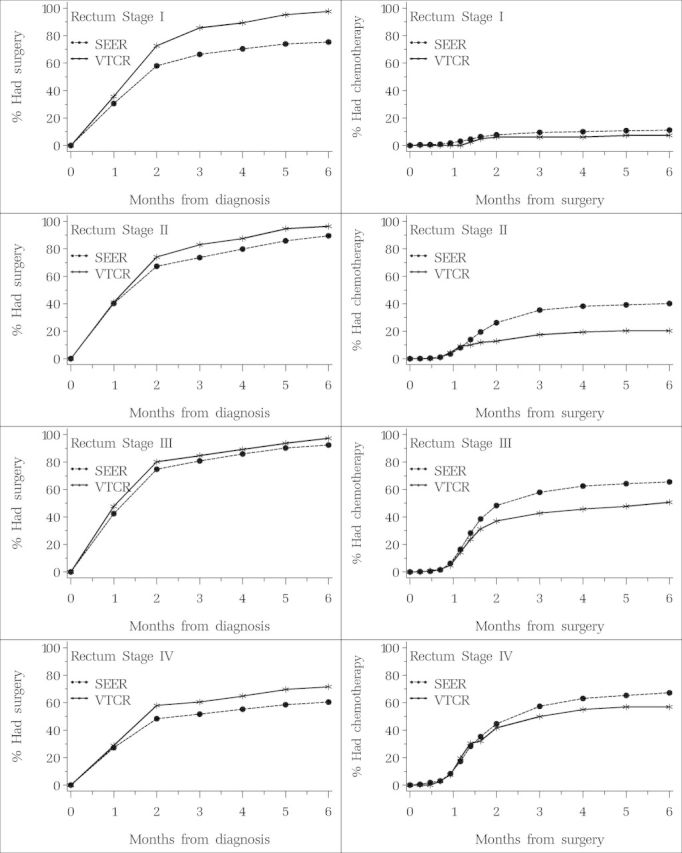
Percent having surgery by time since diagnosis and percent having chemotherapy (with or without radiotherapy) by time since surgery. Patients diagnosed with rectal cancer in Surveillance, Epidemiology, and End Results (SEER)–Medicare and Veneto–Tuscany Cancer Registry (VTCR) by stage.
Hospitalizations
The distribution of the number of hospital admissions in the year after diagnosis was similar in the two cohorts, across stages, although the mean number of inpatient days in VTCR patients was double that of SEER–Medicare patients (30 vs 15 days) (Table 4). This result is similar to the relative hospitalization pattern in the year prior to diagnosis in VTCR and SEER–Medicare patients (3.4 vs 1.7 days) (Table 2) and is consistent across stages. Furthermore, the distribution of patients by number of weeks in hospital showed a mode of 2 weeks in the SEER–Medicare cohort and 4 or more weeks in the VTCR cohort.
Table 4.
Hospitalizations in the first year following colorectal cancer diagnosis in 2000–2001; Surveillance, Epidemiology, and End Results (SEER)–Medicare and Veneto–Tuscany Cancer Registry (VTCR)
| All cases (stages I–IV) | Stage I | Stage II | Stage III | Stage IV | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEER–Medicare (n = 18 438) | VTCR (n = 1396) | SEER–Medicare (n = 5430) | VTCR (n = 213) | SEER–Medicare (n = 5905) | VTCR (n = 463) | SEER–Medicare (n = 4565) | VTCR (n = 412) | SEER–Medicare (n = 2538) | VTCR (n = 308) | |||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Number of hospital admissions | ||||||||||||||||||||
| 0 | 1230 | 7 | 61 | 4 | 726 | 13 | 11 | 5 | 195 | 3 | 21 | 4 | 135 | 3 | 15 | 4 | 174 | 7 | 14 | 5 |
| 1 | 8736 | 47 | 679 | 49 | 2770 | 51 | 121 | 57 | 2975 | 51 | 250 | 54 | 2020 | 44 | 199 | 48 | 971 | 38 | 109 | 35 |
| 2 | 4678 | 25 | 365 | 26 | 1053 | 20 | 53 | 25 | 1537 | 26 | 114 | 25 | 1321 | 29 | 106 | 26 | 767 | 30 | 92 | 30 |
| 3+ | 3794 | 21 | 291 | 21 | 881 | 16 | 28 | 13 | 1198 | 20 | 78 | 17 | 1089 | 24 | 92 | 22 | 626 | 25 | 93 | 30 |
| Number of hospital days | ||||||||||||||||||||
| 0 | 1230 | 7 | 61 | 4 | 726 | 14 | 11 | 5 | 195 | 3 | 21 | 4 | 135 | 3 | 15 | 4 | 174 | 7 | 14 | 5 |
| 1 week (1–7 days) | 4671 | 25 | 23 | 2 | 1809 | 33 | 6 | 3 | 1474 | 25 | 4 | 1 | 967 | 21 | 7 | 2 | 421 | 17 | 6 | 2 |
| 2 weeks (8–14 days) | 5838 | 32 | 336 | 24 | 1538 | 28 | 62 | 29 | 1969 | 33 | 119 | 26 | 1514 | 33 | 95 | 23 | 817 | 32 | 60 | 19 |
| 3 weeks (15–21days) | 2898 | 16 | 235 | 17 | 558 | 10 | 43 | 20 | 997 | 17 | 83 | 18 | 829 | 18 | 65 | 16 | 514 | 20 | 44 | 14 |
| 4+ weeks (22+ days) | 3801 | 21 | 741 | 53 | 799 | 15 | 91 | 43 | 1270 | 22 | 236 | 51 | 1120 | 25 | 230 | 56 | 612 | 24 | 184 | 60 |
| Total number of hospital days | 282 489 | 41 669 | 66 474 | 5620 | 95 121 | 13 200 | 78 692 | 12 354 | 42 202 | 10 495 | ||||||||||
| Mean number of days in hospital | 15 | 30 | 12 | 26 | 16 | 29 | 17 | 30 | 17 | 34 | ||||||||||
Discussion
In this study, we compared the characteristics of newly diagnosed elderly colorectal cancer patients in two cohorts from Italy and the United States and their patterns of care in the first year after diagnosis. Because of the structural differences in health-care organization and delivery between the United States and Italy, we made great efforts to ensure comparability of results. The major challenges of this study were to ensure that we had comparable cohorts and that we were comparing the same procedures and treatments in both data sources. The HDCs available in the VTCR cohort contained complete information for each hospitalization from a single source. For the SEER–Medicare cohort, treatment information was obtained from SEER data and Medicare claims that included inpatient hospitalizations, outpatient clinic services, and physician visits. Elaborate algorithms were needed for the databases to identify treatments.
More patients in the Italian cohort had advanced disease at diagnosis than did patients in the US cohort. The difference in stage at diagnosis can be explained, in part, by differences in use of colorectal screening between the United States and Italy. Screening programs in 2000–2001 in VTCR area barely reached 10% of the population aged 50 and older (25) and consisted mainly of fecal occult blood testing. A national formal screening program was not introduced in Italy until 2003 (26). In the same year, over 50% of the US population aged 65 and older had undergone colorectal cancer screening (27), reflecting the organized promotion of screening by the Medicare program. We also found more rightsided colon cancers in the US cohort than in the Italian cohort. This difference likely reflects the higher use of colonoscopy in the United States, where it is the predominant form for colorectal cancer screening (27).
We observed greater use of adjuvant therapy in the US cohort, especially in chemotherapy for stage III colon patients and chemotherapy and radiotherapy for stage II and III rectal patients. In 1990, the US National Institutes of Health issued a consensus statement for colorectal cancer treatment. The statement, which was based on evidence from clinical trials, concluded that chemotherapy should be offered as care for stage III colon cancer patients and chemotherapy and radiation therapy should be offered to stage II and III rectal cancer patients (28). The higher use of adjuvant therapy in the US cohort likely reflects a greater acceptance by US clinicians of the potential benefit of adjuvant therapy for elderly patients with cancer (29). This is notwithstanding underrepresentation of elderly cancer patients in clinical trials, thus resulting in uncertainty about whether older patients will benefit from adjuvant treatment (30).
US clinicians also gave more chemotherapy to stage IV colon cancer patients than did Italian clinicians: 57% of stage IV colon cancer patients in the SEER–Medicare cohort received chemotherapy, 12% more than what was reported for the VTCR cohort. Whether the higher use of chemotherapy among stage IV SEER–Medicare patients represents overuse of chemotherapy cannot be determined from these data. During the time of this study, oncologists paid by Medicare could profit from the administration of specific chemotherapy agents, potentially resulting in overtreatment, whereas oncologists in Italy did not have a financial incentive to prescribe chemotherapy.
Although use of adjuvant therapy was higher in the US cohort, the percentage of patients undergoing open surgery was higher among VTCR cohort. This was especially true for stage I rectal cancers (95% vs 77%) and stages I and II combined colon cancer (98% vs 90%). To assess if SEER–Medicare patients were more likely to have smaller tumors removed by polypectomy rather than open surgery, we examined SEER data from 2001 to 2002 for persons aged 65 or older who were diagnosed with stage I rectal cancer. We found that 28.5% of these patients had polypectomy reported to the SEER registry as their cancer surgery. Only 3.5% of SEER patients had no cancer surgery reported.
The percentage of IH days in VTCR patients was nearly double that of SEER–Medicare patients, despite the fact that the SEER–Medicare cohort was older and a larger percentage had higher comorbidity scores. The longer length of stay found for the Italian cohort was observed both before and after the cancer diagnosis and may reflect different government policies regarding hospital stays. Both countries adopted the diagnosis-related groups (DRG) system, whereby hospitals receive a lump sum payment for each patient, determined by the patient’s diagnosis, health status, and procedures performed during the hospitalization, thus giving hospitals a strong incentive to discharge patients as soon as possible following admission. However, its country-specific implementation has probably been different: At the time of study, in Italy a patient would stay in hospital during the presurgical period for diagnostic tests, whereas in the United States the same patient would have presurgical tests performed in the outpatient setting and be admitted to hospital on the day of their surgery. Differences in hospice programs in the United States and Italy could also have contributed to the observed shorter hospital stays for Medicare patients in our study. In the United States, Medicare hospice services are primarily home-based and allow patients to die at home instead of in hospital. The Medicare program has covered hospice services since 1986. In 2000–2001, a similar service had not yet been established in Italy and a higher proportion of terminal patients might have been hospitalized for end-of-life care.
Within stage of diagnosis, the patterns in both time to surgery after diagnosis and time from surgery to adjuvant therapy were similar in the VTCR and SEER–Medicare cohorts. Evaluation of time-to-care intervals will be important in future studies across health systems or countries as well as for patient subgroups.
Although this study appears to be the first to compare patterns of care between cohorts of cancer patients in the United States and a European country, there were several limitations. The data for this study are over 10 years old. However, 2000–2001 were among the only years that the VCTR data and HDCs were linked. Additionally, during the period of our study, cancer care, including surgery and adjuvant and neoadjuvant treatments, was hospital-based in Italy, allowing complete capture of cancer-related services. Comparisons of more contemporary patterns of care will be important in future studies. Our study was limited to cancer patients aged 66 and older, and although the majority of newly diagnosed cancer patients are in this older age group, we could not compare treatment patterns in the younger population. In both countries, we relied on administrative data to identify treatment receipt. Our study cohorts represented only a portion of colorectal cancer patients in Italy and the United States, and our results are not necessarily representative of the two countries. These data offer no insight into a physician’s recommendation regarding therapy or a patient’s decision to accept treatment. Finally, information on specific treatments, such as polypectomy, was incomplete and therefore could not be considered in our analysis.
Conclusions and Implications
In spite of structural differences between the United States and Italy in health-care organization and delivery, as well as in data collection, we can conclude that patterns of care and timing of care in the first year after diagnosis are generally similar among patients within stage of disease at diagnosis. The main differences in care were related to hospitalizations and use of adjuvant therapy. In Italy, length of hospital stay has become a major concern in more recent years, as hospitalization is the most costly component of care, and improving its organization represents an opportunity to reduce expenditures without affecting quality of care.
A more challenging question identified from this study relates to the use of chemotherapy for patients with stage IV cancer, where chemotherapy will not cure disease but may increase survival. With the introduction of expensive new agents to treat colorectal cancer, such as bevacizumab and cetuximab, the cost of colorectal cancer treatment has skyrocketed (1,3) and is expected to increase even more in the future. As such, costs of cancer care will put continuing stress on health-care budgets, and new strategies and policies thus become necessary. Presently in the United States under the Medicare program, treatment decisions cannot be made based on costs (2). In Italy since 2006, a national registry for antineoplastic drugs (available at http://antineoplastici.agenziafarmaco.it/) has been activated at the AIFA (Italian Medicines Agency). Assessment of patient eligibility and monitoring of treatment are preconditions for the hospitals to have the approval for use and reimbursement from the National Health System.
Future work with more recent data might include comparisons of biologically targeted therapies and hormonal treatments; different cancer sites, such as prostate cancer, where therapy recommendations are less standardized; and different approaches to end-of-life care. Comparisons between health-care delivery systems and countries, such as this one, can identify opportunities to improve health care and revise practice patterns. These analyses can also increase understanding of patient outcomes and economic consequences of differences in policies related to cancer screening, treatment, and programs of care.
Funding
The work has been partially supported by the National Cancer Institute at the National Institutes of Health, order no. HHSN261201200137P.
Appendix A. International Classification of Diseases, Ninth Revision, Clinical Modifications (ICD-9-CM) and Healthcare Common Procedure Coding System (HCPCS) codes used to define colorectal cancer treatment
| Colorectal treatment | ICD-9-CM procedure | HCPCS |
|---|---|---|
| Chemotherapy | 99.25 | J9000–J9999, 36260, 96400, 96405, 96406, 96408, 96410, 96412, 96414, 96420, 96422, 96423, 96425, 96440, 96445, 96450, 96520, 96530, 96542, 96545, 96549, 95990, 95991, A4301, E0782, E0783, E0784, E0785, E0786, G0355, G0357–G0360, C9411, J0207, J0640, J0880, J1190, J1440, J1441, J1950, J9217, J9218, J9219, J2405, J2430, J2505, J2820, J3487, J8520, J8521, J8530, J8560, J8565, J8600, J8610, J8700, J8999, K0415, KO416, Q0083, Q0084, Q0085, Q0136, Q0137, Q0179, S0177, S0181 |
| Pelvic exenteration | 68.8 | 51597 |
| Colectomy/ proctectomy | 45.71–45.76, 45.79, 45.8, 48.4, 48.41, 48.49, 48.5, 48.61–48.65, 48.69 | 44140, 44141, 44143–44147, 44150–44153, 44155, 44156, 44160, 45110–45114, 45116, 45119, 45123, 45160, 45170 |
| Permanent colostomy | 46.1, 46.10, 46.13 | |
| Radiation therapy | 92.21–92.33, 92.39 | 76370, 76950, 77261–77263, 77280, 77285, 77290, 77295, 77299, 77300, 77301, 77305,77310, 77315, 77321, 77326, 77327, 77328, 77331–77334, 77336, 77370, 77399, 77401–77404, 77406, 77407–77409, 77411–77414, 77416, 77417, 77427, 77431, 77432, 77470, 77499, 77520, 77523, 77750, 77761–77763, 77776–77778, 77781–77784, 77789, 77790, 77799 |
The seminal idea behind this work was born during the international workshop on “Combining Epidemiology and Economics for Measurement of Cancer Costs,” held in Frascati, Italy in September 2010.
References
- 1. Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12(10):933–980 [DOI] [PubMed] [Google Scholar]
- 2. Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626–633 [DOI] [PubMed] [Google Scholar]
- 3. Schrag D. The price tag on progress—chemotherapy for colorectal cancer. N Engl J Med. 2004;351(4):317–319 [DOI] [PubMed] [Google Scholar]
- 4. Elkin EB, Bach PB. Cancer’s next frontier: addressing high and increasing costs. JAMA. 2010;303(11):1086–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Warren JL, Barbera L, Bremner KE, et al. End-of-life care for lung cancer patients in the United States and Ontario. J Natl Cancer Inst. 2011;103(11):853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung D, Hersey K, Fleshner N. Differences between urologists in United States and Canada in approach to bladder cancer. Urology. 2005;65(5):919–925 [DOI] [PubMed] [Google Scholar]
- 7. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907 [DOI] [PubMed] [Google Scholar]
- 8. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90 [DOI] [PubMed] [Google Scholar]
- 9. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, eds. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [database online]. Lyon, France: International Agency for Research on Cancer; 2010. http://globocan.iarc.fr Accessed February 15, 2013 [Google Scholar]
- 10. Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8(9):784–796 [DOI] [PubMed] [Google Scholar]
- 11. Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9(8):730–756 [DOI] [PubMed] [Google Scholar]
- 12. Gatta G, Capocaccia R, Berrino F, Ruzza MR, Contiero P. EUROPREVAL Working Group. Colon cancer prevalence and estimation of differing care needs of colon cancer patients. Ann Oncol. 2004;15(7):1136–1142 [DOI] [PubMed] [Google Scholar]
- 13. Gatta G, Zigon G, Aareleid T, et al. Patterns of care for European colorectal cancer patients diagnosed 1996–1998: a EUROCARE high resolution study. Acta Oncol. 2010;49(6):776–783 [DOI] [PubMed] [Google Scholar]
- 14. Allemani C, Storm H, Voogd AC, et al. Variation in “standard care” for breast cancer across Europe: a EUROCARE-3 high resolution study. Eur J Cancer. 2010;46(9):1528–1536 [DOI] [PubMed] [Google Scholar]
- 15. Groome PA, O’Sullivan B, Irish JC, et al. Management and outcome differences in supraglottic cancer between Ontario, Canada, and the Surveillance, Epidemiology, and End Results areas of the United States. J Clin Oncol. 2003;21(3):496–505 [DOI] [PubMed] [Google Scholar]
- 16. Groome PA, O’Sullivan B, Irish JC, et al. Glottic cancer in Ontario, Canada and the SEER areas of the United States. Do different management philosophies produce different outcome profiles? J Clin Epidemiol. 2001;54(3):301–315 [DOI] [PubMed] [Google Scholar]
- 17. Paci E, Crocetti E, Benvenuti A, et al. Tuscany cancer registry. In: Curado MP, Edwards B, Shin HR, et al., eds. Cancer Incidence in Five Continents. Vol. 9 Lyon, France: IARCPress; 2007. IARC Scientific Publications No. 160. [Google Scholar]
- 18. Zambon P, Andolfo A, Baracco M, et al. Cancer incidence in Italy, Veneto region (1998–2001. In: Curado MP, Edwards B, Shin HR. et al. , eds. Cancer Incidence in Five Continents. Vol. 9 Lyon, France: IARCPress; 2007. IARC Scientific Publications No. 160. [Google Scholar]
- 19. World Health Organization International Classification of Diseases, Ninth Revision, Clinical Modification. Geneva, Switzerland: World Health Organization; 1997 [Google Scholar]
- 20. Cislaghi C, Giuliani F. L’Out of Pocket sanitario nelle Regioni Italiane. In: Ancona A, ed. Approfondimenti sull’indagine Multiscopo ISTAT 2005. Roma, Italy: AGENAS; 2008. Monitor No. 22 (suppl 3). [Google Scholar]
- 21. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl 8):IV-3–IV-18 [DOI] [PubMed] [Google Scholar]
- 22. American Joint Committee on Cancer Colon and rectum. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed New York, NY: Springer-Verlag; 2010:143–164 [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383 [DOI] [PubMed] [Google Scholar]
- 24. Benson AB, III, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408–3419 [DOI] [PubMed] [Google Scholar]
- 25. Ederle A, Grazzini G, Senore C. Lo screening dei tumori colorettali in Italia. In: Rosselli Del Turco M, Zappa M, eds. Terzo Rapporto dell’Osservatorio Nazionale per la Prevenzione dei Tumori Femminili, 2004. http://www.osservatorionazionalescreening.it/node/52 Accessed June 5, 2012 [Google Scholar]
- 26. Masseria C. Colorectal cancer in Italy: a review of current national and regional practice on screening and treatment. Eur J Health Econ. 2010;10(suppl 1):S41–S49 [DOI] [PubMed] [Google Scholar]
- 27. US Department of Health and Human Services Cancer Trends Progress Report—2009/2010 Update. http://progressreport.cancer.gov Accessed June 5, 2012 [Google Scholar]
- 28. NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264(11):1444–1450 [PubMed] [Google Scholar]
- 29. Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–1097 [DOI] [PubMed] [Google Scholar]
- 30. Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol. 2002;20(19):3992–3998 [DOI] [PubMed] [Google Scholar]


